Translate this page into:
Therapeutic uses of post-partum tissue-derived mesenchymal stromal cell secretome
For correspondence: Prof Preethi Vidya Udagama, Department of Zoology & Environment Sciences, Faculty of Science, University of Colombo, Kumarathunga Munidasa Mawatha, Colombo 03, 00300, Sri Lanka e-mail: preethi@zoology.cmb.ac.lk
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Human post-partum tissue mesenchymal stromal cells (hPPT-MSCs) are widely used in research to investigate their differentiation capabilities and therapeutic effects as potential agents in cell-based therapy. This is ascribed to the advantages offered by the use of MSCs isolated from hPPT over other MSC sources. A paradigm shift in related research is evident that focuses on the secretome of the human MSCs (hMSCs), as therapeutic effects of hMSCs are attributed more so to their secreted growth factors, cytokines and chemokines and to the extracellular vesicles (EVs), all of which are components of the hMSC secretome. Positive therapeutic effects of the hPPT-MSC secretome have been demonstrated in diseases related to skin, kidney, heart, nervous system, cartilage and bones, that have aided fast recovery by replacing damaged, non-functional tissues, via differentiating and regenerating cells. Although certain limitations such as short half -life of the secretome components and irregular secreting patterns exist in secretome therapy, these issues are successfully addressed with the use of cutting-edge technologies such as genome editing and recombinant cytokine treatment. If the current limitations can be successfully overcome, the hPPT-MSC secretome including its EVs may be developed into a cost-effective therapeutic agent amenable to be used against a wide range of diseases/disorders.
Keywords
Extracellular vesicles
human mesenchymal stromal cell secretome
post-partum tissue
stem cell therapy
Introduction
Stem cell (SC) research has brought regenerative medicine to the forefront of cell-based clinical research and therapy due to promising outcomes. SC research has produced cells, tissues and whole organ-like structures in vitro using SCs to replace damaged or non-functional tissues or organs by transplantation1. Diseases such as autism2 and muscular dystrophies3 which are labelled as incurable have gained promising results using SC therapy.
Pluripotent embryonic SCs (ESCs) isolated from the blastocyst stage of embryos have ethical concerns than the multipotent adult SCs which are isolated from the bone marrow, brain and heart tissue; post-partum tissue (PPT) such as umbilical cord (UC), UC blood (UCB), amniotic membrane (AM) and placenta and surgical waste such as deciduous teeth pulp and adipose tissue (AD)4. As ESC sources are the stored embryos at assisted reproductive centres, varying ethical issues dependent on the country of use, in addition to their tumourigenic capacity have limited the use of ESCs in research5. Induced pluripotent SCs, adult cells which are reprogrammed to function as embryonic-like SCs, have also shown great potential in therapeutics6. Among the different types of adult SCs, bone marrow SCs (BM-SCs) have widely been used in research. With the ability of establishing SCs from biological and surgical waste, SC research has flourished mainly due to the minimum ethical considerations associated with the use of such waste starting material7. High availability8, absence of tumourigenicity and favourable immune-privileged effects9 are other reasons for the increased use of SCs in biological waste material to fulfil the demand of the ever-rising numbers of therapy trials. Mesenchymal stromal cells (MSCs) and haematopoietic SCs (HSCs) derived from PPT are such adult SC categories that are in the initial stages of clinical trials. As at November 2020, the US National Institutes of Health SC registry lists 5685 SC-related clinical trials, of which 86 are related to HSCs derived from cord blood and 58 trials related to MSCs from other PPTs10.
With the use of relatively easy isolation methods, low rejection rates, high availability and wide differentiation potential, PPT-SCs have gained attention in SC research. hMSCs can be isolated from all PPTs using either digestion or explant methods. UCB provides a source to isolate HSCs by selecting CD34+ cells and expanding them in a suitable medium supplemented with selected cytokines as non-adherent cultures11 or else UCB can be used to isolate MSCs by expanding mononuclear cells (MNCs) as adherent cultures12. UCB-hMSC showed significantly higher proliferation, clonality and/or significantly lower expression of p53, p21 and p16, well known markers of senescence, compared to BM and AD hMSCs11. However, successful isolation of hMSCs from UCB is believed to depend on the time between collection and isolation, the net volume of blood and the MNC count; hence, the isolation process itself becomes laborious and time-consuming, resulting in low yields of hMSCs13. Due to such difficulties confronted, as well as contemplation by parents on storing UCB in blood banks for future use of their child, the use of other types of PPT are considered. Although their self-renewal capacity when compared with other hMSCs was not significantly different, placenta-hMSC showed high proliferative capacity and better growth characteristics than bone marrow, adipose-derived and UCB-hMSCs11. Expression of stemness markers was not significantly different between these hMSCs, making UCB and placenta-hMSCs potential candidates for research akin to bone marrow- or adipose-derived hMSCs11. Although 5-50 per cent of SC marker-positive cells reside within the population of amniotic epithelial cells, a mere 0.01-0.1 per cent SCs are present within the other residing tissue types14, making the AM a rich source of SCs compared to somatic tissues. Human AM-MSCs, UC-MSCs and UCB-MSCs also demonstrate immunosuppressive properties151617. In an in vitro study, human placenta-MSCs have shown a significantly higher ability of immunosuppression compared to human UC-MSCs18.
It is believed that most of the therapeutic effects are due to different bioactive molecules such as growth factors, cytokines, chemokines and angiogenic factors that are secreted by SCs which are collectively known as the 'stem cell secretome’, and all these molecules have been thoroughly investigated19. It is reported that the UC-MSC secretome is significantly different from the bone marrow- and adipose-derived SC secretomes20. This article reviews the therapeutic effects of the UC-derived mesenchymal SC secretome and highlights the pros and cons of its applications, compared to other SC secretomes.
