Translate this page into:
Evaluation of the effects of dexketoprofen trometamol on knee joınt: an in vivo & in vitro study
Reprint requests: Dr Ozlem Sagir, Assistant Professor, Balikesir University, Faculty of Medicine, Department of Anaesthesiology & Reanimation, 10145, Balikesir, Turkey e-mail: ozlemsagir@yahoo.com
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Intra-articular (ia) injections of local anaesthetics and non-steroidal anti-inflammatory drugs (NSAID's) are simple and efficient to ensure post-operative analgesia but some of these have toxic effects on the synovium and cartilage. Dexketoprofen is recently introduced S-enantiomer of ketoprofen with a better analgesic and side effect profile. This study was done to evaluate the possible toxic effects of dexketoprofen trometamol on knee joint cartilage and symovium in vitro and in vivo.
Methods:
Forty one Sprague-Dawley rats were anaesthetized by ketamine. Dexketoprofen trometamol (0.25 ml) was injected into the right knee joint of the 35 rats and 0.25 ml serum physiologic into the left knee joint of the same animals. Six rats were sham operated. Thirty five animals were randomly divided into five equal groups. Seven animals were sacrified at 24th, 48th hours and 7th, 14th, and 21st days of the injections. Haematoxylin eosin stained sections from the knee joints were evaluated for the signs of inflammation according to five point scale. Primary chondrocytes were isolated from the articular cartilages of rats for in vitro studies. Cells were exposed to 0.25 ml dexketoprofen trometamol or 0.25 ml dexketoprofen medium mixture at 1:1 ratio for 15, 30, 45 and 60 min. Cell viability was determined by 3-(4, 5- dimethylthiazole-2-yl)-2.5-diphenyl tetrazolium bromide (MTT) assay, 24, 48 and 72 h after drug treatment.
Results:
No significant histopathologic differences were found between dexketoprofen trometamol and physiologic serum (control) applied joints at all time intervals in in vivo study. Cell proliferation in dexketoprofen trometamol treated chondrocytes was inhibited for all time intervals compared to control. In dexketoprofen-medium mixture groups significant differences were only seen 24 h after the 30 and 45 min application of medium: drug mixture.
Interpretation & conclusions:
Intra-articular application of dexketoprofen trometamol into the rat knee joints did not cause significant histopathological changes, but its in vitro application in primary chondrocyte culture caused significant cytotoxicity. The effects of dexketoprofen at different concentrations need to be further investigated in culture of rat and human chondrocytes.
Keywords
Dexketoprofen trometamol
intra-articular injection
primary cell culture
rat
Orthopaedic operations, like arthroscopic knee surgery, are performed as ambulatory surgery in order to decrease the infection risk and the cost of the operation12. Therefore, constitution of a suitable and effective analgesic protocol for such operations is quite important because pain elongates the hospitalization period of the patient and causes impairment in patient satisfaction. Various pain treatment modes like oral analgesics, intravenous (iv) opioids, peripheral nerve blocks, epidural analgesia, intra-articular (i.a.) and intrasynovial non-steroidal anti-inflammatory drugs (NSAID)/ opioids/ local anaesthetics have been practised for many years3. Among these, i.a. injection bears a special value especially for the treatment of post operative pain after arthroscopic knee surgery, because of its simplicity, safety and low cost4. However, the toxic effects of some drugs on cartilage and bone restrict their usage by i.a. route5.
Dexketoprofen trometamol is a NSAID that belongs to the aryl-proprionic acid group which is an active S-enantiomer of racemic ketoprofen6. It is a better analgesic than ketoprofen, with fewer side effects7. These properties make the dexketoprofen trometamol a good candidate for the ia usage. Although it has been safely used orally, intravenously (iv) and intramuscularly (im), information about the i.a. usage of this drug is not available. Further, the available forms of the drug are used in major orthopaedic surgery but no data are found about the usage of these after knee arthroscopy8.
The aim of this study was to evaluate the possible toxic effects of dexketoprofen trometamol on knee joint cartilage and synovium. For this purpose in vitro primary chondrocyte cell culture and in vivo i.a. injection studies in animal model were performed.
Material & Methods
This study protocol was approved by the local ethics committee for animal experiments and carried out between December 2010 and December 2011 at the research laboratories of Balikesir University, Balikesir, Turkey. In vivo studies were performed in Animal Laboratory and Histology Laboratory of Faculty of Medicine. In vitro studies were conducted in Cell Culture Laboratory of Faculty of Science and Arts, at Balikesir, University. Forty one male Sprague-Dawley rats weighing 250-300 g and 10 five days old rats were used for the study. The rats were housed with a 12-h light/dark cycle and provided with food and water ad libitum.
