Translate this page into:
In vitro activity of daptomycin & linezolid against methicillin resistant Staphylococcus aureus & vancomycin resistant enterococci isolated from hospitalized cases in Central India
Reprint requests: Dr D.S. Chitnis, Head, Department of Microbiology, Immunology & Molecular BiologyChoithram Hospital & Research Centre, Manik Bagh Road, Indore 452 014, India e-mail: ds_chitnis@rediffmail.com
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Growing incidence of methicillin resistant Staphylococcus aureus (MRSA) and vancomycin resistant enteroccoci (VRE) is posing a therapeutic problem due to limited drug options. Therefore, the present study was undertaken to check susceptibility of MRSA and VRE isolates against new antimicrobials such as daptomycin and linezolid.
Methods:
A total of 586 Gram-positive isolates comprising 442 S. aureus and 144 enterococci isolated from hospitalized cases included in the study, were subjected to in vitro antimicrobial susceptibility testing by disc diffusion method. One hundred twenty four enterococci obtained from rectal swabs of neonates were also included. Minimum inhibitory concentration (MIC) was determined for daptomycin, linezolid, vancomycin and teicoplanin against 50 each isolates of MRSA and VRE by E strip.
Results:
Among the staphylococci, 326 (73.85%) isolates were MRSA. MIC for vancomycin and teicoplanin among MRSA was ≤3 μg/ml. MIC for daptomycin among MRSA was found to be in the range of 0.064-1.5 μg/ml. Percentage of VRE among clinical samples was 14.29 per cent while it was 47.06 per cent among enterococci from rectal swabs of neonates. MIC was >256 μg/ml for vancomycin among VRE and was associated with van A genotype. MIC range for daptomycin among VRE was 0.38-3 μg/ml. MIC for linezolid among MRSA and VRE was in the range of 0.25 to 1 and 0.38 -1.5 μg/ml, respectively.
Interpretation & conclusions:
The present study showed a rise in MIC to vancomycin for sizable number of MRSA and growing percentage of VRE at our centre. Daptomycin and linezolid showed 100 per cent activity against MRSA and VRE.
Keywords
Daptomycin
linezolid
methicillin resistant Staphylococcus aureus
minimum inhibitory concentration
vancomycin resistant enterococci
Gram-positive organisms are the most common bacterial pathogens causing serious infections such as complicated skin and soft tissue infection1, bacteraemia and infective endocarditis2. The grave concern is the growing incidence of drug resistant pathogens, such as methicillin resistant Staphylococcus aureus (MRSA) and vancomycin resistant enterococci (VRE), for which therapeutic options are limited3.
Daptomycin a cyclic lipopeptide derived from the fermentation of Streptomyces roseosporus, is composed of a hydrophilic 13 member amino acid core and lipophlilic 10 carbon tails that confer its unique mechanism of antibacterial action involving calcium dependent binding of the drug to the cytoplasmic membrane. This causes alteration in membrane function which results into impairment of potassium dependent macromolecular synthesis following efflux of potassium from the cell3. It is approved for use in the treatment of complicated skin and soft tissue- structure infections caused by Gram-positive bacteria and S. aureus bacteraemia including right sided infective endocarditis3. Rapid concentration-dependent bactericidal activity against a variety of Gram-positive organisms, including S. aureus (both methicillin sensitive and resistant-MSSA and MRSA), Enterococcus faecalis (both vancomycin susceptible and resistant) has been described for daptomycin3.
Linezolid has a broad spectrum of activity against Gram-positive bacteria including multiple drug resistant isolates. Linezolid inhibits bacterial protein synthesis by binding to the 50s ribosomal subunit near to the interface with the 30s subunit, causing inhibition of 70S initiation complex formation. It is active against both MSSA and MRSA, and inhibits all strains at a concentration of 4 μg/ml or less4.
Several studies have reported in vitro susceptibility to daptomycin and linezolid against Gram-positive bacterial isolates4–12, but there have been only a few published studies from India on the antimicrobial susceptibility to daptomycin and linezolid against MRSA and enterococci13–15. In view of the limited information available from India, the present study was aimed to evaluate the antimicrobial susceptibility of MRSA and VRE isolates obtained from hospitalized patients against daptomycin, vancomycin and linezolid.