Composition of the PPT-MSC secretome
A typical hMSC secretome is known to contain growth factors, cytokines, extracellular vesicles, lipid mediators, extracellular membrane proteases and hormones21, causing differential effects on the treated cells.
Growth factors and cytokines
Composition of the Wharton's jelly (WJ) hMSC secretome was investigated using homonuclear magnetic resonance and multiplexing laser bead technology in a study, where it was discovered that compared to unconditioned medium, conditioned medium consisted of increased levels of transforming growth factor-β1, epidermal growth factor (EGF), granulocyte colony-stimulating factor (G-CSF), granulocyte macrophage colony-stimulating factor (GM-CSF), platelet-derived growth factor-AA and vascular endothelial growth factor (VEGF) as well as a range of cytokines such as interleukin (IL)-12p70, interferon-gamma, IL-17A and IL-1022. VEGF and fibroblast growth factor (FGF) possess cardioprotective and cardioregenerative effects23; the inclusion of VEGF in the UC-hMSC secretome may lead the secretome to manifest such properties. Wound healing capacity of the secretome may be attributed to the presence of IL-6, IL-8 and MCP-1 that enhance monocyte migration into injured sites, thereby suggesting the migration of other cell types such as fibroblasts into the wound sites with the help of mentioned cytokines when treated with the secretome24. There is a wide range of proliferative and anti-apoptotic growth factors, immunomodulatory, immunosuppressive cytokines and chemokines listed as constituents of the secretome22, which may well surmount to different activities and effects exerted by the secretome.
Extracellular vesicles (EVs)
Other than the soluble factors of the secretome, extracellular vesicle (EV) is an additional distinct component with a size range of 80 nm to 1 μm25 and categorized into three subtypes: exosomes, microvesicles and apoptotic bodies26. Components such as proteins, lipids and functional genetic material [DNA, microRNA (mRNA) and fragmented DNA] present in these vesicles are transferred into other target cells aiding regulation requirements for therapeutic procedures in SC therapy27. UC-MSC-derived nanovesicles have been reported to confer therapeutic effects on skin burn rat models by accelerating skin damage repair via Wnt-signalling pathway28 and murine models on hypoxic pulmonary hypertension by exerting lung protection and reducing pulmonary hypertension via STAT-3-mediated signalling pathway29. Both these studies report that the exosome-carrying EVs are responsible for the therapeutic effects, suggesting mRNA-mediated cell signalling.
Large-scale manufacturing of EVs is required to be used in therapeutic platforms. Different culture systems with varying parameters such as thermal stress, hypoxia, radiation, increase of intracellular calcium levels and sulphydryl-blocking agents have been identified as potential factors which enhance the EV-secreting ability30.
Significance of the hPPT-MSC secretome
Of the MSC secretomes, the UC-MSC secretome has proven to be significantly different from BM-MSC and adipose-derived MSC secretomes31. UC-MSCs show significantly reduced synthesis of important proangiogenic factors but increased secretion of angiogenic growth factors and chemokines when compared to BM-MSCs and AD-MSCs323334. UC-MSCs have also demonstrated significantly higher increased secretion of neurotrophic factors35, important cytokines and haematopoietic growth factors than the BM-MSC and AD-MSC secretomes36, pointing towards the potential benefits of therapy specific to the UC-MSC secretome. A study comparing the effects of BM-MSC and WJ-MSC secretomes on neural differentiation demonstrated different temporal profiles regarding stimulation of neurite outgrowth and the gene expression of neuronal markers37. Although proteomic-based mass spectrometry has shown differences of protein profiles among the BM, AD and UC-MSC secretomes, it also confirmed that the UC-MSC secretome is a potential candidate for neuroregenerative research as much as the other two secretomes31. UC-MSCs cultured using post-partum waste and the resultant secretome obtained with ease, together with minimum ethical considerations, will augment its value in cell-free therapeutic procedures. The following subsections highlight research in which UC-MSC secretome was investigated in different therapeutic procedures against a wide range of diseases.
Therapeutic effects of hPPT-MSC secretome
Anti-ageing and other skin repair therapies
Skin is the main target of most cosmetic products. Anti-ageing and skin tone-lightning products are enormously marketed by pharmaceutical and cosmetic companies. Importance of using naturally derived stimulants or inhibitors for cosmetic purposes is highly recommended as skin is a very sensitive organ and the consumers are extremely cautious about the side effects and toxicity of such products. Pharmaceutical companies are focusing on naturally derived components to reduce their production costs which may target a wide array of the population regardless of their economic status.
Late recovery of skin wounds caused by different injuries is also a growing concern as it decreases the quality of life of the patient by scar formation and increased risk of infection38. Diabetic wounds result only in 50 per cent short-term recovery, even under high standard treatment methods39, which suggests that the current therapeutic methods require change. Conversely, burn wounds also require critical care to stabilize and functionally recover the patients40. Table I lists the research where PPT-derived SC-conditioned medium was used to manifest anti-ageing and anti-melanogenesis effects, as well as to successfully recover wounds of different origin, i.e., diabetic wounds and burn wounds. Type of animal model or human cell lines used for these experiments, the outcomes and plausible underlying mechanisms of each report are also summarized in Table I.
