Translate this page into:
Similar regeneration of articular cartilage defects with autologous & allogenic chondrocytes in a rabbit model
For correspondence: Dr P.R.J.V.C. Boopalan, Department of Orthopaedics, Christian Medical College, Vellore 632 004, Tamil Nadu, India e-mail: jpboopy@gmail.com
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Articular cartilage defects in the knee have a very poor capacity for repair due to avascularity. Autologous chondrocyte transplantation (ACT) is an established treatment for articular cartilage defects. Animal studies have shown promising results with allogenic chondrocyte transplantation since articular cartilage is non-immunogenic. In addition to being economical, allogenic transplantation has less morbidity compared to ACT. This study was undertaken to compare ACT with allogenic chondrocyte transplantation in the treatment of experimentally created articular cartilage defects in rabbit knee joints.
Methods:
Cartilage was harvested from the left knee joints of six New Zealand white rabbits (R1-R6). The harvested chondrocytes were cultured to confluence and transplanted onto a 3.5 mm chondral defect in the right knees of 12 rabbits [autologous in 6 rabbits (R1-R6) and allogenic in 6 rabbits (R7-R12)]. After 12 wk, the rabbits were euthanized and histological evaluation of the right knee joints were done with hematoxylin and eosin and safranin O staining. Quality of the repair tissue was assessed by the modified Wakitani histological grading scale.
Results:
Both autologous and allogenic chondrocyte transplantation resulted in the regeneration of hyaline/mixed hyaline cartilage. The total histological scores between the two groups showed no significant difference.
Interpretation & conclusions:
Allogenic chondrocyte transplantation seems to be as effective as ACT in cartilage regeneration, with the added advantages of increased cell availability and reduced morbidity of a single surgery.
Keywords
Autologous chondrocyte transplantation
autologous versus allogenic
cartilage culture
Wakitani score
Articular cartilage is a multiphasic tissue, which has the ability to bear large compressive loads. It has limited self-healing capability as it lacks vascular, neural and lymphatic network1. The poor intrinsic capability for repair has led to a wide variety of biological and non-biological treatment modalities with varying levels of success2345. The goal for treatment of cartilage defects is restoration of hyaline cartilage. Among the current cell-based therapeutics in treatment of cartilage defects, chondrocyte implantation and marrow stromal studies have been the mainstay options246.
Autologous chondrocyte transplantation (ACT) in treatment of cartilage lesions has progressed from the use of chondrocytes in suspension with periosteal flaps to chondrocytes embedded in collagen membranes and bio-scaffolds and as a therapeutic option worldwide78. ACT in short-term follow up studies has shown both favourable and failure outcomes910. The available evidence to test its outcomes with other treatment options is inadequate and requires long-term follow up to clinically define its effectiveness and its benefit-risk ratios. ACT requires in vitro expansion of cells to obtain sufficient cells to meet the surface area to volume ratio of the cartilage defects. However, serial passaging results in dedifferentiation of chondrocytes with expression of hypertrophic markers11. Chondrocyte being a mature cell by nature either dedifferentiate to become fibrocartilage or terminally differentiate to become hypertrophic cartilage, either way resulting in transplantation failure.
Allogenic transplantation of chondrocytes has also been used with some success in animal models involving rabbits121314. Despite the strong expression of antigens on chondrocytes, these have been shown to be non-immunogenic and possess positive immunomodulatory properties15. Rat studies using isogeneic and allogenic chondrocytes in the healing of osteochondral defects did not show any differences between the two groups16. Allogeneic chondrocytes with positive immunomodulatory property could serve as the ideal alternative source15. The advantages of the allogenic approach are single surgery, high seeding density with early culture and decreased dedifferentiated cell use. The purpose of this study was to compare allogenic chondrocyte transplantation versus ACT in the treatment of experimentally created cartilage defects in rabbit knee joints.
Material & Methods
Cartilage harvest: The study was conducted in the departments of Orthopaedics and Physiology, and animal house facility of the Christian Medical College (CMC), Vellore, India, during December 2008 to July 2010. Twelve adult New Zealand white rabbits (average age - 6 months) were used for the study. The Institutional Animal Ethics Committee approved the study protocol. The rabbits were anaesthetized with intramuscular ketamine (50 mg/kg) and 2 per cent xylazine (4 mg/kg). Under sterile condition, cartilage was harvested from the left knee joints of six rabbits (R1-R6). Care was taken not to damage the underlying subchondral area. The harvested cells were cultured and used as autologous chondrocytes for the right femorotibial joints of six rabbits (R1-R6) and as allogenic cells in the right femorotibial joints of six other rabbits (R7-R12).