Material & Methods
The study was carried out at Choithram Hospital and Research Centre (CHRC), Indore, India. This hospital is a 350 bedded multispeciality referral centre in central India with facilities in intensive cardiac care, nephrology, neurology, gynaecology, paediatrics, general medicine and various surgical specialities. Consecutive isolates during study period were from admitted cases in medical, surgical and ICU wards.
Five hundred eighty six isolates of non duplicate Gram-positive cocci were included in the present study. These comprised S. aureus (442) and E. faecium (144) from suspected cases of hospital acquired infections (hospital stay >3 days) during January 2008 - December 2010. The staphylcocci isolates were from samples such as urine (n=26), pus (n=147), body fluid (n=50) and blood (219). The enterococci isolates were from samples such as urine (n=48), pus (n=60), body fluid (n=6) and blood (n=30). Blood agar and nutrient agar plates were used as non selective media and mannitol salt agar plate and enterococcosel agar plate as selective media for isolation of S. aureus and enterococci, respectively. All staphylococci were identified by standard biochemical tests16. The identification of the enterococcal isolates was done by standard methods17.
Antimicrobial susceptibility testing: Gram-positive cocci (n=586) were subjected to antimicrobial susceptibility testing by the disk diffusion technique18. The isolates were screened for susceptibility to a panel of antibiotics using Mueller-Hinton agar (BD, India) medium. The antibiotic discs (BD, India) containing the following antibiotic concentrations (in μg) as per CLSI guidelines19 were used : ampicillin (10), oxacillin (1), cefoxitin (30), cephaloridine (30), cefotaxime (30), ceftriaxone (30), cefoperazone (75), cefoparazone/sulbactum (75/30), cefepime (30), teicoplanin (30), vancomycin (30), gentamicin (10), amikacin (30), netilmicin (30), erythromycin (15), ciprofloxacin (5), clindamycin (2), trimethoprim-sulphamethoxazole (1.25/23.75), chloramphenciol (30), and linezolid (30). For enterococci only ampicillin (10), vancomycin (30), gentamicin (10), erythromycin (15), ciprofloxacin (5), chloramphenciol (30), and linezolid (30) were tested.
The inhibition zone diameters were measured to the nearest millimeter and recorded. Each bacterial isolate was classified as susceptible (S), intermediate (I) and resistant (R) to antibiotic according to the zone diameter interpretation standard recommended by the Clinical Laboratory Standards Institute (CLSI)20. E. faecalis ATCC 29212 and S. aureus ATCC 25923, S. aureus ATCC 29213 were used as quality control strains (Sanofiaventis, India) to check antibiotic discs and accuracy of the testing procedure. The identification of MRSA was confirmed by cefoxitin and oxacillin disc diffusion test as described by the CLSI20. The identification of the van A genotype (van A or van B) for each isolates of VRE was performed by using multiplex polymerase chain reaction as described earlier21.
Minimum inhibitory concentration (MIC): A total of 100 randomly selected isolates including 50 MRSA and 50 VRE were included for MIC determination. Of the 50 VRE, 20 were from clinical samples collected during the study period and 30 were from 124 rectal swabs collected from newborns admitted in neonatal ICU and general maternity ward. MRSA and VRE were tested for MIC for daptomycin, linezolid, vancomycin and teicoplanin by Epsilometer test (E-test, AB Biodisk Solna, Sweden). The daptomycin E test contained a concentration gradient of daptomycin with a standard amount of calcium throughout the strip. MIC values were read as per the manufacturers recommendation and interpretation made as per CLSI criteria20.
Susceptibility breakpoint for daptomycin was considered as <1 μg/ml for staphylococci and <4 μg/ml for enterococci, as recommended by the CLSI20. A linezolid susceptible breakpoint of <4 μg/ml was used for staphylococci whereas <2 μg/ml was used for enterococci as approved by CLSI20. E. faecalis ATCC 29212 and S. aureus ATCC 29213, S. aureus ATCC 43300 (MRSA) E. faecalis ATCC 51299 (VRE) were tested concurrently as quality control strains.