| Type of secretome/CM | Disease | Animal models or cell type | Outcome | Possible mechanisms | References |
|---|---|---|---|---|---|
| WJ-hMSC | Ageing effects | UVA irradiated human dermal fibroblasts (in vitro) | Increased proliferation, migration rates and TGF-β signalling41 | Increased cell migration via TGF- β smad signalling pathway | Sánchez-A, 200542 |
| UC-hMSC | Ageing effects | Human dermal fibroblasts in high glucose induced diabetic microenvironment (in vitro) | Decreased ROS production and senescence | Antioxidant and anti-ageing effects through downregulating expression of senescence-related genes | Li et al, 201743 |
| UC-hMSC Subcutaneous administration into animal model | Diabetic wounds | Delayed wound healing mouse models (diabetic wounds) (in vivo) | Significantly higher wound closure rates, capillary densities and PDGF-β, KGF, VEGF expression levels | By increasing expression of important growth factors related to dermal healing | Shrestha et al, 201344 |
| UC-hMSC Burn wounds were topically treated | Burn wounds | Rats with induced burn wounds (in vivo) | Acceleration of wound closure. High density of collagen fibers, increased numbers of fibroblasts and blood vessels | bFGF-mediated cell regeneration | Padeta et al, 201745 |
| Hypoxic AF-hMSC Subcutaneous administration into animal model | Skin wounds | Rats with induced wounds (in vivo) and human skin fibroblasts (in vitro) | Enhanced proliferation and migration of human dermal fibroblasts in vitro and wound healing in rat model | Via TGF-β/SMAD2 and PI3K-PKB/Akt pathways | Jun et al, 201446 |
| UC-hMSC Wounds were topically treated | Wound healing | Human umbilical vein endothelial cells (in vitro) and rats with induced wounds (in vivo) | Decreased inflammation at initial stage, cell migration and angiogenesis stimulation in vitro and in vivo | - | Kusindarta et al, 201647 |
| UC-hMSC infected with Wnt7a-expressing virus Subcutaneous administration into animal model | Cutaneous wounds | Mice with full thickness skin injury (in vivo) | Stimulation of wound closure and regeneration of hair follicles | Via activating fibroblasts enhanced secretory expression of ECM components which promotes keratinocyte migration and reepidermalization. Also enhances crosstalk between cells in complex wound microenvironment | Dong et al, 201748 |
| UCB-hMSC | Melanin synthesis (cosmetic use) | Hyperpigmented melanoma cells and normal human epidermal melanocytes (in vitro) | Inhibition of melanogenesis | Via degradation of MITF expression via the ERK signalling pathway | Kim et al, 201549 |
| UCB-hMSC Topical treatment with CM on wrinkle sites | Skin ageing | Dermal fibroblasts and women with wrinkles | Stimulate growth and production of HDFs by ECM, promoted antiwrinkle effect and dermal density was significantly increased in women | GDF-11 aided in promoting skin rejuvenation via upturned growth and ECM production of human dermal fibroblasts | Kim et al, 201850 |
| WJ-hMSC | Radioactive dermatitis | UVEC and rat models with radiation induced skin wounds | Stimulated proliferation of UVECs, sebaceous glands were regenerated and stimulated angiogenesis and wound healing in vivo | - | Sun et al, 201951 |
CM, conditioned medium; ECM, extracellular matrix; MITF, microphthalmia-associated transcription factor; HDFs, human dermal fibroblasts; ECM, extracellular matrix; GDF-11, growth differentiation factor-11; UVEC, umbilical vein endothelial cells; TGF- β, transforming growth factor beta; ROS, reactive oxygen species; hMSC, human mesenchymal stromal cells; PDGF, platelet-derived growth factor; VEGF, vascular endothelial growth factor; KGF, keratinocyte growth factor; BFGF, basic fibroblast growth factor; PI3K, phosphoinositol-3-kinase; WJ, Wharton’s jelly; UCB, umbilical cord blood; UC, umbilical cord; AF, amniotic fluid; ERK, extracellular signal-regulated kinase; UVA, ultraviolet A, ; PKB, protein kinase B
Anticancer therapy and other cancer-related therapy
As projected in 2012, by 2030, of the global cancer burden, new cancer cases will account to 21.7 million and cancer deaths are calculated around 13 million52. Despite cancer screening programmes for early detection, public awareness programmes and treatment methods linked with novel technological advances, cancer had struck globally with no impact of the economic status of the countries53 Effective treatment is a major component of a balanced approach to cancer54, where anticancer drugs and other methods to eliminate cancer are investigated to match the ever-rising numbers of cancer patients and different cancer types. Of the small molecules approved as anti-cancer drugs from 1940s to 2012, 48.5 per cent were reported to be natural products or derivatives of natural products54. However, cytokines, growth factors and other compounds extracted from human biological material appear to be equally effective in anticancer therapy; hence, the hMSC secretome was investigated for its anticancer potential. Table II elaborates the use of human PPT (hPPT)-MSC secretome on anticancer-related therapy for human laryngeal carcinoma, lung cancers, leukaemia, hepatic and cervical cancers investigated in in vitro studies using human cancer cell lines. The outcomes were apoptosis, inhibition of drug resistant effects, antiproliferative and cell viability effects; the associated mechanisms are listed in Table II. In addition, Zimmerlin et al61, reported on many MSC-secreted factors effective on a wide range of cancers including non-specified paracrine factors secreted from UC-MSCs.
