Translate this page into:
International open trial of uniform multidrug therapy regimen for leprosy patients: Findings & implications for national leprosy programmes
Reprint requests: Dr P. Manickam, National Institute of Epidemiology, Indian Council of Medical Research, R127, TNHB, Ayappakkam, Chennai 600 077, Tamil Nadu, India e-mail: manickam@nie.gov.in
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Uniform therapy for all leprosy patients will simplify leprosy treatment. In this context, we evaluated six-month multidrug therapy (MDT) currently recommended for multibacillary (MB) patients as uniform MDT (U-MDT) in a single-arm open trial under programme conditions. Primary objective was to determine efficacy to prevent five-year cumulative five per cent relapse. Secondary objectives were to assess acceptability, safety and compliance.
Methods:
Newly detected, treatment-naive leprosy patients were enrolled in India (six sites) and P. R. China (two sites). Primary outcome was clinically confirmed relapse of occurrence of one or more new skin patches consistent with leprosy, without evidence of reactions post-treatment. Event rates per 100 person years as well as five-year cumulative risk of relapse, were calculated.
Results:
A total of 2091 paucibacillary (PB) and 1298 MB leprosy patients were recruited from the 3437 patients screened. Among PB, two relapsed (rate=0.023; risk=0.11%), eight had suspected adverse drug reactions (ADRs) (rate=0.79) and rate of new lesions due toreactions was 0.24 (n=23). Rates of neuritis, type 1 and type 2 reactions were 0.39 (n=37), 0.54 (n=51) and 0.03 (n=3), respectively. Among MB, four relapsed (rate=0.07; risk=0.37%) and 16 had suspected ADR (rate=2.64). Rate of new lesions due to reactions among MB was 1.34 (n=76) and rates of neuritis, type 1 and type 2 reactions were 1.37 (n=78), 2.01 (n=114) and 0.49 (n=28), respectively. Compliance to U-MDT was 99 per cent. Skin pigmentation due to clofazimine was of short duration and acceptable.
Interpretation & conclusions:
We observed low relapse, minimal ADR and other adverse clinical events. Clofazimine-related pigmentation was acceptable. Evidence supports introduction of U-MDT in national leprosy programmes. [CTRI No: 2012/ 05/ 002696]
Keywords
Chemotherapy
leprosy
uniform multidrug therapy
The mainstay of leprosy treatment until 1984 was dapsone monotherapy. Although it resulted in reduction of leprosy prevalence globally and the leprosy trends started plateauing, deformities and complications continued to occur and dapsone resistance was documented1. Subsequently, from 1985 onwards, multidrug therapy (MDT) was the key public health intervention that helped in reducing the global leprosy burden substantially1. Initially, the duration of MDT was recommended as two years or until smear negativity for multibacillary (MB) leprosy. For paucibacillary (PB) leprosy, a two-drug combination of rifampicin and dapsone for six months and rifampicin once a month were recommended. Subsequently, over the years, based on the collective experience, the WHO through its two expert committees and a study group modified the treatment regimen. The key modifications were two years of fixed period for MB (1988) and later reduced duration for MB to 12 months (1998). Further, the WHO recommended single-dose regimen (rifampicin, ofloxacin and minocycline) for single-lesion PB patients1. During the implementation of MDT, national vertical programmes focussed on early case detection and treatment of all leprosy patients with MDT2. Most countries were successful in achieving leprosy elimination by the end of first decade of the current century, and vertical leprosy programmes were integrated into the primary health care services34. Such integration demanded further simplification of patient management practices including follow up. The WHO strategy for 2011-2015 focuses on sustaining the initiatives to reduce burden of leprosy in all the endemic communities5.