The study groups were arranged as follows: Group I (n=35, dexketoprofen trometamol injected group); 0. 25 ml standard dexketoprofen trometamol (Arveles®, I.E. Ulagay, Istanbul, Turkey) solution was injected into the right knee joint of the animals. Group II (n=35, control group); 0.25 ml physiologic serum was injected into the left knee joints of the same animals in Group I. Group III (n=6, shame operated group), empty needles with no drugs or solutions, were applied to the right and left knee joints.
The rats were anaesthetized by 6-8 mg/kg ketamine hydrochloride (i.m.) (Ketalar®, Pfizer Warner-Lambert, Istanbul, Turkey). The skin over the joint was shaved and swabbed with 70 per cent ethanol. Injections were given using a 30 gauge 0.5 inch needle inserted through the suprapatellar ligament while the joint was held in 90[0] of flexion, and 0.25 ml standard dexketoprofen trometamol solution which contains 36.9 mg/ml dexketoprofen trometamol was applied to the right knee joints of the 35 rats and 0.25 ml serum physiologic was injected into the left knee joints of the same animals as control group under aseptic conditions. Sham operation was applied to six rats. Empty needles, with no drugs or solutions, were applied to the right and left knee joints of these six animals. After waiting for a time, which is approximately equal to the injection time, the needles were drawn back.
Joint preparation and evaluation: The animals were randomly divided into five equal groups and were returned to their cages. Subgroups of seven animals were sacrificed with intraperitoneal (ip) injection of pentobarbital 150 mg /kg at 24th, 48th h and 7th, 14th, and 21st days of the i.a. injections. Three animals in the sham-operated group were sacrificed at 24th h and three animals at 48th h similarly (Fig. 1). The knee joints were separated and examined for the gross signs of haematoma. After being labelled (left/right and time of death), these were fixed in 10 per cent neutral buffered formalin for two weeks and decalcified in 14 per cent ethylenediaminetetraacetic acid (EDTA) solution for 3 wk. Decalcified samples were processed and embedded in paraffin. Five μm thick sections were obtained from the samples and stained with haematoxylin and eosin (H&E). The same histologist, who was blinded to the treatment, examined all the slides and classified the inflammatory changes in the joints according to a five point scale: Grade I- no inflammation, Grade II- minimal inflammation (mild congestion and oedema), Grade III- mild inflammation (congestion and oedema, small number of neutrophils), Grade IV- moderate inflammation (neutrophils and macrophages, synoviocyte hyperplasia), Grade V- severe inflammation (neutrophils and macrophages, synoviocyte hyperplasia, fibrin exude)9.
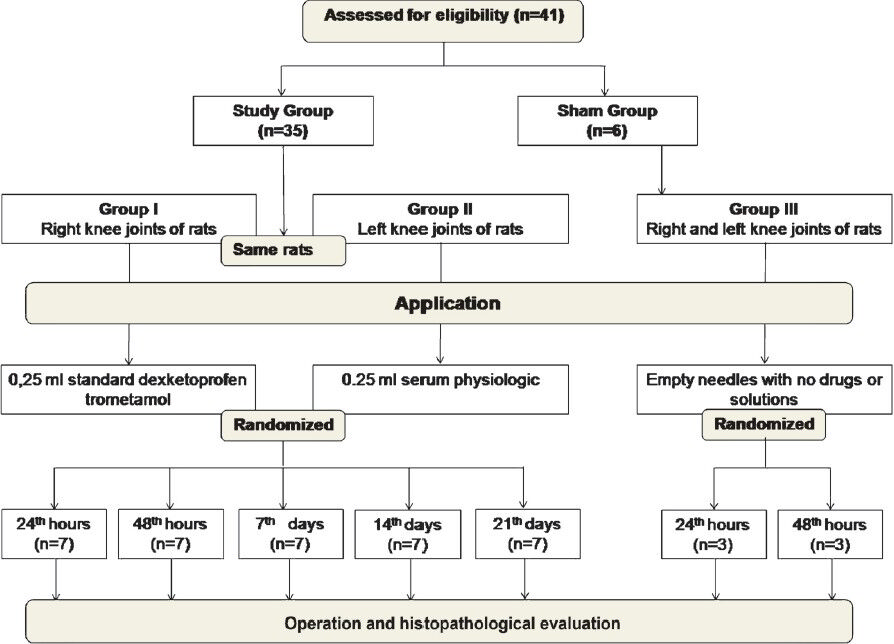
- CONSORT flow diagram of this study.