Chondrocyte isolation and culture: The harvested cartilage was washed with Dulbecco's modified Eagle's medium F12 (DMEM-F12) and sliced into small pieces. The cartilage pieces were digested overnight at 37°C in a five per cent carbon dioxide incubator using 1.5 per cent collagenase type II (Worthington, USA). The chondrocytes were isolated by centrifugation and washed with culture medium containing DMEM-F12, 10 per cent foetal bovine serum, 50 μg/ml ascorbic acid, 200 μg/ml streptomycin, 200 units/ml penicillin and 0.8 μg/ml amphotericin B. For primary cultures, the chondrocytes were counted in a hemocytometer (trypan blue dye exclusion technique) and grown in T-75 flasks under standard culture conditions. The culture medium was changed every 2-3 days. The chondrocytes reached confluency by the 18th day. The primary culture monolayer cells were isolated by 0.25 per cent trypsin-ethylenediaminetetraacetic acid (EDTA), centrifuged to get a pellet that was further counted and used for transplantation.
Creation of articular cartilage defect and transplantation of the cultured chondrocytes: The second operation was performed to create the cartilage defect and transplant the cells. Under aseptic precautions with confirmed anaesthesia, the right knee joint was exposed. A 3.5 mm chondral defect was created in the trochlear groove using a circular stainless steel punch, and care was taken not to enter the subchondral bone (Fig. 1A). A periosteal flap was harvested from the lateral femoral condyle to match the defect size. The cultured chondrocytes as cell suspension was placed into created chondral defects and covered with a periosteal flap (Fig. 1B). The cultured cells from - six rabbits were autologous for six and allogenic for the other six. The periosteal flap was glued with fibrin glue. The skin was closed with 3-O Vicryl (Johnson and Johnson, USA). Postoperatively, all animals were given intramuscular enrofloxacin (10 mg/kg) and meloxicam (5 mg/kg) for three days. The animals were provided with food and water ad libitum. All the animals were maintained in separate cages and were allowed to move freely.
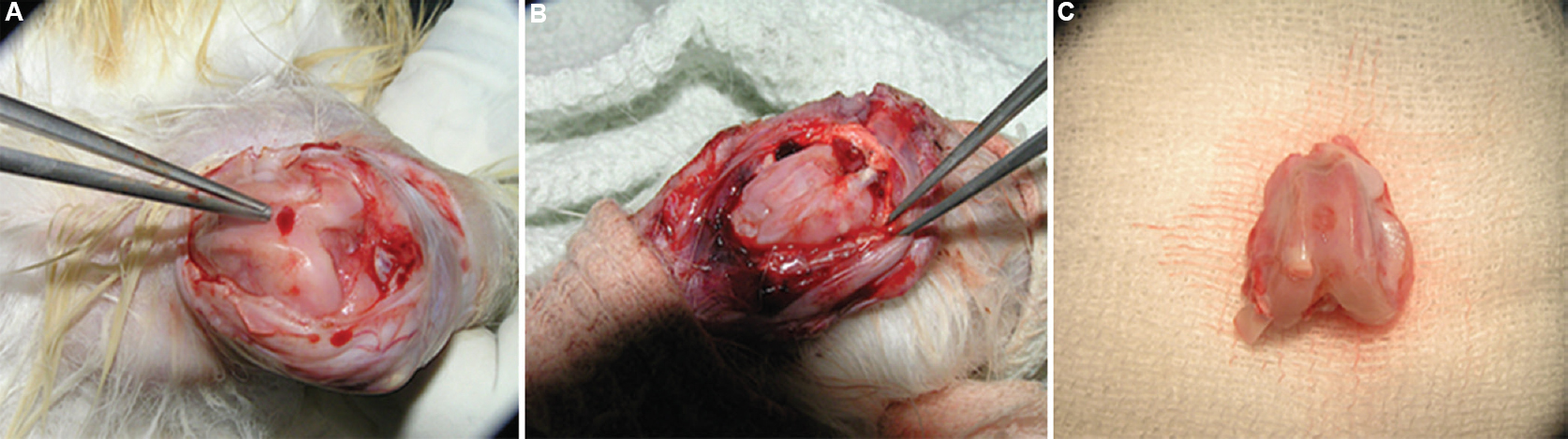
- Perioperative photograph showing (A) 3.5 mm chondral defect created in the trochlear groove of the right knee joint (B) chondral defect filled with cells and covered with periosteal patch (C) 12 wk postoperative gross appearance of the transplanted site (R3) showing healing of the defect.