A simplified approach to leprosy diagnosis and treatment is deemed important for the sustainability of leprosy control services under programmatic conditions. In this context, MB-MDT regimen given for six-month duration was proposed as uniform MDT (U-MDT) regimen for all types of leprosy. Ji and Saunderson6 expressed concerns regarding this approach and the trial design not having a control group. These have been addressed in our earlier publication7. The goal of chemotherapy should be to shorten and optimize treatment regimen to achieve desired outcomes with minimum/acceptable side effects. For reducing the duration of MB-MDT, supportive evidence was available from experimental and clinical trials. Experimental studies suggested that MDT for 2-3 months was capable of killing almost all viable bacilli in the mouse footpad model89. Further, the rifampicin-resistant mutants in an untreated lepromatous patient were likely to be eliminated by three months’ daily treatment with dapsone-clofazimine combination and by that time rifampicin with three monthly doses would have killed over 99.9 per cent of the viable Mycobacterium leprae8. This was further confirmed by a clinical trial, in which loss of infectivity of M. leprae after only one month of the WHO MB-MDT or with a single dose of rifampicin was documented10. It is, therefore, reasonable to believe that patients would respond to six months’ MB-MDT, but a smaller number of them may relapse, who could continue on MDT without any risk of drug resistance. Second issue of importance is the addition of clofazimine for PB-MDT. Evidence from a randomized controlled clinical trial of PB-MDT plus daily clofazimine versus routine PB-MDT suggested that the proportion with persisting active skin patches was considerably lower in the clofazimine arm (7.5%) compared to PB-MDT arm (16%), and in the six month post-PB-MDT follow up, clofazimine group demonstrated better response than the control group (80 vs. 30%)11. Further, clofazimine could be potentially beneficial against type 2 reactions in leprosy patients12. In addition, the combination of three drugs may possibly reduce the chance of drug resistance. A controlled trial with control group could be justified only for a small fraction of highly bacteriologically positive patients (about 2% of newly diagnosed leprosy patients), who could be at risk of possible inadequate treatment and increased risk of relapse. However, based on the principle of equivalence, one would require a substantially large sample size for such a trial, which is practically not feasible. In view of the discontinuation of skin smears in the programmes1, it will not be possible to identify such high-risk patients. U-MDT trial was undertaken as programme implementation research with phase IV clinical trial perspective. National Institute of Epidemiology (NIE) of the Indian Council of Medical Research (ICMR), in Chennai, India, coordinated the U-MDT trial. The primary objective of this trial was to assess treatment response to U-MDT in terms of relapse rate not exceeding a maximum cumulative level of five per cent at the end of five years. The secondary objectives were to assess acceptability, safety and compliance to the U-MDT regimen. Here, we present the final results of the trial.
Material & Methods
It was a single-arm open-field trial. The trial was initiated in October 2003 and the final five years’ follow up at the last site (Rohtas in Bihar, India) was completed in January 2014.
Sample size: Considering the five year maximum relapse rate of five per cent as acceptable limit (Poisson distribution; Po=5%; Pa=3%) with the power of 90 per cent, type 1 error of 5 per cent (one-tailed test) and loss to follow up of 30 per cent in field situations, the required sample size was 2223 which was rounded off to 2500 for each type of leprosy.
Study settings: During 2003-2004, the trial was initiated at six sites - four districts in India (Pune, Kanpur, Tiruvannamalai and Villupuram) and two provinces in P. R. China (Guizhou and Yunnan). Two sites from India - Gaya and Rohtas districts were subsequently included in 2005 and 2007, respectively. The trial was conducted at the district level by leprosy control programme officers in three sites in India (Tiruvannamalai and Villupuram in Tamil Nadu and Pune in Maharashtra). At Kanpur in Uttar Pradesh, the trial was conducted by the National JALMA Institute for Leprosy and Other Mycobacterial Diseases (ICMR), Agra. In two sites of Bihar (Gaya and Rohtas), Damien Foundation India Trust, Chennai, conducted the trial in collaboration with the leprosy programme. In PR China, the trial was conducted as part of national leprosy control programme.
Study participants: Newly detected and treatment-naive leprosy patients were recruited in the trial. Patients with access to the clinic and available to receive U-MDT under supervision and willing for long-term follow up were included after obtaining written informed consent. Patients who had only neuritic manifestations or who had been previously treated for leprosy, were excluded.
Study drugs and treatment schedule: Study participants were given monthly-supervised doses of U-MDT in the presence of the investigators for six months. For adults, the regimen consisted of supervised pulse of 600 mg rifampicin, 300 mg clofazimine and 100 mg dapsone every four weeks along with daily-unsupervised course of 50 mg clofazimine and 100 mg dapsone. The supervised dosage for children aged 10-14 years was 450 mg rifampicin, 150 mg clofazimine and 50 mg dapsone every four weeks and 50 mg clofazimine every alternate day and 50 mg dapsone daily. For children <10 yr, the dose (mg) was adjusted to body weight (kg) as follows: rifampicin 10-20 mg/kg, clofazimine 1-2 mg/kg and dapsone 1-2 mg/kg of the body weight. All the drugs were supplied by the WHO with a special labelling of U-MDT for adult and child blister packs separately for the entire duration of the trial.