Primary chondrocyte culture for in vitro study: Primary chondrocytes were isolated from articular cartilage from knees of 5 days old rat according to the protocol described by Gosset and colleague10. Rats were sacrified with pentobarbital overdose and knee joint cartilages were excised. These were rinsed in calcium and magnesium-free phosphate buffered saline (PBS) and diced into approximately 1 mm3 segments. Cells were enzymatically digested from cartilage tissue segments using 3 mg/ml and 0.5 mg/ml Collagenase-D (Roche, Germany) in Dulbecco's Modified Eagles Medium (DMEM) (Sigma, USA) supplemented with 0.5 mg/ml penicillin and 0.5 mg/ml streptomycin. Digestion was performed in an incubator with 5% CO2 at 37°C with overnight incubation. A cell strainer (70 μm) was used to filter the cells suspended and washed twice with PBS to remove residual collagenase and collagen from the cells. The isolated primary cells were cultured in DMEM supplemented with penicillin/streptomycin and 10 per cent (v/v) heat-inactivated foetal calf serum (HIFCS). The cultures were maintained at 37 °C in an incubator with air containing 5 per cent (v/v) CO2.
Drug treatment to cultured chondrocytes: Primary culture of chondrocytes were seeded into 24 well plates (12.5×104) and cultured overnight before the drug treatment. Cells were then exposed to 0.25 ml standard dexketoprofen trometamol (only drug treated group) or 0.25 ml standard dexketoprofen trometamol and medium mixed at 1:1 ratio (½ diluted drug treated group) for 15, 30, 45 and 60 min as experimental groups; 0.25 ml medium or 0.25 ml medium and normal saline mixed at 1:1 ratio treatment groups were used as controls.
Cell viability: Cell viability was determined by MTT [3-(4, 5-dimethylthiazole-2-yl)-2,5-diphenyl tetrazolium bromide] assay.11 Results from at least three independent experiments with data from at least three wells in each experiment, were averaged and then expressed relative to control groups. At the end of the time intervals, cells were washed with PBS and fresh growth medium was added. Cell viability was measured by MTT assay at 24, 48 and 72 h after treatment. Cells were incubated with MTT (final concentration 0.5 mg/ml) for 4h at 37°C. Subsequently, the medium containing MTT was removed and acidified isopropanol (in 0.04 N HCl) was added to each well. The absorbance of formazan was recorded at 550 nm using a micro plate reader. Inhibition percentage was calculated as follows:
% inhibition = [1-(T/C)] X100
where T is the mean absorbance of the treated cells and C the mean absorbance in the controls.
Statistical analysis: Statistical analysis was performed using SPSS version 15.0. for windows (SPSS Inc, Chicago, Il, USA) The probability of the differences between the experimental and control groups at 24th, 48th h and 7th, 14th, and 21st days was calculated by Fisher's Exact test. Statistical significance of repeated measurements was evaluated with Friedman test. One-way ANOVA was used to investigate the statistical significance of the in vitro results.
Results
Histopathological changes: Articular cartilages had a normal histological structure in all groups. In the specimens of the control groups, there was no sign of synovial inflammation in most cases. Same results were also obtained in the drug treated groups (Fig. 2). There were also no inflammatory changes in the specimens from the six sham-operated rats.
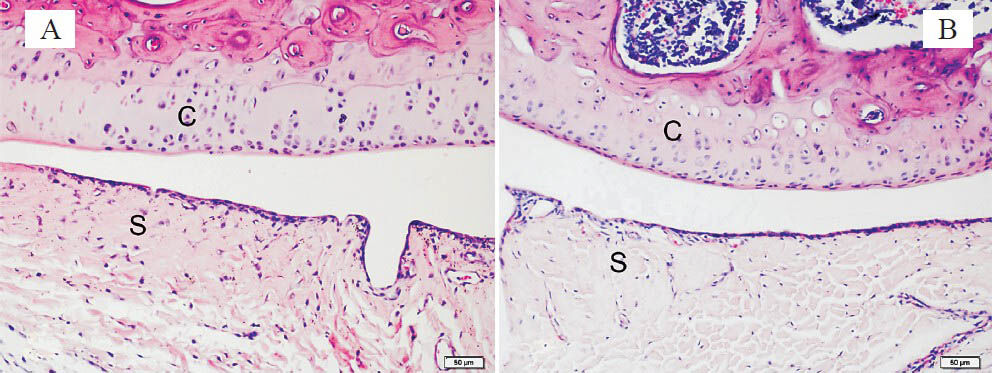
- Histopathological difference between the knee joints of rats 48 h after injection in Group I (dexketoprofen trometamol) (A) and Group II (control) (B). C, cartilage; S, synovium; H&E staining. 200×.
