Translate this page into:
Genetic polymorphism in Plasmodium falciparum: Differentiation of parasite isolates of high & low virulence by RAPD
Reprint requests: Prof. R.C. Mahajan, Emeritus Professor, Department of Parasitology, Postgraduate Institute of Medical Education & Research, Chandigarh 160 012, India. e-mail: indurc43@gmail.com
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
The increase in Plasmodium falciparum infections which are associated with severe and complicated malaria and drug resistance has made control of malaria a difficult task. Extensive genetic polymorphism in P. falciparum has been reported from several parts of the world which affects the efficacy of sub-unit vaccines. The knowledge of genotypes of the parasite in a geographical region is therefore, important for effective management and control. The aim of the present study was to investigate the usefulness of random amplified polymorphic DNA (RAPD)-PCR technique for differentiation of P. falciparum isolates from patients presenting with severe (cerebral malaria) and mild malaria.
Methods:
Genetic polymorphism in 21 P. falciparum isolates obtained from patients found positive for P. falciparum by light microscopy was studied by RAPD-PCR analysis. Eleven RAPD primers were used for analysis of 21 P. falciparum isolates obtained from cerebral and non-cerebral malaria patients.
Results:
Of the 11 primers, only three (E-4, E-8, and R-8) produced useful polymorphic patterns. The cluster analysis based on UPGMA demonstrated that isolates causing cerebral malaria cluster separately from those causing uncomplicated malaria. However, the analysis of phylogenic tree showed that P. falciparum isolates causing non-cerebral and cerebral malaria clustered separately but showed relatedness.
Interpretation & conclusions:
The results of the present study showed that the RAPD-PCR was able to differentiate the isolates causing severe and mild malaria. The cluster analysis of the phylogenic tree suggested that the virulent strains evolved from less virulent strains as it clustered separately. RAPD technique may be useful in discriminating between the different isolates of the same species resulting in different clinical profiles.
Keywords
Cerebral malaria
genetic polymorphism
Plasmodium falciparum
RAPD
virulence
The magnitude of malaria in terms of morbidity and mortality in humans makes it a major public health problem in tropical and sub-tropical countries. It is estimated that about 300-660 million cases of malaria occur every year, of whom around 90 per cent are in sub-Saharan Africa12. The spread of drug resistant strain of Plasmodium falciparum is an increasing problem. Much effort is currently being made for the development of an effective malaria vaccine for control of this disease. P. falciparum genome has a G+C content of 17-19 per cent which is the lowest for any reported organism3. Plasmodium spp. have 14 chromosomes as demonstrated by the presence of 14 kinetochores4 as well as by pulse field gel electrophoresis (PFGE)5. The size of the chromosomes ranges from 800 to 3500 kb and the chromosomes show size polymorphism in cultured parasites as well as in parasite from wild strains. The presence of size polymorphism is due to the deletion of either coding or repetitive sequences6. Random amplified polymorphic DNA (RAPD) is a simple and rapid technique which requires very small quantity of genomic DNA and no sequencing, cloning and hybridization representing distinct advantages over other molecular techniques generally used in genomic characterization. RAPD has been used to obtain genetic marker for many organisms as well as for taxonomic identification78.
Several techniques based on DNA fingerprinting for microorganisms such as restriction fragment length polymorphism (RFLP)9–11, microsatellites12 and single stranded confirm polymorphism (SSCP) 5 have been used for genotyping of P. falciparum. In the present study, we have used RAPD technique for genotyping of P. falciparum field isolates prevalent in northern region of India. The aim of the study was to know whether the RAPD was able to differentiate between the clinical isolates of parasite causing complicated and uncomplicated malaria.
Material & Methods
Subjects and blood samples: A total of 21 blood samples were collected from different individuals presenting with acute symptoms of malaria and found positive for P. falciparum by light microscopy of Giemsa stained peripheral blood smears. The study protocol was approved by the Ethics Committee of the Institute. Written informed consent was taken from the patient before drawing the blood samples. These patients were referred to general medicine out patients department attached to the Nehru hospital, Postgraduate Institute of Medical Education & Research (PGIMER), Chandigarh during June 2002 to July 2003. Since Nehru Hospital is a referral, tertiary care centre, patients from various States in north and north-western India are referred to this hospital. The majority of patients were from northern States of India including six from Punjab, four from Haryana, two from Himachal Pradesh and six from Uttar Pradesh while three samples were from north-western State of Rajasthan. The small sample size was because the number of the patients referred to PGIMER, Chandigarh, in that year was small (n=21). Most of these patients were suffering from severe malaria i.e. cerebral malaria and/or severe anaemia which is a common presentation of malaria. These isolates were divided into three groups:
-
Cerebral malaria group (CM): These were isolates from 10 patients presenting with signs of involvement of central nervous system (CNS) like altered sensorium and coma. All these patients were positive for P. falciparum in peripheral blood and did not have any evidence of infection with other microbes as checked microbiologically.