Data collection: The investigators of all the sites assessed every new leprosy patient for suitability for inclusion in the study as per the protocol. Patients who decided not to join the study or found ineligible were given regular MDT as per the national leprosy programme guidelines in India or P. R. China. During the treatment period patients were interviewed and carefully examined for adverse drug reactions (ADRs), leprosy reactions and neuritis at the time of their monthly visit for receiving the supervised dose of treatment. Subsequently, occurrence of clinical events such as relapse, reactions, disability and neuritis and other events such as migrations and deaths was recorded during the yearly follow up visits after completion of treatment. Patients developing new lesions, pain in the nerves, joint pains, fever and any other complaint were requested to report and were examined and treated as early as possible. The NIE, Chennai, monitored the trial for its duration and ensured adherence to the trial protocol at the trial sites. In addition, reporting forms were collected, scrutinized and entered in the trial database at NIE. Discrepancies found during scrutiny were clarified with the study sites. Further, quality checks were conducted through on-site supervision visits and periodic monitoring throughout the study period. Operational definitions used in the trial are given elsewhere7.
The study protocol was approved by the Institutional Human Ethics Committees of the participating organizations. All the participants in the study provided written informed consent administered in their local languages. (Clinical Trials Registry of India: 2012/05/002696).
Data analysis: Baseline characteristics of the study participants at all the study sites were analyzed and frequencies were estimated. Per protocol analysis was done and person years (PY) for study participants were calculated from the time of completion of treatment to the observation of primary outcome (relapse) or from the time of recruitment till the time of lost to follow up due to suspected ADR (during treatment period) or non-clinical events or completion of five years post-treatment. Those with relapse, suspected ADR or any of the non-clinical events were right censored and thereafter they ceased to contribute to the person-time of observation. For those who had temporarily migrated and then joined the study later, the maximum PYs contributed by them, i.e. from enrolment to each of those follow up time-points, were calculated. Event rates per 100 PY were also calculated. The rates were compared using Chi-square test. Further, cumulative risk [risk=1-e(−rate× period)] of relapse for five years was computed. We used SPSS18.0 (SPSS Inc., Chicago, IL, USA) and OpenEpi13 were used for data analysis.
Results
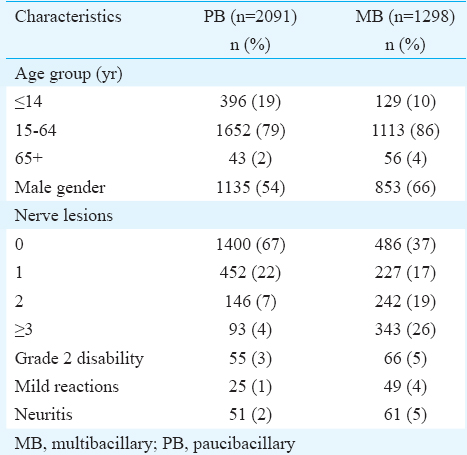
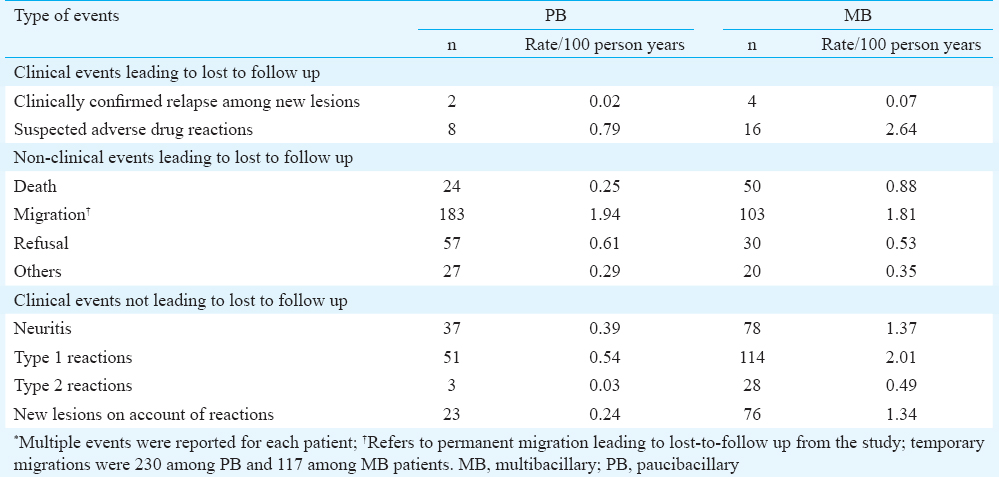
During October 2003 and June 2008, 3389 (98.6%) (PB=2091; MB=1298) of the 3437 new patients screened for the trial were enrolled (Fig. A, B). Forty eight patients could not be enrolled for various reasons including ineligibility (n=34), duplication of records (n=7), other reasons (n=6) and declined to participate (n=1). Of these ineligible patients,19 had pure neuritic leprosy and were put on routine MDT. Of the total recruited, MB% ranged between 27 per cent (168 of 631) in Gaya and 67 per cent (111 of 166) in P. R. China (Tiruvannamalai: 46% of 520; Villupuram: 45% of 505; Pune: 34% of 812; Kanpur: 40% of 316; Rohtas: 33% of 439). Of the total enrolled, 3169 completed the prescribed treatment. Thirty participants (PB=21 and MB=9) completed the treatment beyond nine months after initiation, and hence, they were excluded from subsequent analysis.