| Type of secretome/CM | Type of cancer | Human cell line used | Outcome | Possible mechanisms | References |
|---|---|---|---|---|---|
| WJ-hMSC | Human laryngeal carcinoma | Hep-2 cell line (in vitro) | Caused apoptosis in Hep-2 cells | Via increase p53 and decrease of Bcl-2 | Elias et al, 201655 |
| WJ-hMSC | Lung cancer | A549 lung cancer cells (in vitro) | Drug resistant effects inhibited | - | Hendijani et al, 201556 |
| WJ-hMSC | Leukaemia cells | K562 leukaemia cells | Significant antiproliferative effects | - | Hendijani et al, 201457 |
| AM-hMSC | Hepatic carcinoma | HepG2 cell line (in vitro) | Decreased cell proliferation and cell viability | Via increased expression of p53, p21 and Caspase 3 (proapoptotic mRNA) and diminished expression of Ki-67 (cell proliferation marker) | Riedel et al, 201758 |
| UCB-hMSC | Cervical cancer | HeLa cell line (in vitro) | Significantly induced apoptosis | Via mitochondrial apoptotic pathway | Sandra et al, 201459 |
| WJ-MSC | Breast Cancer | MCF-7 cell line (in vitro) | Induced apoptosis | Not specified | Mirabdollahi et al, 201960 |
CM, conditioned medium; WJ, Wharton’s jelly; hMSC, human mesenchymal stromal cells; AM, amniotic membrane; UCB, umbilical cord blood; mRNA, microRNA
Use of hPPT-MSC secretome in therapeutic procedures against various other diseases
In vitro differentiated cells have the advantage of aiding fast recovery of the patient, rather than the time-consuming method of transplanting undifferentiated SCs which would differentiate and then replace the non-functional or injured tissue. Procedures to differentiate SCs into a variety of mature cell types in vitro and in vivo under different stimulated conditions such as by adding synthetic and natural compounds have been investigated; and, the hMSC secretome rich in various growth factors and cytokines has also been explored. In 2014, a phase 1 clinical trial was set up with 20 patients, to investigate the effect of microvesicles derived from UCB-MSCs, to decrease the inflammatory state and enhance the β-cell mass as well as the glycaemic control62. Two recent studies have also been registered on NIH clinical trials, US data base, on uses of secretome of adipose derived MSC for the treatment of Osteoarthritis and for Articular Regeneration and using hypoxia-MSC secretome to treat COVID-19 patients6364. Table III summarizes such research where the secretome was used in cell differentiation and cell protection protocols for therapeutic applications in cartilage disorders, Parkinson's disease, ischaemia, cardiotoxicity, acute myocardial infarction, pulmonary artery hypertension, chronic renal disease and skeletal muscle atrophy.
| Type of secretome/CM | Therapeutic application | Animal models or cell type | Outcome | Possible mechanisms | References |
|---|---|---|---|---|---|
| Thrombospondin-2 secreted by UCB-hMSC | Cartilage disorders | Chondro progenitor cells and rabbits with full-thickness osteochondral defects | Increased chondrogenic effects | Through signalling pathways such as PKCα, ERK, p38/MAPK and notch | Jeong et al, 201365 |
| WJ-hMSC | Cartilage disorders | Chondrocytes | Increased expression of cartilage specific genes | Via significantly enhanced expression of collagen type II, Sox-9, aggrecan and COMP genes | Hassan Famian et al, 201766 |
| Amniotic epithelial cells | Dopaminergic neuron to treat Parkinson’s disease | UCB-hMSC | Differentiation into dopaminergic neuron-like cells | Through neurotrophic factor BDNF and NGF, derived in brain | Yang et al, 201367 |
| UCB-hMSC | Protection of ischaemic cardiomyocytes | Murine HL-1 cardiomyocytes subjected to stimulated ischaemia | Decreased number of dead cells and increased viability | Via enhancement of Akt, ERK and transcription factor STAT3 (cell survival promoting kinases) phosphorylation | Bader et al, 201368 |
| Amniotic fluid SC | Protection from cardiotoxicity | H9c2 cardio myoblasts and primary mouse neonatal ventricular cardiomyocytes | Blockage doxorubicilin induced cardiotoxicity senescence and apoptosis | Via activation of PI3K/Akt signalling cascade and upregulation of its related genes | Lazzarini et al, 201669 |
| AM-hMSCs Injecting CM into infarcted rat hearts | Acute myocardial infarction | Rat models with heart infarcts | Infarct size limitation, reduced cardiomyocyte apoptosis, ventricular remodeling and increased capillary formation | Via activation of prosurvival ERK1/2 MAPK pathway and inhibition of SAPK/JNK and p38 MAPK proapoptotic pathways68 | Danieli et al, 201570 |
| UCB-hMSCs Infused CM into rat models via tail vein | PAH | monocrotaline induced PAH rat model | Reduced ventricular pressure, the right ventricle/(left ventricle + interventricular septum) ratio and respiratory functions properly managed | Via enhanced IL-1α, CCL5 and TIMP-1 levels | Lee et al, 201671 |
| UC-hMSC Prior to administration of CM via the left renal artery, total ligation of the left ureter was done | Chronic renal disease | Rat model with unilateral ureteral obstruction | Positive treatment of renal interstitial fibrosis | Via significant reduction of MDA and ROS and enhanced activity of GSH | Liu et al, 201772 |
| UC-hMSC Soleus muscles of both hind legs were injected with CM | Skeletal muscle atrophy | Hind limb muscle atrophy models | Significantly improved muscle mass and muscle fiber size | Via enhancing the PI3K-PKB/Akt signalling cascade | Kim et al 201673 |
| UC-hMSC | Irradiation myocardial fibrosis | Irradiated primary HCF | Improved cell viability, reduced collagen deposition, prevented oxidative stress, increased antioxidant status and reduced pro-fibrotic cytokines | Via inhibition of the NF-κB signalling pathway | Chen et al, 201874 |
| UC-hMSC Administered via left renal artery | Renal fibrosis | Rat models with renal interstitial fibrosis | Decreased deposition of extracellular matrix, inflammatory cell infiltration and release of inflammatory factors | Via inhibiting TLR4/NF-κB signalling pathway activation | Liu et al, 201875 |
| Extra cellular vesicles of Adipose derived MSC Intravenous administration | Autoimmune Encephalomyelitis (AE) | Mice models with induced experimental (AE) | Reducing proliferative potency of T cells, leukocyte infiltration, and demyelination | Not reported | Jafarinia et al, 202076 |
BDNF, brain-derived neurotrophic factor; NGF, nerve growth factor; PI3K, phosphoinositol-3-kinase; SC, stem cell; PAH, pulmonary artery hypertension; HCF, human cardiac fibroblasts; MDA, malondialdehyde; ROS, reactive oxygen species; GSH, glutathione; hMSC, human mesenchymal stromal cells; UCB, umbilical cord blood; UC, umbilical cord; AM, amniotic membrane ; PKCα, Protein Kinase C-alpha ; ERK, extracellular signal-regulated kinase; p38/MAPK, p38 mitogen-activated protein kinases; TLR4/NF-κB, Toll-like receptor 4/nuclear transcription factor-κB; PKB, Protein Kinase B
In addition, HSCs were also reported to increase their proliferation rates due to paracrine factors secreted by WJ-hMSCs such as IL1a, IL-6, IL-7, IL-8 cytokines, hyaluronic acid, cell adhesion molecules, cadherins and growth factors [stem cell factor and hepatocyte growth factor (HGF)] which are secreted in high amounts than BM-hMSC3677. Figure presents the gist of the review in a nutshell.