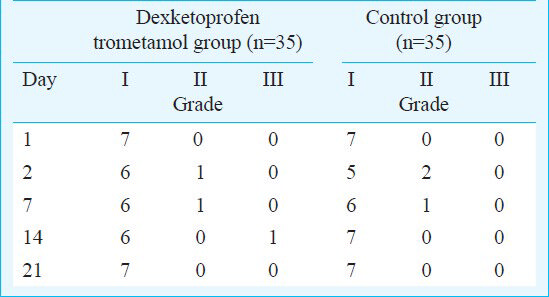
The inflammatory scores of the samples in physiologic serum and dexketoprofen trometamol injected groups are shown in the Table. There were no grade IV and V inflammatory changes in any of the groups. Grade III inflammatory changes occurred only in one joint, in the dexketoprofen trometamol group at 14 days after the injection. But there were no significant differences in inflammation and cartilage degeneration between these two groups at any time intervals. When repeated measurements were evaluated, no significant differences over time were determined in both the dexketoprofen trometamol group and the control group.
The knee joints of all animals were examined for the gross signs of haematoma. Haematoma was detected in only one knee joint in dexketoprofen trometamol administered group, 48 h after the injection.
In comparison to non drug treated control cells, cell proliferation in drug treated chondrocytes was inhibited for all time intervals. This inhibition was approximately >70 per cent in only drug treated groups and these differences were significant (P<0.05). But, in ½ diluted drug treated groups significant differences were only seen at 24 h after the 30 and 45 min application of medium: drug mixture (P<0.05) (Fig. 3).
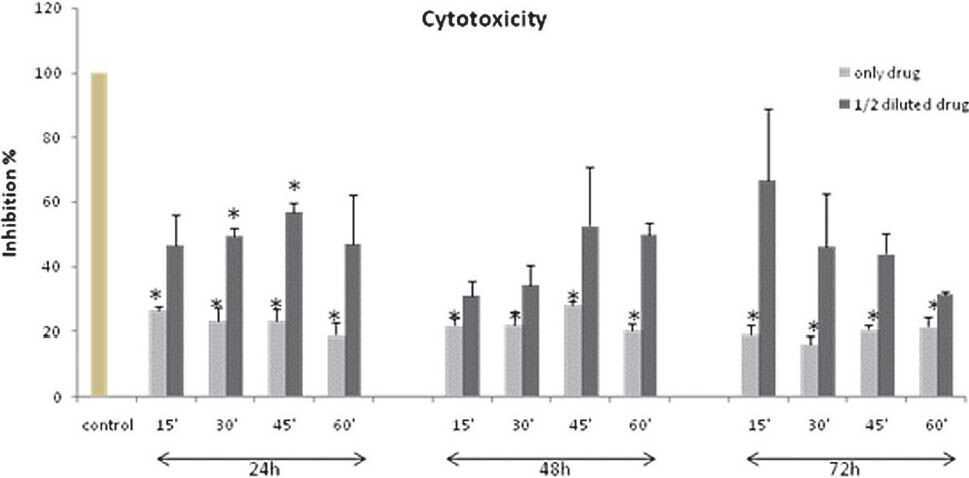
- Cytotoxic effects of dexketoprofen trometamol (only drug) and 1/2 diluted groups compared to non-treated control group on viability of primary chondrocyte cells. The means of three experiments performed in triplicate are shown with SD. (*P<0.05 in comparison with the control group).
Discussion
Intra-articular application of dexketoprofen trometamol into the rat knee joints did not cause significant histopathological changes. But in vitro application of the same drug in primary chondrocyte cultures caused significant cytotoxicity and decreased the cell proliferation.
NSAIDs are among the most widely used medications in the world because of their demonstrated efficacy in reducing pain and inflammation. Besides, NSAIDs have principal effects peripherally and, therefore, their local application to the site of injury can produce good analgesia while still minimizing the drugs side effects owing to their regional usage12.
The water soluble dexketoprofen trometamol salt contributed to a faster onset of analgesic action as was seen in various clinical trials performed in patients with moderate to severe pain13. It has been used both alone and in combination with other drugs for post-operative analgesia14.