![Intake and follow up of paucibacillary leprosy patients from all the study sites, uniform multidrug therapy trial, 2003-2014. *Of the 3437 new leprosy patients screened for the trial, 48 (1.4%) were not enrolled due to various reasons [exclusion criteria=34; duplicates=7; other reasons=6; declined=1]. No details about PB/MB status of these 48 patients are available.](/content/175/2016/144/4/img/IJMR-144-525-g001.png)
- Intake and follow up of paucibacillary leprosy patients from all the study sites, uniform multidrug therapy trial, 2003-2014.
- *Of the 3437 new leprosy patients screened for the trial, 48 (1.4%) were not enrolled due to various reasons [exclusion criteria=34; duplicates=7; other reasons=6; declined=1]. No details about PB/MB status of these 48 patients are available.
![Intake and follow up of multibacillary leprosy patients from all the study sites, uniform multidrug therapy trial, 2003-2014. *Of the 3437 new leprosy patients screened for the trial, 48 (1.4%) were not enrolled due to various reasons [exclusion criteria=34; duplicates=7; other reasons=6; declined=1]. No details about PB/MB status of these 48 patients are available.](/content/175/2016/144/4/img/IJMR-144-525-g002.png)
- Intake and follow up of multibacillary leprosy patients from all the study sites, uniform multidrug therapy trial, 2003-2014.
- *Of the 3437 new leprosy patients screened for the trial, 48 (1.4%) were not enrolled due to various reasons [exclusion criteria=34; duplicates=7; other reasons=6; declined=1]. No details about PB/MB status of these 48 patients are available.
Findings among PB type of patients: Of the total 2091 PB patients enrolled,19 per cent (n=396) were younger than 15 years (mean age±SD of 29.3±15.1 yr) and 54 per cent (n= 1135) were male (Table I). Grade 2 disability (G2D) was present in three per cent (n=55) of them at recruitment, and nerve lesions were present in 33 per cent (n=691) of the patients. Evidence of mild reactions was found in one per cent (n=25) of the patients and 51 (2%) had neuritis at the time of enrolment.
Primary outcome: Two PB patients had clinically confirmed relapse (Table II). The relapse rate per 100 person years (PY) was 0.02 (total PY=8780) and the cumulative risk over five years was 0.11 per cent. One of the relapses occurred in the second year and the patient was put on routine MDT by the site investigator. The second relapse occurred in the third year (Table III) of follow up and was put on one more course of U-MDT. Both had their skin lesions ‘improved’ at the completion of the trial.

Secondary outcomes: Acceptance of the U-MDT regimen was 100 per cent for all the sites. Totally, 94 per cent completed U-MDT within nine months (52% within six months and rest in nine months). There were no complaints about clofazimine pigmentation. The investigators reported that skin pigmentation due to clofazimine was of short duration and acceptable to the enrolled patients with PB leprosy.
During the study period, a total of 645 special events were reported among PB patients. Of these, 301 events resulted in lost to follow up due to clinical (n=10) or non-clinical events (n=291). The remaining 344 were events that did not lead to lost to follow up (clinical events=114 and temporary migrations=230) (Table II).
At the end of five years post-treatment follow up, the death rate was 0.25 per 100 PY (n=24) among PB patients. Of these deaths, one was reportedly due to complications following leprosy reactions from Guizhou site in P. R. China. Seven deaths were due to injuries (suicide=2, snake bite=1 and motor vehicle accidents=4), followed by four cardiac problem-related deaths. Cause of death was unknown for four deaths.
Of the total PB patients recruited, 2.7 per cent (n=57) refused to continue in the study for various reasons. Majority of them were self-refusal for clinical examination during follow up (n=30). Twelve participants did not report any reason for discontinuation.
Among the lost to follow up, 27 were due to various reasons such as shifting outside the study area (n=20) and being found ineligible during the treatment period (wrong diagnosis or pregnancy). P. R. China site removed four patients from the trial since they were either put on routine MDT by investigators (n=3) or as opted by the patient (n=1).