Results
Number of staphylococci and enterococci isolates from each ward/ ICU/OPD and their methicillin and vancomycin resistant vs sensitive patterns are detailed in the Table. Among the 442 non duplicate staphylococcal isolates included in the study, 326 (73.85%) were MRSA. Resistance among MRSA to chloramphenicol, amikacin, gentamicin, clindamycin, erythromycin, ciprofloxacin and lincomycin was 11.5, 11.6, 28.8, 29.4, 68.03, 77 and 54.9 per cent, respectively. More than 70 per cent isolates of S. aureus (73.33%) were resistant to oxacillin, cefoxitin, cephaloridine, cefotaxime, ceftazidime, cefoparazone alone or in combination with sulbactum, and cefpime (Table). All S. aureus isolates were sensitive to linezolid, vancomycin and teicoplanin.
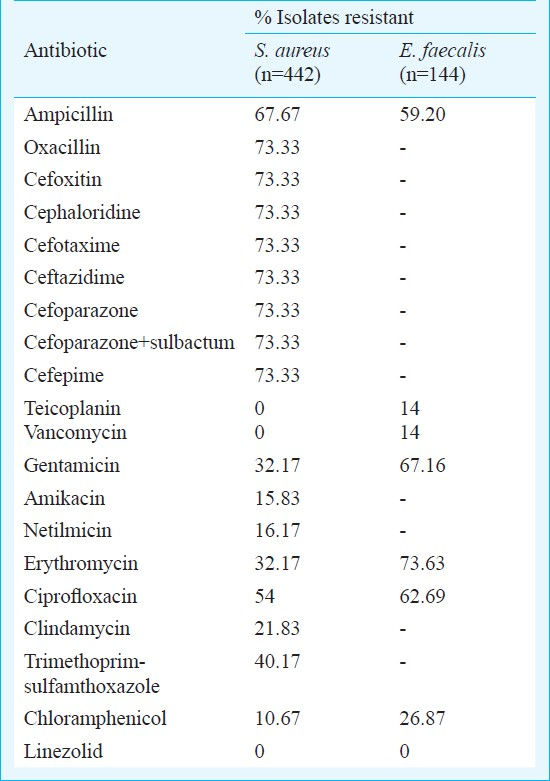
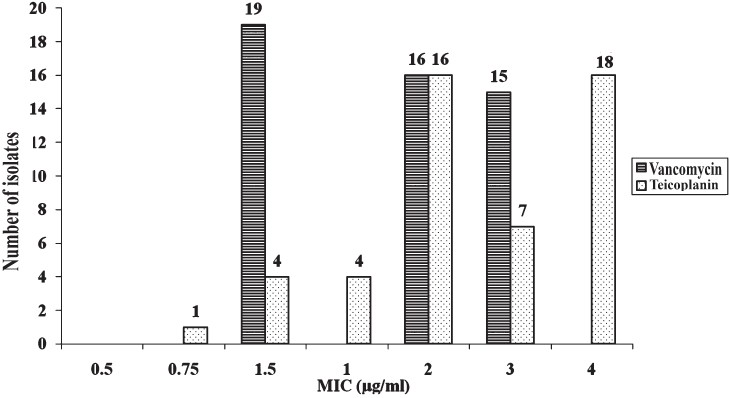
The MIC values for daptomycin and linezolid for the MRSA and VRE isolates are shown in Figs 1 and 2, respectively. MIC for daptomycin among MRSA was found to be in the range of 0.064-1.5 μg/ml and for linezolid was in the range of 0.25 to 1 μg/ml and only one isolates had MIC 1.5 μg/ml. MIC for vancomycin and teicoplanin among MRSA was in the range of 1.5-3 μg/ml and 1.5-4 μg/ml respectively (Fig. 1).
- MIC values for vancomycin (50) and teicoplanin for MRSA.