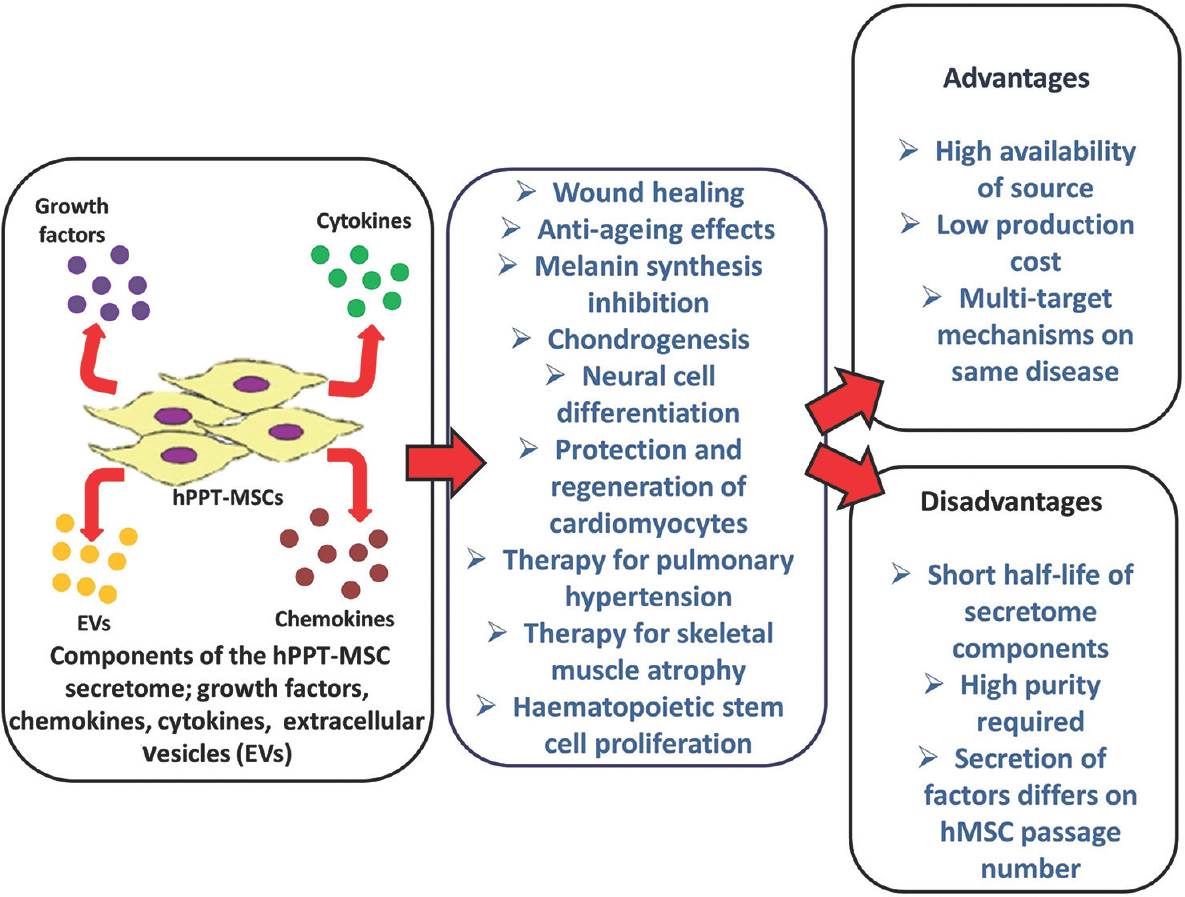
- Human post-partum tissue mesenchymal stromal cells secrete growth factors, chemokines, cytokines and extracellular vesicles which form the components of its secretome. These secretome components manifest different therapeutic effects. List of advantages and disadvantages of the therapeutic use of human post-partum tissue mesenchymal stromal cell secretome over current cell therapy methods is provided.