The clinical events leading to lost to follow up included eight suspected ADR (total PY=1009; rate=0.79). As per the WHO/TDR guidelines (http://www.who.int/tdr/publications/documents/investigator.pdf?ua=1) and based on available clinical notes, one of the ADR was classified as ‘probably’(exfoliative dermatitis with jaundice) and seven as ‘possibly’ related to the drug. Of the reported clinical events, rate of occurrence (per 100 PY) of new lesions on account of reactions was 0.24 (n=23) and that of neuritis was 0.39 (n=37). Of the total neuritis, 24 were reported independently and 13 were reported along with type 1 reaction. Rate of occurrence of type 1 reaction was 0.54 (n=51). Type 2 reaction was 0.03 (n=3) per 100 PY from two PB patients (first year=2 and fourth year=1) who also had nerve lesions at the time of enrolment.
Status of skin lesions during follow up: Of the total PB patients, 97 per cent patients had either inactive or improved skin lesions at the time of completion of treatment and 0.5 per cent had static lesions at the end of fifth year of post-U-MDT (Table IV).
Findings among MB type of patients
Of the 1298 MB patients enrolled (mean age 35.3±16.1 yr), 10 per cent (n=129) were children younger than 15 years and 66 per cent (n=853) were male (Table I). G2D was present in five per cent (n=66) at recruitment and nerve lesions were present in 63 per cent (n=812) of the study participants. At enrolment, four per cent (n=49) had evidence of mild reactions and five per cent (n=61) had neuritis.
Primary outcome: Of the MB patients, four had clinically confirmed relapse (Table II) and the relapse rate was 0.07 per 100 PY (total PY=5379) and cumulative risk for five years was 0.37 per cent. Three relapses occurred during the second year and one in the first year. All of them were put on one more course of U-MDT. At the fifth year of post-treatment follow up, one patient from P. R. China had static skin lesions (Table III) and the rest had either ‘inactive’ (n=2) or improved (n=1) lesions.
Secondary outcomes: All of the MB patients accepted U-MDT regimen in all the sites. There were no complaints about clofazimine. The skin pigmentation due to clofazimine was reported to be of short duration and acceptable to the enrolled patients with MB leprosy. Of the total 1298 who accepted U-MDT, 94 per cent (n=1220) completed the regimen and 52 per cent (n=675) consumed doses within six months.
In all, 636 special events were reported among MB patients. Of these, 223 were clinical (n=20) or non-clinical (n=203) leading to lost to follow up. The remaining 413 events (clinical=296 and temporary migrations=117) did not result in lost to follow up (Table II). Fifty MB patients died during the follow up period (rate: 0.88 per 100 PY). Of these, nine each were due to respiratory failure and liver diseases and eight deaths were due to cardiac problems. Seven deaths were reportedly due to injuries (suicide=4; drowning=2; homicide=1). Ten MB patients died due to various causes. Cause of death was unknown for seven patients.
Of the 30 patients who refused to continue in the study for various reasons, 12 patients refused clinical examination during follow up, and for five of them, the regimen was changed and nine did not report any reason for discontinuation. Three patients refused because they were not interested in continuing in the study and one patient refused on account of stigma.
Among the lost to follow up reported under ‘others’ events, 20 were due to various reasons such as shifting outside the study area (n=13). P. R. China site removed seven patients from the trial since five of them were put on routine MDT [either by the investigators (n=4) or as opted by patient (n=1)] and two patients received additional dose of clofazimine.
Of the clinical events leading to lost to follow up, 16 were due to suspected ADRs (total PY=605; rate=2.64 per 100 PY). Of these, seven had dapsone-induced exfoliative dermatitis and were classified as ‘probably’ and rest as ‘possibly’ related to the drug. Of the reported clinical events, rate of occurrence (per 100 PY) of new lesions on account of reactions was 1.34 (n=74) and that of neuritis was 1.37 (n=78). Of the neuritis, 43 were reported independently and 29 were reported along with type 1 and six with type 2 reactions. Rate of occurrence of type 1 reaction was 2.01 (n=114) and that of type 2 reaction was 0.49 (n=28) per 100 PY (Table II). Type 2 reactions (28 events from 24 patients) occurred during treatment and throughout the follow up.
Status of skin lesions during follow up: Proportion of MB patients with inactive and improved skin lesions was 95 per cent at the end of the completion of treatment. Static lesions were present in 1.1 per cent at the end of fifth year of post-U-MDT (Table IV).
Discussion
Our observation of low level of relapse was consistent with the findings from the most recent randomized controlled trial from Brazil that compared U-MDT with regular MDT (0.09 per 100 PY; two relapses during 2139 PY)1415. Rate documented in our trial was much lower than the reported relapse rates from programmatic settings and other field trials161718192021 [maximum rates (per 100 PY): 0.65 in PB and 2.04 in MB]. Based on information available from leprosy programmes, the WHO reports frequency of relapse per year as 0.1 per cent for PB and 0.06 per cent for MB22. According to India's leprosy programme, the country as a whole reported 433 clinical relapses for the year 2013-2014 with one larger province reporting the maximum (n=236)21.