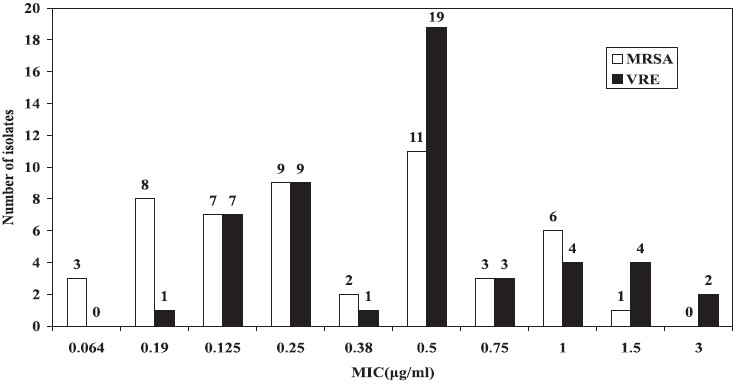
- MIC values for daptomycin among MRSA (n=50) and VRE (n=50).
Vancomycin resistance among 144 clinical isolates of enteroccocci was 14.29 per cent whereas it was 47.06 per cent for enterococci from 124 rectal swabs of neonates. Resistance to other drugs such as ampiciilin was 59.20 per cent, ciprofloxacin 62.69 per cent, erythromycin 73.63 per cent and chloramphenicol 26.87 per cent. MIC for vancomycin and teicoplanin among VRE isolates was in the range of 64 to >256 μg/ml. Ten of the VRE isolates tested had vanA containing genotypes. Among VRE, MIC for daptomycin was 0.19-1.5 μg/ml but two isolates had MIC of 3 μg/ml. Linezolid exhibited very good activity against VRE as well (MIC 0.38 -1.5 μg/ml) (Figs 2, 3).
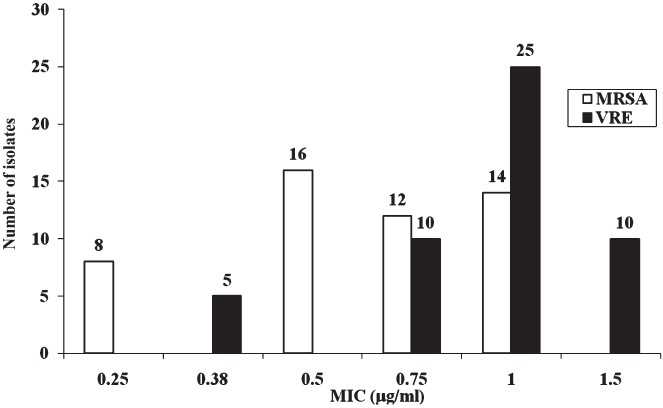
- MIC values for linezolid among MRSA (n=50) and VRE (n=50).
Discussion
Methicillin resistance was seen in 73.85 per cent of S. aureus isolates in the present study. We have earlier reported MRSA in the range of 6.9-80.89 per cent22. Methicillin resistance was often associated with simultaneous resistance for macrolide, quinolones and co-trimoxazole. Resistance for clindamycin among MRSA was 29.4 per cent as against 40 per cent reported by other group13. Clindamycin has been used successfully for the treatment of infection caused by MRSA23. Low percentage of resistance 11.5 per cent was observed for chloramphenicol in the present study. However, adequate data for the use of chloramphenciol to treat MRSA are lacking. None of the MRSA were resistant or intermediate resistant to vancomycin but 16 of the MRSA had vancomycin MIC 3 μg/ml and 34 of the MRSA had MIC of 1.5-2 μg/ml. Among MRSA, MIC for daptomycin was found to be in the range of 0.064-1 μg/ml. Only one isolate had MIC of 1.5 μg/ml. The same isolate had shown MIC of 3 μg/ml for vancomycin. The isolates cannot be labelled as vancomycin intermediate S. aureus (VISA) as these simultaneouly had elevated MIC for both vancomycin and daptomycin. Reduced susceptibility to vancomycin has been reported to be associated with reduced susceptibility to daptomycin. Diederen et al5 reported 7 of the 17 VISA isolates to have daptomycin MIC of 2 μg/ml and one isolate to have MIC 4 μg/ml. Others67 have also shared similar experience. Sader et al24 showed bactericidal activity of daptomycin against heterogenous VISA (hiVISA) isolated from blood. The use of vancomycin to treat infections caused by MRSA having vancomycin MIC 2 μg/ml also needs caution since therapeutic efficacy of vancomycin in such situation may not be rewarding25.