Histopathology: At the end of 12 wk, the rabbits were euthanized using sodium thiopental (70 mg/kg). Each right knee joint was excised and decalcified in 10 per cent formic acid, processed in automatic tissue processor (Leica ASP300 Leica Micro-systems GmbH, Wetzlar, Germany) and embedded in paraffin. Longitudinal sections of five micrometre thickness were prepared (LeicaRM2255) and stained (Leica Autostainer XL) with hematoxylin and eosin, to assess tissue morphology, Safranin O and fast green and Masson's trichrome, to identify the presence of proteoglycan-rich matrix. The quality of the repair tissue in the articular defect that had autologous transplantation (R1-R6) was compared with that of the allogenic transplantation (R7-R12) by the modified Wakitani histological grading scale14. The sections were graded according to: (i) cell morphology; (ii) matrix staining (metachromasia); (iii) surface regularity; (iv) thickness of cartilage; and (v) integration of donor with adjacent host cartilage. The scoring (0 to 14) was performed by two observers who were blinded to the animal study groups.
Statistical analysis: Statistical analysis was done using SPSS 11.0 (SPSS, Inc., Chicago, IL, USA). Median and range were presented for both groups separately. Mann-Whitney test was used for statistical comparison between the autologous (n=5) and allogenic transplant groups (n=6).
Results
There was complete arthrofibrosis with degeneration of joint cartilage in one rabbit that received autologous transplantation and was excluded from the final analysis.
Histopathology: None of the joints were infected. There was no synovitis or osteophyte formation. In most of the animals, the transplant area was seen and showed resemblance to the adjacent normal cartilage (Fig. 1C). The articular surface in some of the animals had a bump due to periosteal tissue.
Autologous cell transplantation: The chondral defects of the right knee joints of rabbits (R1-R6) were treated with autologous chondrocytes. R1 showed the absence of cells in the transplanted site with only periosteal tissue bridging the adjacent normal cartilage. There was hyaline cartilage formation seen in R2 and R6 with hypertrophy of the overlying periosteum (Figs 2A, 2C and 3A). Rabbit R3 and R4 showed only fibrocartilage formation with smooth and intact surface with normal cellularity and staining; R5 was excluded due to arthritis/arthrofibrosis.
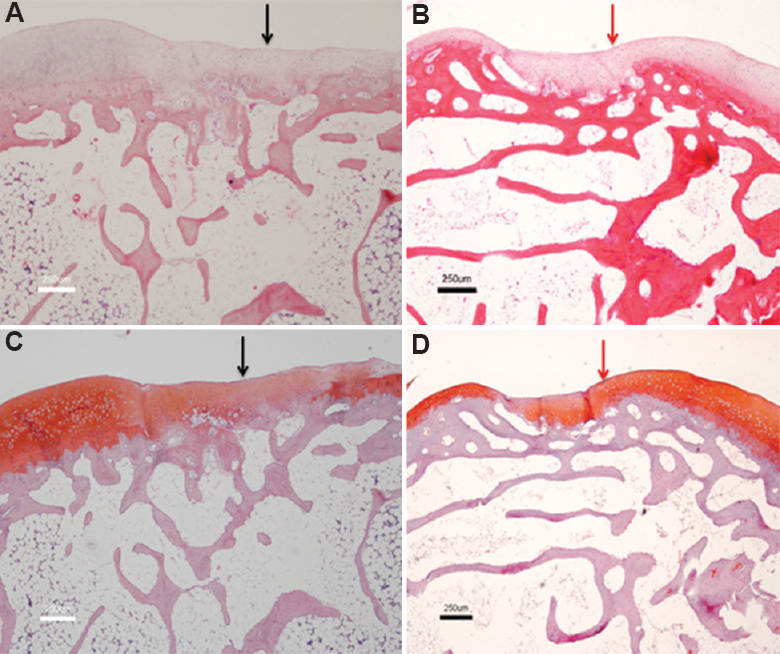
- Comparative histology showing cartilage defect healed with autologous chondrocytes versus allogenic chondrocytes. (A) (Autologous, H and E, ×4) (black arrow); (B) (Allogenic, H and E, ×4) (red arrow); (C) (Autologous, Safranin O, ×4) - Predominantly mixed hyaline and fibrocartilage at the transplanted site (black arrow); (D) (Allogenic, Safranin O, ×4) - Predominant hyaline cartilage at transplanted site with good integration with surrounding normal cartilage (red arrow).