In the present study, almost all the new patients in the eight centres (98.6%) were enrolled and 94 per cent of them completed U-MDT treatment in nine months indicating good acceptability and compliance. The profile of study participants represented the actual scenario of new leprosy cases at the community level. Among these patients, low relapse rates were observed after completion of U-MDT. Thus, in this trial, apart from the question of extent of relapses in PB and MB patients, it was possible to consider overall effectiveness of this treatment regimen under routine programmatic conditions. Since this study was taken up for patient treatment, case detection became more proactive from the point of view of recruitment. This would explain a lower level of MB proportion among the new cases in this study.
With regard to safety of the regimen, the addition of clofazimine could potentially offer clinical and cost benefits. In terms of clinical benefits, clofazimine possibly reduces incidence of neuritis in PB and type 2 reactions in MB. The present study was not designed to test these beneficial effects. However, the observed incidence rates of neuritis and type 2 reactions and cumulative risk of neuritis (1.94% and 6.63% in PB and MB, respectively) and type 2 reactions (0.16% and 2.43% in PB and MB, respectively) were lower than those reported in the literature. For instance, the overall incidence of neuritis reported ranges between 6.1 and 34 per cent23242526272829. Similarly, reported rates (range) of type 2 reactions are higher in hospital-based studies (overall: 2-28.9%) than in the field leprosy programmes (overall: 0.2-4.6%; MB: 1-8.9%)232425262730313234. India's national leprosy programme reported 12,901 episodes of reactions/neuritis episodes for 2013-2014 for the entire country18. In the programmatic context, addition of clofazimine may theoretically add to the cost to treat leprosy. However, such costs will be offset by reduction in morbidity among PB patients and hence reduced cost of management of such morbidities. Reduced duration of regimen for MB will further halve the cost of regimen. Thus U-MDT regimen will actually reduce the cost of leprosy treatment.
Advantages and implications for leprosy programmes
U-MDT trial was essentially a programmatic implementation research. Hence, it is worth considering the findings in the context of its implications for programmes. Nearly all new treatment naive patients from the study areas were included. Proportion of MB was lower than PB (38 vs. 62%) and MB patients had nerve involvement. We expect this to be generally representative of the real-life situation in the programme. We tried to keep implementation of the U-MDT as per the programme routine. However, the case detection had been proactive and the follow up of the patients was more rigorous. It is expected that if U-MDT is implemented in the programme situation with appropriate sensitization of patients and providers, it will help in effectively reducing leprosy prevalence at the district/regional levels as well.
In the national leprosy programme (India), skin smear and skin biopsies are not performed. In the absence of such testing, it is essential to consider how much could be the probable misclassification in the present study. PB-MB grouping is employed primarily on the assumption that the protective immunity is inversely correlated with the number of lesions35. In programmatic conditions, it was thus possible that some of the leprosy patients would have been misclassified as PB or MB36. However, the extent of such misclassification in the present study seems to be minimal. For instance, a low rate of type 2 reactions among PB (rate=0.03; risk=0.16%) was observed as compared to 0.49 per 100 PY among MB patients (risk=2.43%, P<0.001).
Two study sites carried out skin smear test as part of their implementing agency's or country's policy and practice although skin smear examination was not required as per common protocol. P. R. China sites performed skin smear examination and documented rapid fall in bacteriological index with almost 95 per cent MB patients becoming smear negative at the end of five years of follow up3738. This information further supports the applicability of U-MDT in the programme.
Finally, there is a need to consider implications of trial findings on the follow up strategy while adopting U-MDT in programmes. All the suspected ADRs were reported within a maximum of three months, and all the relapses occurred within first three years after treatment completion. Further, it was noted that the occurrence of type 2 reactions was continuing during post-treatment follow up. Hence, the primary health care physicians will require necessary clinical expertise to recognize and manage such clinical events. There is a need to educate and counsel patients to be alert about any such event and report immediately to the primary health care providers.
Only a small number of patients in PB and MB had static lesions at the end of five years post-U-MDT. Since relapses occurred within first three years after U-MDT, a carefully crafted strategy for periodic follow up algorithm during the first three years after MDT might help in picking up relapse patients relatively early. In 2013-2014, India's leprosy programme confirmed that a sizeable number of suspected relapses at the primary health care level (n=486) were referred and confirmed at the district hospital level (n=433)21. Hence, the national leprosy programmes could implement such a strategy of identification, referral and management at appropriate levels.