Limitations and the way forward with the hPPT-MSC secretome
Although many potential beneficial therapeutic advantages of the PPT-MSC secretome are apparent, yet the most important issue involved is controlling the MSCs to continuously secrete the required factors in adequate amounts, because the secretion of secretome factors varies due to the state of the cells and the passage number of the cell line7879. In therapeutic procedures where hPPT-MSCs are transplanted, the short half-life of the secreted factors, such as HGF which only remains viable for 3-5 min, raises another concern; administering continuous doses of such secreted stimulants with extremely short half-lives to patients is required for positive therapeutic effects80. Solution to this problem was provided with the use of genome-editing technologies, where the genome of UCB-hMSCs was edited to render the cells continuously secrete HGF, but in an induced manner76. Furthermore, treatment of hMSCs with different cytokine cocktails modified the hMSC secretome by directing hMSCs to secrete specific required factors to render considerable therapeutic effects against, for example, liver inflammation by improving the immunomodulatory capacity81.
Existing literature supporting the presence of the therapeutic effects of EVs is another future aspect of the hMSC secretome to be examined. Purification of these EVs from the secretome is important as a study demonstrated that the purity of EVS secreted by UC-hMSCs is a limiting factor for their immunosuppressive effects82. Provision of solutions to issues related to the therapeutic uses of MSC secretome by means of gene editing, cytokine therapy and extrapurification procedures may however, lead to increased charges of such therapeutics, rendering these unavailable for the developing world. This single reason could mar the beneficial use of the hMSC secretome or its secreted components in therapy; hence, when producing at the commercial scale, it is crucial to adapt to procedures where the current limitations will be overcome in a cost-effective manner. Furthermore, the mRNAs transported by exosomes had been reported to be mediators of cancer communication and also associated with a number of neurodegeneration disorders; hence, further analysis of these derivatives should be done before clinical applications83. Use of standardized herbal extracts as an alternative may be an inexpensive option as a range of herbal extracts have shown proliferation and differentiation abilities when used on SCs7, suggestive of induced changes to the secretome. Countries rich in biodiversity and traditional medicine knowledge can actively contribute to achieve this goal, collaborating with countries which possess cutting edge technological advances, so that ‘induced secretome therapy’ may be affordable globally.
Conclusions
The properties of the hPPT-MSC secretome, provided through a strong and growing body of evidence, bear ample testimony to the potential therapeutic usage of it. However, extensive clinical trials are warranted to reinforce facts and figures obtained by in vitro and in vivo animal studies. Limiting factors of the hPPT-MSC secretome in therapeutic usage can be surmounted by strategies with the help of cutting-edge technologies. However, there is a risk in decreasing the cost-effectiveness of the proposed secretome therapy by the use of such novel technological advances using expensive reagents and equipment; hence, as an alternative, standardized herbal extracts may be used which are naturally available, cheaper, non-toxic and scientifically proven and are effective on hMSCs that will render these cells and their secretome therapeutically feasible, by inducing hMSCs to secrete its components selectively, in a continuous manner. If the current limitations posed may be successfully overcome, the secretome of PPT MSCs inclusive of its EVs may become an effective therapeutic agent which could be used against a wide range of diseases/disorders.
Financial support & sponsorship: National Science Foundation, Sri Lanka (RG/2015/HS/01), is acknowledged for funding.
Conflicts of Interest: None.
References
- Transplantation of human cord blood mononuclear cells and umbilical cord-derived mesenchymal stem cells in autism. J Transl Med. 2013;11:196.
- [Google Scholar]
- Human umbilical cord mesenchymal stem cells in the treatment of duchenne muscular dystrophy: Safety and feasibility study in India. J Stem Cells. 2015;10:141-56.
- [Google Scholar]
- Clinical Trials with mesenchymal stem cells: An Update. Cell Transplant. 2016;25:829-48.
- [Google Scholar]
- A pathologist's perspective on induced pluripotent stem cells. Lab Invest. 2017;97:1126-32.
- [Google Scholar]
- Potential role of herbal remedies in stem cell therapy: Proliferation and differentiation of human mesenchymal stromal cells. Stem Cell Res Ther. 2016;7:110.
- [Google Scholar]
- Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011;9:12.
- [Google Scholar]
- Wharton's jelly-derived mesenchymal stem cells: Phenotypic characterization and optimizing their therapeutic potential for clinical applications. Int J Mol Sci. 2013;14:11692-712.
- [Google Scholar]
- NIH US National Library of Health: Clinical trial register. Available from: https://clinicaltrials.gov/ct2/results?term=Stem+cells&Search=Search
- Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. Int J Mol Sci. 2013;14:17986-8001.
- [Google Scholar]
- Expansion of human cord blood hematopoietic stem cells for transplantation. Cell Stem Cell. 2010;7:427-8.
- [Google Scholar]
- Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cells. 2004;22:625-34.
- [Google Scholar]
- Comparison of molecular profiles of human mesenchymal stem cells derived from bone marrow, umbilical cord blood, placenta and adipose tissue. Int J Mol Med. 2016;37:115-25.
- [Google Scholar]
- Amnion-derived stem cells: In quest of clinical applications. Stem Cell Res Ther. 2011;2:25.
- [Google Scholar]
- Dose-dependent immunomodulatory effect of human stem cells from amniotic membrane: A comparison with human mesenchymal stem cells from adipose tissue. Tissue Eng. 2007;13:1173-83.
- [Google Scholar]
- Human umbilical cord mesenchymal stem cells hUC-MSCs exert immunosuppressive activities through a PGE2-dependent mechanism. Clin Immunol. 2010;135:448-58.
- [Google Scholar]
- The immunomodulatory activity of human umbilical cord blood-derived mesenchymal stem cells in vitro. Immunology. 2009;126:220-32.
- [Google Scholar]
- Stem cells as drug delivery methods: Application of stem cell secretome for regeneration. Adv Drug Deliv Rev. 2015;82-83:1.
- [Google Scholar]
- Umbilical cord as prospective source for mesenchymal stem cell-based therapy. Stem Cells Int. 2016;2016:6901286.