All MRSA and VRE strains were 100 per cent susceptible to daptomycin in the present study. The other group6 reported >99 per cent susceptibility of daptomycin against staphylococci from Asia pacific region. Activity of daptomycin against MRSA has been reported from India13–15. India Daptomycin Study Group14 reported daptomycin MIC in the of range 0.047-1 μg/ml.
A high level resistance to vancomycin was observed for 10 isolates of the enterococci (MIC >256 μg/ml) and the presence of Van A gene was documented in all of these VRE isolates as evident by PCR method. The enterococcal isolates with high MIC for vancomycin also had high MIC for teicoplanin suggesting cross-resistance between the two drugs. The increased MIC for vancomycin in S. aureus and vancomycin resistance among enterococci could possibly be due to increased usage of the drug at our centre. The presence of VRE among clinical isolates was 14 per cent but among isolates from rectal swabs it was as high as 47 per cent. The high percentage of VRE from rectal swabs suggests increased gut colonization with VRE among neonates. The reason for gut colonization appeared to be selective pressure of vancomycin. Further, it needs to be mentioned that clonal relatedness was not checked in the study for VRE from clinical isolates and rectal swabs isolates.
To conclude, elevated MIC for vancomycin among MRSA and enterococci is a cause of concern and daptomycin/linezolid remain good therapeutic alternatives to treat infections caused by MRSA and VRE. The only limitation of daptomycin is that it is not indicated for treatment of pneumoniae because of its inhibition by pulmonary surfactants26. Adverse events such as thromobocytopenia for linezolid27, neutorpenia and allergic reaction for vancomycin28 and rare effects on skeletal muscles for daptomycin29 have been reported. Therefore, clinician should be aware of side effect while using these drugs empirically. Also, strict infection control measures need to be emphasized to control the prevalence of MRSA and VRE in the hospital practice. The selection of antibiotic should be based on in vitro susceptibility and the hospital based antibiotic policies must be strictly followed and constant surveillance of drug resistance for all bacterial pathogens is needed.
References
- Complicated infections of skin and skin structures: when the infection is more than skin deep. J Antimicrob. 2004;53(Suppl S2):ii37-50.
- [Google Scholar]
- Datpomycin a review of its use in the management of complicated skin and soft tissue infections and Staphylococcus aureus bacteraemia. Drug. 2007;67:1483-512.
- [Google Scholar]
- The oxazolidinone linezolid inhibits intiation of protein synthesis in bacteria. Antimicrob Agents Chemother. 1998;42:3251-5.
- [Google Scholar]
- In vitro activity of daptomycin against methicillin resistant Staphylococcus aureus including heterogeneously glycopeptide resistant strains. Antimicrob Agents Chemother. 2006;50:3189-91.
- [Google Scholar]
- Antimicrobial susceptibility of gram positive bacterial isolates from the Asia Pacific region and an in vitro evaluation of the bactericidal activity of daptomycin, vancomycin and teicoplanin: a SENTRY program report (2003-2004) Int J Antimicrob Agents. 2007;30:143-9.
- [Google Scholar]
- In vitro activities of daptomycin, vancomycin, linezolid and quinupristin-dalfopristin against staphylococci and enterococci including vancomycin intermediate and resistant strains. Antimicrob Agents Chemother. 2000;44:1062-6.
- [Google Scholar]
- Daptomycin antimicrobial activity tested against methicillin resistant staphylococci and vancomycin resistant enterococci isolated in European medical centres (2005) BMC Infect Dis. 2007;18:29.
- [Google Scholar]
- Antimicrobial susceptibility of gram positive bacteria isolated from European medical centres: results of the daptomycin surveillance programme (2002-2004) Clin Microbiol Infect. 2006;12:844-52.