Limitations and biases
Our study had few limitations and biases. Key limitation was that of inability to meet the sample size requirements for MB. Due to overall reduction in prevalence, adequate number of patients could not be enrolled in the given geographic areas of the study sites. Further, the sample size was calculated for an expected relapse rate of three per cent (Pa) in the study groups, i.e., two per cent less than an assumed level of five per cent (Po). At the end of the trial, we observed relapse of <1 per cent. The power to detect this two per cent difference (i.e., between 3 and 1%) was 100 per cent for PB and 99.9 per cent for MB group. Therefore, even with the recruited number of participants, we had closer to 100 per cent power to support our conclusions of efficacy of the six-month U-MDT regimen to prevent relapses in PB and MB types of leprosy patients.
In terms of biases, two types of selection biases might be considered. The study sites were purposively selected on the basis of ability to recruit patients and to offer better services and follow up. Further, as only those patients who were willing, were enrolled, there could be some level of selection bias at the level of participants. However, in most of our field sites, almost all the patients opted for U-MDT, and hence, such selection bias would be minimal. Further, due to the active nature of follow up from the investigators and the coordinating centre, it is possible that research bias might have contributed to the higher treatment completion rates than the reported figures in programme settings.
On the basis of our findings, it is concluded that the observed low relapse among the newly detected PB and MB leprosy patients from India and P. R. China demonstrates efficacy and effectiveness of U-MDT regimen in both PB and MB patients. The regimen was found to be acceptable and safe for both the groups of patients. The negligible proportion of static lesions in the MB patients of our trial documented the effectiveness of shortened duration of regimen. Treating physicians need to be aware as well as vigilant about monitoring leprosy patients for special events during and after completion of MDT for about three years. Based on such monitoring and assessments, treating physicians can decide to prolong treatment duration for individual patients. The global and national programmes should consider the evidence for programmatic adaptation of U-MDT strategy for all types of leprosy patients.
Acknowledgment
Authors acknowledge the Indian Council of Medical Research (ICMR), Department of Health Research (DHR), Government of India: Dr N.K. Ganguly [Former Director-General (DG)-ICMR], Dr V.M. Katoch (Former DG-ICMR & Secretary DHR); NIE (ICMR): Dr S. Balasubramanyam, Servshri P.A. Tamby, K. Boopathi, S. Satish, M. Gangadhara Rao, Dr B. Kishore Kumar, WHO-GLP: Drs S.K. Noordeen, D. Daumerie, Myo Thet Htoon, Sumana Barua, P.V. Ranganadha Rao; Indian State Health Services: Dr P. Krishnamurthy, Director of Public Health and Preventive Medicine, Government of Tamil Nadu; Dr Subhash Salunke, Director General of Health Services, Government of Maharashtra; Expert Committee for review of relapses and reactions: Dr V.V. Pai, Director, Bombay Leprosy Project; Dr P Vijayakumaran, formerly from Damien Foundation India Trust (DFIT); Dr B. Nagaraju, formerly with NIE-ICMR; Study sites: DFIT: Dr Santhosh Kumar; NJILOMD: Dr S.K. Tripathy, Dr Avi Kumar Bansal, Project Assistants at all the study sites.
Conflicts of Interest: None.
References
- Multidrug therapy against leprosy: development and implementation over the past 25 years. Geneva: WHO; 2004.
- Leprosy elimination campaigns – Detecting and curing patients. Wkly Epidemiol Rec. 1999;74:329-34.
- Guide to eliminate leprosy as a public health problem (1st ed). Geneva: WHO; 2000.
- National programme managers for leprosy elimination: Report of an intercountry meeting, Kathmandu, Nepal. New Delhi: WHO; 2005.
- Enhanced global strategy for further reducing the disease burden due to leprosy (Plan Period: 2011-2015), (SEA/GLP/2009.3). New Delhi: WHO; 2009.
- Uniform MDT (U-MDT) regimen for all leprosy patients – Another example of wishful thinking. Lepr Rev. 2003;74:2-6.
- [Google Scholar]
- International open trial of uniform multi-drug therapy regimen for 6 months for all types of leprosy patients: rationale, design and preliminary results. Trop Med Int Health. 2008;13:594-602.
- [Google Scholar]
- Bactericidal activities of combinations of new drugs against Mycobacterium leprae in nude mice. Antimicrob Agents Chemother. 1996;40:393-9.
- [Google Scholar]
- Experimental evaluation of possible new short-term drug regimens for treatment of multibacillary leprosy. Antimicrob Agents Chemother. 1997;41:326-30.
- [Google Scholar]
- Bactericidal activity of single dose of clarithromycin plus minocycline, with or without ofloxacin, against Mycobacterium leprae in patients. Antimicrob Agents Chemother. 1996;40:2137-41.