- [Google Scholar]
- Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell. 2012;10:244-58.
- [Google Scholar]
- MSCs conditioned media and umbilical cord blood plasma metabolomics and composition. PLoS One. 2014;9:e113769.
- [Google Scholar]
- Vascular endothelial growth factor (VEGF) as a key therapeutic trophic factor in bone marrow mesenchymal stem cell-mediated cardiac repair. Biochem Biophys Res Commun. 2009;390:834-8.
- [Google Scholar]
- Therapeutic applications of mesenchymal stromal cells: Paracrine effects and potential improvements. Tissue Eng Part B Rev. 2012;18:101-15.
- [Google Scholar]
- Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009;20:1053-67.
- [Google Scholar]
- MSC secretes at least 3 EV types each with a unique permutation of membrane lipid, protein and RNA. J Extracell Vesicles. 2016;5:29828.
- [Google Scholar]
- Molecular signatures of secretomes from mesenchymal stem cellsmesenchymal stemromal cells: Therapeutic benefits. Mol Cel Toxicol. 2017;13:133-41.
- [Google Scholar]
- HucMSC-exosome mediated-wnt4 signaling is required for cutaneous wound healing. Stem Cells. 2015;33:2158-68.
- [Google Scholar]
- Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation. 2012;126:2601-11.
- [Google Scholar]
- Mesenchymal stem cell-derived extracellular vesicles for renal repair. Curr Gene Ther. 2017;17:29-42.
- [Google Scholar]
- Unveiling the differences of secretome of human bone marrow mesenchymal stem cells, adipose tissue-derived stem cells, and human umbilical cord perivascular cells: A proteomic analysis. Stem Cells Dev. 2016;25:1073-83.
- [Google Scholar]
- Mesenchymal stromal cell characteristics vary depending on their origin. Stem Cells Dev. 2013;22:2606-18.
- [Google Scholar]
- Protein synthesis and secretion in human mesenchymal cells derived from bone marrow, adipose tissue and Wharton's jelly. Stem Cell Res Ther. 2014;5:53.
- [Google Scholar]
- Paracrine factors secreted by umbilical cord-derived mesenchymal stem cells induce angiogenesis in vitro by a VEGF-independent pathway. Stem Cells Dev. 2015;24:437-50.
- [Google Scholar]
- Higher propensity of Wharton's jelly derived mesenchymal stromal cells towards neuronal lineage in comparison to those derived from adipose and bone marrow. Cell Biol Int. 2013;37:507-15.
- [Google Scholar]
- Umbilical cord mesenchymal stem cells: Adjuvants for human cell transplantation. Biol Blood Marrow Transplant. 2007;13:1477-86.
- [Google Scholar]
- The Secretome of Bone marrow and Wharton jelly derived mesenchymal stem cells induces differentiation and neurite outgrowth in sh-sy5y cells. Stem Cells Int. 2014;2014:438352.
- [Google Scholar]
- Therapeutic potential of stem cells in skin repair and regeneration. Chin J Traumatol. 2008;11:209-21.
- [Google Scholar]
- Reducing the incidence of foot ulceration and amputation in diabetes. Curr Diab Rep. 2004;4:413-8.
- [Google Scholar]
- Regenerative effects of Wharton Jelly stem cells-conditioned medium in UVA-Irradiated human dermal fibroblasts. Malaysian J Med Biol Res. 2016;3:45-50.
- [Google Scholar]
- Umbilical cord-derived mesenchymal stromal cell-conditioned medium exerts in vitro antiaging effects in human fibroblasts. Cytotherapy. 2017;19:371-83.
- [Google Scholar]
- Enhanced healing of diabetic wounds by subcutaneous administration of human umbilical cord derived stem cells and their conditioned media. Int J Endocrinol. 2013;2013:592454.
- [Google Scholar]
- Mesenchymal Stem Cell conditioned Medium Promote the recovery of skin burn wound 41. Asian J Anim Vet Adv. 2017;132:141.
- [Google Scholar]
- Hypoxic conditioned medium from human amniotic fluid-derived mesenchymal stem cells accelerates skin wound healing through TGF-β/SMAD2 and PI3K/Akt pathways. Int J Mol Sci. 2014;15:605-28.
- [Google Scholar]
- Human umbilical mesenchymal stem cells conditioned medium promote primary wound healing regeneration. Vet World. 2016;9:605-10.
- [Google Scholar]
- A conditioned medium of umbilical cord mesenchymal stem cells overexpressing Wnt7a promotes wound repair and regeneration of hair follicles in mice. Stem Cells Int. 2017;2017:3738071.
- [Google Scholar]
- Conditioned media from human umbilical cord blood-derived mesenchymal stem cells inhibits melanogenesis by promoting proteasomal degradation of MITF. PLoS One. 2015;10:e0128078.
- [Google Scholar]
- Conditioned media from human umbilical cord blood-derived mesenchymal stem cells stimulate rejuvenation function in human skin. Biochem Biophys Rep. 2018;16:96-102.
- [Google Scholar]
- The healing effects of conditioned medium derived from mesenchymal stem cells on radiation-induced skin wounds in rats. Cell Transplant. 2019;28:105-15.
- [Google Scholar]
- Global cancer transitions according to the human development index (2008-2030): A population-based study. Lancet Oncol. 2012;13:790-801.
- [Google Scholar]
- Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2018;68:394-424.
- [Google Scholar]
- Natural products derived from plants as a source of drugs. J Adv Pharm Technol Res. 2012;3:200.
- [Google Scholar]
- The effect of Wharton's Jelly mesenchymal stem cells on a squamous cell carcinoma cell line. Arc Cancer Res. 2016;4:45.