-
Severe anaemia group (SA): The two isolates were obtained from two patients who had very low haemoglobin level of <5 g/dl, but did not have any symptoms indicative of CNS involvement.
-
Uncomplicated malaria group (UM): Nine isolates were obtained from patients with high fever who did not have any symptom and signs suggestive of severe and complicated malaria.
The patients showing mixed infection (with P. falciparum and P. vivax) by microscopy of Giemsa stained peripheral blood smear examination, were excluded from the study. Blood (2 ml) was collected by venepuncture in sterile vial containing citrated anticoagulant and stored in refrigerator till used.
Extraction of parasite DNA: DNA was extracted from the infected RBCs by using a rapid and simple method described elsewhere1113. Briefly, 50 μl of parasitized blood was washed thrice with 1 ml of ice-cold sodium phosphate (5 mM, pH 8.0). The suspension was centrifuged at 10000 × g for 10 min. The supernatant was discarded and the pellet re-suspended in 50 μl of sterile distilled water and heated in a boiling water bath for 10 min. The suspension was cooled slowly at room temperature and centrifuged at 10000 × g. Supernatant (40 μl) was transferred into another vial and 3 μl of this was used as DNA template in 25 μl PCR mixture for RAPD analysis.
RAPD analysis: To obtain RAPD patterns, the following 11 primers were used for amplifications14.

For RAPD amplification,all PCR reactions were carried out in 25 μl volume in a thermal cycler (Eppendorf Master Cycler Gradient, Germany), using 4 mM MgCl2, 10 μg/ml BSA, 0.2 mM of each of dNTPs (Bangalore Genei Pvt. Ltd., India), 1 × Taq Polymerase buffer, 1.25 units of Taq DNA polymerase (Bangalore Genei, Pvt. Ltd., India), 25 pmol each of the primers and 3 μl of DNA14. The initial denaturation of DNA was done at 94°C for 3 min, and subjected to 47 cycles at the following conditions: 94° C for 1 min, 36° C for 1.30 min, and 72° C for 2 min and final extension was done at 72° C for 10 min. PCR products were run on 1.5 per cent agarose gel containing ethidium bromide. The DNA bands were visualized under UV in a gel documentation system (UVI Pro, England).
Results & Discussion
DNA based markers play an important role in estimating diversity and identification of genotypes in an organism prevalent in an area. Earlier, we have used PCR-RFLP to determine the extent of genetic polymorphism in genes encoding potential vaccine candidate antigens101115. In our earlier study using PCR-RFLP analysis of MSP-1 gene of P. falciparum isolates, we observed predominance of certain genotypes in cerebral malaria cases10. In the present study, RAPD-PCR was used to determine the genetic diversity in P. falciparum clinical isolates collected from north and north-western regions of India and to relate the genetic diversity with the severity of disease caused by P. falciparum isolates. The PCR-RFLP analysis is restricted to a particular gene while RAPD amplifies random segment of genomic DNA by using a short primers arbitrarily chosen without prior sequence information is helpful to determine the evolutionary patterns of the organism to be studied8. Of the 11 primers used for RAPD analysis, seven primers (R-1, R-2, R-6, R-8, E-4, E-8 and E-10) generated amplicon while four primers (R-3, R-4, R-5, and R-7) did not produce any band. The amplicon band patterns of all the isolates were similar with four primers and only three primers (E-4, E-8, and R-8) produced useful polymorphic patterns. A total of 350 DNA bands were evaluated by all the primers. The RAPD analyses of P. falciparum clinical isolates suggested that different genotypes are prevalent in north and north-western India.
The polygenetic tree constructed using Unweighted Pair Group Method with Arithmetic Mean (UPGMA) demonstrated that isolates causing cerebral malaria clustered separately from those causing uncomplicated malaria and severe anaemia (Fig.). The analysis of theses isolates by E-4 primer showed distinct differences among the isolates. The isolates causing severe anaemia (SA) formed a sister group among the isolates causing uncomplicated malaria (UM), suggesting that the isolates causing SA and UM differ slightly from each other (Fig.). This suggested that the isolates with varying virulence are prevalent concurrently in all parts of north-western India.