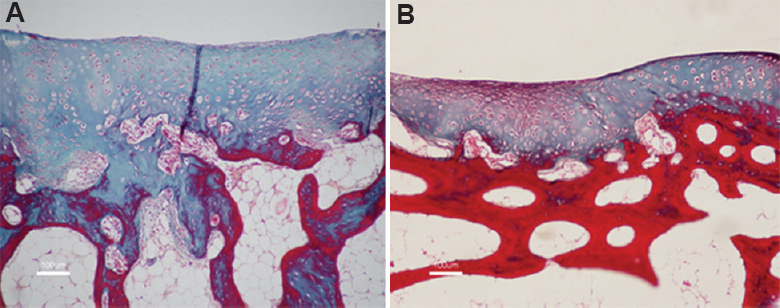
- Histology of the cartilage (Masson's Trichrome, ×10) treated with (A) autologous chondrocytes; (B) allogenic chondrocytes showing the presence of hyaline and fibrocartilage formation with good integration with adjacent normal cartilage.
Allogenic cell transplantation: The right knee chondral defects of rabbit (R7-R12) received allogenic chondrocytes as treatment. Rabbits R7 and R8 showed hyaline cartilage formation with normal cellularity devoid of chondrocyte clustering. The edges were well integrated with the adjacent normal cartilage, and thickness of cartilage varied from 75 to 100 per cent of the adjacent normal cartilage. There was no periosteal hypertrophy (Figs. 2B, 2D and 3B). Rabbits R9 and R10 showed only fibrocartilage formation with varied thickness but bonding at both ends of the graft. R11 and R12 showed mixed fibrohyaline cartilage with predominance of hyaline cartilage.
The modified Wakitani histological score graded the healing of chondral defects. The total histological score was compared between the groups, which received allogenic and autologous cells. The total histological scores between the two groups showed no significant difference (Table).
| Wakitani’s histological grading score | Median score (minimum, maximum) | |
|---|---|---|
| Autologous knees (n=5) | Allogenic knees (n=6) | |
| Cellular morphology | 3.5 (0, 4) | 4 (1, 4) |
| Matrix staining (metachromasia) | 2.5 (1, 3) | 3 (1, 3) |
| Surface regularity | 1 (0, 3) | 2 (0, 3) |
| Thickness of cartilage | 2 (0, 2) | 2 (1, 2) |
| Integration of host to adjacent donor cartilage | 0 (0, 0) | 0 (0, 2) |
| Total | 9.5 (1, 11) | 10 (5, 14) |
Discussion
The present study showed that in experimentally created chondral defects, cartilage regeneration secondary to autologous and allogenic chondrocyte transplantation had similar outcomes. ACT has been established as a treatment modality for articular cartilage defects1718. However, it is still considered as a procedure under investigation requiring more prospective clinical studies for defining its efficiency. Management of articular cartilage repair using both autologous and allogenic transplantation has been described with varying success. In our previous study, allogenic cartilage culture and transplantation showed promising results12.
Allogenic cartilage culture and transplantation has certain advantages compared to autologous cell-based therapy. Patient requires only a single surgery unlike autologous where an additional surgical procedure is required for harvest of cartilage. Chondrocytes can be harvested from joints of patients who are undergoing amputation post-trauma. With single surgery, the morbidity of anaesthesia and the cost can be minimized. There is also less chance of infection. Large area of cartilage is available for harvest unlike autologous where cartilage has to be harvested from non-articular region, which is very narrow. It is also known that when a defect is made in the non-articular region, cartilage degeneration can start and progress to other areas of joint1.
Immune rejection to allogenic chondrocytes has been described in rabbit studies where premature degeneration of regenerated cartilage was seen19. However, other studies with allogenic chondrocyte transplantation have shown good outcomes12131420. Studies have shown chondrocytes to be hypoimmunogenic lacking immunogenic surface molecules and immunosuppressive properties in co-culture studies with increased expression of soluble immunomodulatory factors1519. In the current study, allogenically transplanted joints did not show any inflammation or any immune reaction.