- [Google Scholar]
- Effect of human Wharton's jelly mesenchymal stem cell secretome on proliferation, apoptosis and drug resistance of lung cancer cells. Res Pharm Sci. 2015;10:134-42.
- [Google Scholar]
- Human Wharton's Jelly mesenchymal stem cell secretome display significant antiproliferative effect on K562 leukemia cells. Royan international twin congress, 10th congress on stem cell biology and technology. 2014;16:86.
- [Google Scholar]
- Amniotic membrane conditioned medium promotes cell death and inhibits proliferation of hepatocarcinoma HepG2 cells. Placenta. 2017;51:114.
- [Google Scholar]
- Conditioned media of human umbilical cord blood mesenchymal stem cell derived secretome induced apoptosis and inhibited growth of hela cells. Indones Bio. 2014;6:57-62.
- [Google Scholar]
- An anticancer effect of umbilical cord-derived mesenchymal stem cell secretome on the breast cancer cell line. Cell tissue bank. 2019;20:423-34.
- [Google Scholar]
- Mesenchymal stem cell secretome and regenerative therapy after cancer. Biochimie. 2013;95:2235-45.
- [Google Scholar]
- NIH US National Library of Health: Clinical Trial Register. Available from: https://clinicaltrials.gov/ct2/show/NCT02138331?term=NCT02138331&rank=1
- NIH US National Library of Health: Clinical trial register. Effects of ASC secretome on human osteochondral explants (ASC-OA). Available from: https://www.clinicaltrials.gov/ct2/show/NCT04223622?cond=mesenchymal+stem+cells+secretome&draw=2&rank=2
- NIH US National Library of Health: Clinical trial register. Treatment of Severe COVID-19 Patients Using Secretome of Hypoxia-Mesenchymal Stem Cells in Indonesia. Available from: https://www.clinicaltrials.gov/ct2/show/NCT04753476?cond=mesenchymal+stem+cells+ secretome&draw=2&rank=1
- Thrombospondin-2 secreted by human umbilical cord blood-derived mesenchymal stem cells promotes chondrogenic differentiation. Stem Cells. 2013;31:2136-48.
- [Google Scholar]
- Conditioned Medium of Wharton's Jelly Derived Stem Cells Can Enhance the Cartilage Specific Genes Expression by Chondrocytes in Monolayer and Mass Culture Systems. Adv Pharm Bull. 2017;7:123-30.
- [Google Scholar]
- Conditioned medium from human amniotic epithelial cells may induce the differentiation of human umbilical cord blood mesenchymal stem cells into dopaminergic neuron-like cells. J Neurosci Res. 2013;91:978-86.
- [Google Scholar]
- Cardioprotection by cord blood mesenchymal stromal cells through activation of Akt, ERK and STAT3 signaling. Thorac Cardiovasc Surg. 2013;61:OP86.
- [Google Scholar]
- The human amniotic fluid stem cell secretome effectively counteracts doxorubicin-induced cardiotoxicity. Sci Rep. 2016;6:29994.
- [Google Scholar]
- Conditioned medium from human amniotic mesenchymal stromal cells limits infarct size and enhances angiogenesis. Stem Cells Transl Med. 2015;4:448-58.
- [Google Scholar]
- Therapeutic effects of umbilical cord blood derived mesenchymal stem cell-conditioned medium on pulmonary arterial hypertension in rats. J Anat Soc India. 2016;65:15-9.
- [Google Scholar]
- Human umbilical cord-derived mesenchymal stem cells conditioned medium attenuate interstitial fibrosis and stimulate the repair of tubular epithelial cells in an irreversible model of unilateral ureteral obstruction. Nephrology (Carlton). 2018;23:728-36.
- [Google Scholar]
- Conditioned medium derived from umbilical cord mesenchymal stem cells regenerates atrophied muscles. Tissue Cell. 2016;48:533-43.
- [Google Scholar]
- The conditioned medium of human mesenchymal stromal cells reduces irradiation-induced damage in cardiac fibroblast cells. J Radiat Res. 2018;59:555-64.
- [Google Scholar]
- Human umbilical cord mesenchymal stem cell conditioned medium attenuates renal fibrosis by reducing inflammation and epithelial-to-mesenchymal transition via the TLR4/NF-κB signaling pathway in vivo and in vitro. Stem Cell Res Ther. 2018;9:7.
- [Google Scholar]
- Therapeutic effects of extracellular vesicles from human adipose-derived mesenchymal stem cells on chronic experimental autoimmune encephalomyelitis. Journal of cellular physiology. 2020;235:8779-90.
- [Google Scholar]
- Proteome analysis of human Wharton's jelly cells during in vitro expansion. Proteome Sci. 2010;8:18.
- [Google Scholar]
- Adult stem cell plasticity: Fact or artifact? Annu Rev Cell Dev Biol. 2003;19:1-22.
- [Google Scholar]
- Inducible HGF-secreting human umbilical cord blood-derived MSCs produced via TALEN-mediated Genome editing promoted angiogenesis. Mol Ther. 2016;24:1644-54.
- [Google Scholar]
- Cytokine treatment optimises the immunotherapeutic effects of umbilical cord-derived MSC for treatment of inflammatory liver disease. Stem Cell Res Ther. 2017;8:140.
- [Google Scholar]
- Nanosized UCMSC-derived extracellular vesicles but not conditioned medium exclusively inhibit the inflammatory response of stimulated T cells: Implications for nanomedicine. Theranostics. 2017;7:270-84.
- [Google Scholar]
- The current landscape of the mesenchymal stromal cell secretome: A new paradigm for cell-free regeneration. Cytotherapy. 2016;18:13-24.
- [Google Scholar]