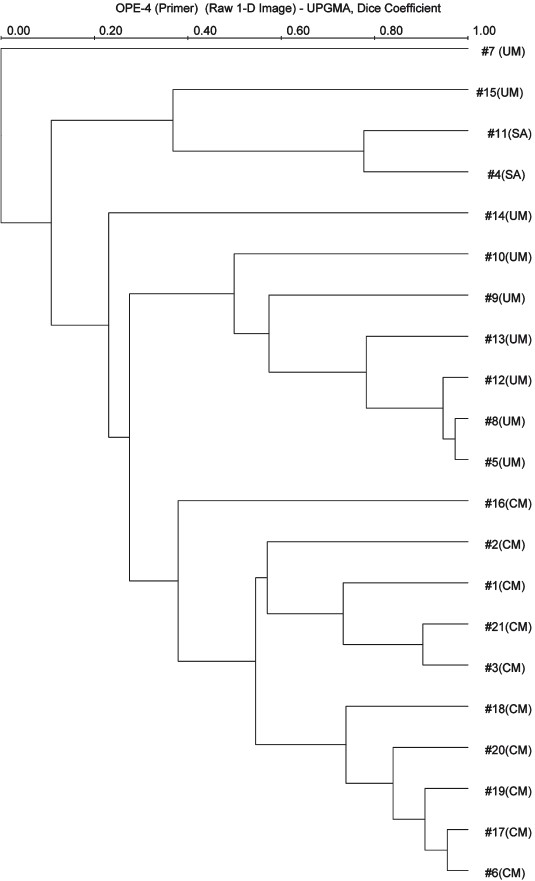
- Phylogenetic tree depicting the genetic diversity of the north Indian P. falciparum isolates based on E-4 RAPD primer. The values shown at the nodes of the major and minor sub-divisions are bootstrap values. The number of isolates of P. falciparum used to generate tree are shown at the end of each branch of tree. Within the tree, “UM”, “CM” and “SA” indicates the isolates from uncomplicated malaria, cerebral malaria and severe anaemia, respectively.
The analysis of phylogenic tree showed relatedness among the P. falciparum clinical isolates causing non cerebral and cerebral malaria which formed sister groups with each other suggesting that the high virulence parasite originated from less virulent one. Our findings also show that the RAPD-PCR technique is a powerful and rapid method for detecting the genetic polymorphism in P. falciparum by which we can differentiate the parasite isolates causing varying clinical manifestations.
Acknowledgment
Authors acknowledge the Indian National Science Academy, New Delhi, for providing the financial support for this study.
References
- Pediatric mortality in Africa: Plasmodium falciparum malaria as a cause or risk? Am J Trop Med Hyg. 2004;71(Suppl 2):16-24.
- [Google Scholar]
- The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434:214-7.
- [Google Scholar]
- The genome of Plasmodium falciparum. I: DNA base composition. Nucleic Acids Res. 1982;10:539-46.
- [Google Scholar]
- The karyotype of Plasmodium falciparum determined by ultrastructural serial sectioning and 3D reconstruction. J Parasitol. 1986;72:731-6.
- [Google Scholar]
- Molecular karyotype of P. falciparum conserved linkage groups and expandable histidine rich genes. Proc Natl Acad Sci USA. 1987;84:7672-6.
- [Google Scholar]
- Chromosome size polymorphisms in Plasmodium falciparum can involve deletions and are frequent in natural parasite populations. Cell. 1986;44:87-95.
- [Google Scholar]
- Applications of random amplified polymorphic DNA (RAPD) in molecular ecology. Mol Ecol. 1992;1:55-63.
- [Google Scholar]
- Fingerprinting genomes using PCR with arbitrary primers. Nucleic Acids Res. 1990;18:7213-8.
- [Google Scholar]
- P. falciparum extensive polymorphism in MSP-2 in an area with endemic malaria in Papua New Guinea. Exp Parasitol. 1994;79:106-16.
- [Google Scholar]
- Plasmodium falciparum: Polymorphism in MSP-1 gene in Indian isolates and predominance of certain alleles in cerebral malaria. Exp Parasitol. 2006;112:139-43.
- [Google Scholar]
- Genetic polymorphism in MSP-2, AMA-1 and CSP genes in P. falciparum strains prevalent in North-Western India. J Vect Borne Dis. 2009;46:109-16.
- [Google Scholar]
- Towards a high resolution P. falciparum linkage map: polymorphic markers from hundreds of simple sequence repeats. Genomics. 1996;33:430-44.
- [Google Scholar]
- Rapid and simple method for isolating malaria DNA from finger prick samples of blood. Mol Biochem Parasitol. 1992;53:241-4.
- [Google Scholar]
- Use of RAPD technique in inheritance studies of P. falciparum. J Parasitol. 1996;96:941-6.
- [Google Scholar]
- Polymorphism in merozoite surface protein-1 gene in North Indian field isolates of Plasmodium vivax. Indian J Med Res. 2009;130:736-41.
- [Google Scholar]