- [Google Scholar]
- Susceptibility to daptomycin, quinupristin-dalfopristin and linezolid and some other antibiotics in clinical isolates of methicillin resistant and methicillin sensitive S. aureus from the Oslo area. Scand J Infect Dis. 2007;39:1059-62.
- [Google Scholar]
- Comparative in vitro activity of daptomycin against gram positive microorganisms : SENTRY surveillance program, Spain (2002-2006) Enferm Infect Microbiol Clin. 2008;26:489-94.
- [Google Scholar]
- Canadian Antimicrobial Resistance Alliance (2008).Antimicrobial susceptibility of 3931 organisms isolated from intensive care units in Canada:Canadian National intensive care unit study, 2005-2006. Diagn Microbiol Infect. 2008;62:67-80.
- [Google Scholar]
- In vitro activity of daptomycin against Staphylococcus aureus and vancomycin resistant enterococcus faecium isolates associated with skin and soft tissue infections: first results from India. Diagn Microbiol Infect Dis. 2009;65:196-8.
- [Google Scholar]
- Activity of daptomycin against Gram positive bacterial isolates from Indian medical centre (2006-2007) Int J Antimicrob Agents. 2009;34:497-9.
- [Google Scholar]
- Antimicrobial susceptibility profiles of methicillin susceptible and resistant Staphylococcus aureus: focus on daptomycin minimum inhibitory concentration at a tertiary care centre in Mumbai, India. Int J Antimicrob Agents. 2010;36:267-70.
- [Google Scholar]
- Staphylococcus: cluster-forming Gram positive cocci. In: Collee AG, Fraser BP, Marmion JG, Simmons A, eds. Mackie and McCartney practical medical microbiology (14th ed). New York: Churchill Livingstone; 1996. p. :245-61.
- [Google Scholar]
- Identification of Enterococcus species isolated from human infections by a conventional test scheme. J Clin Microbiol. 1989;27:731-4.
- [Google Scholar]
- Antibiotic susceptibility by standardized single disc method. Am J Clin Pathol. 1966;45:493-6.
- [Google Scholar]
- Clinical and Laboratory Standards Institute. In: Performance standards for antimicrobial susceptibility testing. Wayne, Pa, USA: Clinical and Laboratory Standards Institute; 2010. 21st Informational Supplement. (M100-S21)
- [Google Scholar]
- Clinical and Laboratory Standards Institute. In: Performance standards for antimicrobial susceptibility testing. Wayne, Pa, USA: Clinical and Laboratory Standards Institute; 2009. 19th Informational Supplement. (M100-S19)
- [Google Scholar]
- Detection of glycopeptide resistance genotypes and identification to the species level of clinically relevant enterococci by PCR. Clin Microbiol Infect. 1995;33:24-7.
- [Google Scholar]
- Growing problem of methicillin resistant staphylococci - Indian scenario. Indian J Med Sci. 2000;54:535-40.
- [Google Scholar]
- Should clindamycin be used in the treatment of patients with infections caused by erythromycin-resistant staphylococci? J Antimicrob Chemother. 2000;45:709-17.
- [Google Scholar]
- Occurrence of vancomycin tolerant and heterogenous vancomycin intermediate strains (hiVISA) among S. aureus causing blood stream infection in nine USA hospital. J Antimicrob Chemother. 2009;64:1024-8.
- [Google Scholar]
- Vancomycin therapeutic guideline. A summary of consensus recommendation from infectious disease society of America, the American society of health system pharmacists and the society of infectious diseases pharmacists. Clin Infect Dis. 2009;49:325-7.
- [Google Scholar]
- Inhibition of daptomycin by pulmonary surfactant: in vitro modeling and clinical impact. J Infect Dis. 2005;191:2149-52.
- [Google Scholar]
- Linezolid: a review of its use in the management of serious gram positive infections. Drugs. 2001;61:525-51.
- [Google Scholar]
- Daptomycin 98-01 and 99-01 Investigators. The safety and efficacy of daptomycin for the treatment of complicated skin and skin structure infections. Clin Infect Dis. 2004;38:1673-81.
- [Google Scholar]