- [Google Scholar]
- Chemotherapy trial in paucibacillary leprosy using clofazimine. Indian J Lepr. 1999;71:311-24.
- [Google Scholar]
- Chemotherapy of tuberculosis, Mycobacteriumavium complex disease, and leprosy. In: Brunton LL, Lazo JS, Parker KL, eds. Goodman and Gilman's the pharmacological basis of therapeutics. New York: McGraw-Hill; 2006. p. :1220.
- [Google Scholar]
- OpenEpi: Open source epidemiologic statistics for public health, version 3.03a. Available from: http://www.OpenEpi.com
- Primary results of clinical trial for uniform multidrug therapy for leprosy patients in Brazil (U-MDT/CT-BR): reactions frequency in multibacillary patients. Lepr Rev. 2012;83:308-19.
- [Google Scholar]
- Results from the clinical trial of uniform multidrug therapy for leprosy patients in Brazil (U-MDT/CT-BR):decrease in bacteriological index. Lepr Rev. 2014;85:262-6.
- [Google Scholar]
- WHO expert committee on leprosy. 8th report. WHO technical report series 968. Geneva: WHO; 2012.
- Leprosy. 2010. BMJ Clin Evid. pii: 0915 Available from: http://clinicalevidence.bmj.com/x/systematic-review/0915/archive/06/2010.html
- [Google Scholar]
- Relapse study in smear positive multibacillary (MB) leprosy after 1 year WHO-multi-drug therapy (MDT) in Cebu, Philippines. Lepr Rev. 2011;82:65-9.
- [Google Scholar]
- Directorate General of Health Services (DGHS) 2015. NLEP – Progress Report for the year 2013-14. New Delhi: Government of India;
- [Google Scholar]
- Global leprosy update, 2013; reducing disease burden. Wkly Epidemiol Rec. 2014;89:389-400.
- [Google Scholar]
- Leprosy beyond MDT: study of follow-up of 100 released from treatment cases. Indian J Lepr. 2010;82:189-94.
- [Google Scholar]
- Incidence of acute nerve function impairment and reactions in leprosy: a prospective cohort analysis after 5 years of follow-up. Int J Epidemiol. 2004;33:337-43.
- [Google Scholar]
- The INFIR cohort study: investigating prediction, detection and pathogenesis of neuropathy and reactions in leprosy. Methods and baseline results of a cohort of multibacillary leprosy patients in North India. Lepr Rev. 2005;76:14-34.
- [Google Scholar]
- Occurrence and management of leprosy reaction in China in 2005. Lepr Rev. 2009;80:164-9.
- [Google Scholar]
- Risk factors for leprosy reactions in three endemic countries. Am J Trop Med Hyg. 2015;92:108-14.
- [Google Scholar]
- Reversal reactions in the skin lesions of AMFES patients: incidence and risk factors. Lepr Rev. 2000;71:309-17.
- [Google Scholar]
- Occurrence of reactions, their diagnosis and management in leprosy patients treated with multidrug therapy; experience in the leprosy control program of the All Africa Leprosy and Rehabilitation Training Center (ALERT) in Ethiopia. Int J Lepr Other Mycobact Dis. 1992;60:173-84.
- [Google Scholar]
- Identification of clinical, epidemiological and laboratory risk factors for leprosy reactions during and after multidrug therapy. Mem Inst Oswaldo Cruz. 2013;108:901-8.
- [Google Scholar]
- Clinical course of erythema nodosumleprosum: an 11-year cohort study in Hyderabad, India. Am J Trop Med Hyg. 2006;74:868-79.
- [Google Scholar]
- A systematic review on the epidemiological data of erythema nodosumleprosum, a type 2 leprosy reaction. PLoS Negl Trop Dis. 2013;7:e2440.
- [Google Scholar]
- Reactions following completion of 1 and 2 year multidrug therapy (MDT) Am J Trop Med Hyg. 2010;83:637-44.
- [Google Scholar]
- WHO expert committee on leprosy. 7th report. WHO technical report series 874. Geneva: WHO; 1998.
- Report of the International Leprosy Association Technical Forum. Paris, France, 22-28 February 2002. Int J Lepr Other Mycobact Dis. 2002;70(1 Suppl):S1-62.
- [Google Scholar]
- Six years’ follow-up of multibacillary leprosy patients treated with uniform multi-drug therapy in China. Int J Dermatol. 2015;54:315-8.
- [Google Scholar]
- Bacteriological results and leprosy reactions among MB leprosy patients treated with uniform multidrug therapy in China. Lepr Rev. 2012;83:164-71.
- [Google Scholar]