Complications were encountered in both the study arms. In rabbits, it was not possible to suture the periosteum to hard subchondral bone. Thus, the technique of sealing the periosteal edge with fibrin glue was used after implantation of cells as the cells could have been lost from the site of transplantation. This was evident by the empty defect seen in one rabbit. Both the groups showed hyaline and mixed hyaline and fibrocartilage formation with no discernable difference in the histological appearances. Histological examination in both groups showed variable filling of the defects with good integration of the transplanted site to the adjacent cartilage. A limitation to our study was having a single end point. It would have been interesting to understand the morphological changes taking place at various time points - early, middle and late. Our study did not show any significant difference in the outcome between the two groups. This finding opens doors for tissue engineering and more research towards the use of alternative sources such as allogenic chondrocytes or stem cells with chondrogenic potential or combination therapy for repair techniques. This also indicates towards the use of mature cells as chondrocytes in treatment of cartilage defects, which in long term may result in suboptimal biomechanical joint function.
In conclusion, our preliminary findings showed that the outcome of cartilage regeneration was similar with autologous and allogenic chondrocyte-cultured cell transplantation in an experimental model. Further comparative study using a biocompatible implantable scaffold should be considered as a delivery vehicle to avoid loss of cells that can occur occasionally with periosteal arthroplasty.
Acknowledgment
Authors acknowledge Dr Elizabeth Vinod for her contributions towards this article preparation.
Financial support & sponsorship: The study was funded by the Department of Biotechnology, Government of India, New Delhi (grant no. BT/PR8940/GBD/27/42/2007).
Conflicts of Interest: None.
References
- The basic science of articular cartilage: Structure, composition, and function. Sports Health. 2009;1:461-8.
- [Google Scholar]
- Return to sport after articular cartilage repair in athletes’ knees: A systematic review. Arthroscopy. 2016;32:651-68 e1.
- [Google Scholar]
- A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J Bone Joint Surg Br. 2003;85:223-30.
- [Google Scholar]
- Articular cartilage repair: Basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage. 2002;10:432-63.
- [Google Scholar]
- Articular cartilage: From formation to tissue engineering. Biomater Sci. 2016;4:734-67.
- [Google Scholar]
- Autologous bone-marrow mesenchymal cell induced chondrogenesis (MCIC) J Clin Orthop Trauma. 2016;7:153-6.
- [Google Scholar]
- Cartilage repair: Past and future – Lessons for regenerative medicine. J Cell Mol Med. 2009;13:792-810.
- [Google Scholar]
- Autologous chondrocyte implantation for full thickness articular cartilage defects of the knee. Cochrane Database Syst Rev. 2010;10:CD003323.
- [Google Scholar]
- Failures and reoperations after matrix-assisted cartilage repair of the knee: A systematic review. Arthroscopy. 2016;32:386-92.
- [Google Scholar]
- Current clinical therapies for cartilage repair, their limitation and the role of stem cells. Curr Stem Cell Res Ther. 2012;7:143-8.
- [Google Scholar]
- The loss of phenotypic traits by differentiated cells in vitro, I. Dedifferentiation of cartilage cells. Proc Natl Acad Sci U S A. 1960;46:1533-42.
- [Google Scholar]
- Rabbit articular cartilage defects treated by allogenic chondrocyte transplantation. Int Orthop. 2006;30:357-61.
- [Google Scholar]
- Repair of osteochondral defects with allogeneic tissue engineered cartilage implants. Clin Orthop Relat Res. 1999;367S:S382-95.
- [Google Scholar]
- Repair of large full-thickness articular cartilage defects with allograft articular chondrocytes embedded in a collagen gel. Tissue Eng. 1998;4:429-44.
- [Google Scholar]
- Culture expanded primary chondrocytes have potent immunomodulatory properties and do not induce an allogeneic immune response. Osteoarthritis Cartilage. 2016;24:521-33.
- [Google Scholar]
- Repair of osteochondral defects with grafts of cultured chondrocytes. Comparison of allografts and isografts. Clin Orthop Relat Res. 1994;302:251-8.
- [Google Scholar]
- Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889-95.
- [Google Scholar]
- Articular cartilage engineering with autologous chondrocyte transplantation. A review of recent developments. J Bone Joint Surg Am. 2003;85-A(Suppl 3):109-15.
- [Google Scholar]
- Alloreactivity and immunosuppressive properties of articular chondrocytes from osteoarthritic cartilage. J Orthop Surg (Hong Kong). 2016;24:232-9.
- [Google Scholar]
- Repair of the rabbit ear cartilage defects with transforming growth factor-beta1 and allogenic chondrocyte/poly-DL-lactide higher porosity polymer. Zhonghua Er Bi Yan Hou Ke Za Zhi. 2004;39:340-3.
- [Google Scholar]