The i.a. drug administration is one of the simplest techniques in arthroscopic knee surgery and does not require any additional equipment. Many studies have shown that opioids, local anaesthetics and NSAIDs, which have been introduced into the joint for prevention of pain after arthroscopic interventions, were effective12151617. The most important handicap of i.a. drug usage is the histopathological changes created on the articular cartilage and synovium by locally administered drugs. These changes are variable depending on the concentration of the drugs and the exposure time to the drugs12. Webb and Ghosh5 have reported that bupivacaine has negative effects on synovial cartilage in a dose and time dependent manner and a short term application of low dose bupivacaine is safe. Dragoo et al18 have suggested severe chondrolysis after the use of i.a. infusions of bupivacaine on articular cartilage. Bupivacaine decreases proteoglycan synthesis and causes inflammatory responses in articular cartilage and synovial membrane cells. Chemical effects of the local anaesthetics on chondrocytes and synovial fluid, pH, and preservatives in the solution have implications in the development in this condition19. Several studies evaluated the effects of i.a. administered NSAIDs such as lornoxicam, tenoxicam and ketorolac in rat knee joint9202122. Irwin and colleagues9 showed that i.a. application of ketorolac resulted in significantly more inflammatory reactions in articular cartilage and synovium at all times of examination especially at the 5th day of the injection in rats. Lornoxicam did not have significant anti-inflammatory effect on rat synovia20. But conflicting results have been reported about the histopathological alterations created by i.a. application of ketorolac and tenoxicam92122.
Dexketoprofen trometamol is licensed in the Republic of Turkey for i.v., i.m. and oral administrations, but is not approved for ia usage in humans. Several factors are responsible for this limitation. One of these is the formulation of the drug. Most preparations contain potentially harmful preservatives such as the sodium metabisulphide in preparations of diclofenac. The pH of the formulation is another important factor. Dogan and colleagues21 showed that ia application of morphine and ketolorac into the knee joint caused histopathological alterations and these effects were more prominent in morphine because of the acidic nature of this drug. Additionally Dragoo and colleagues18 have suggested that i.a. administration of the epinephrine added local anaesthetics have important chondrotoxic effects and the acidic pH of the epinephrine may be responsible for this. Injectable dexketoprofen trometamol preparations contain only ethanol, sodium chloride, sodium hydroxide and water in addition to the active drug and commercial form of the dexketoprofen trometamol has a 6.5-8.5 pH value. Another important factor about the i.a. usage of the dexketoprofen trometamol was its protein binding rate. Owen et al23 were reported that drugs which bind to the proteins at a high rate stay in the synovial fluid for a long time. Because dexketoprofen trometamol has a high protein binding rate, it stays in the synovial fluid for a long period of time614. This enables a longer period of analgesic action. But the same property may turn into a disadvantage if the drug creates adverse effects on joint cartilage or synovium.
Although several studies have indicated that non-selective NSAIDs inhibit bone formation and fracture healing in vivo, there is only limited information available about the effects of NSAIDs on the chondrocytes in vitro24. Chang and colleagues25 have shown that the four non-selective NSAIDs indomethacin, diclofenac, piroxicam and ketorolac have significant cytotoxic effect on culture of chondrocytes isolated from epiphyseal-articular cartilage.
According to our in vivo studies, dexketoprofen trometamol treatment did not cause any significant changes on chondrocytes and synovial cells. Dexketoprofen trometamol treatment on the primary chondrocytes caused significant cytotoxicty and decrease in cell proliferation for 24, 48 and 72 h time intervals, in vitro.
Risk of haemorrhage in the knee joint following intra-articular injections is found26, unnoticed at the time of injection. Irwin and colleagues9 reported that four knee joint capsule developed haematoma. In our study, the haematoma was observed in only one knee joint.
This study had some limitations. Firstly, because it was performed on animal models, our results might be different from those observed in humans. Second, we did not investigate the histopathological effects of drug on knee joint in a dose-dependent manner, because we intended to use the standard parenteral form of the drug which has been used safely by iv and im routes. Attainment of the chondrocytes from young, healthy rats was the third limitation of our study because i.a. surgery is more commonly done in adult groups and diseased knees. Fourth, primary cell culture was done only for chondrocytes, so in vitro effects of the drug were investigated only in the chondrocytes but not in the synovial cells.
In conclusion, though dexketoprofen trometamol i.a. treatment did not cause significant histopathological changes in rat knee joint, in vitro application of the dexketoprofen trometamol in primary chondrocyte cultures caused significant cytotoxicity. Further investigations are required to study the effects of dexketoprofen at different concentrations covering therapeutic concentration in chondrocyte cultures obtained from healthy or diseased cartilages of adult animals and humans.
References
- Cost-effectiveness analysis of the most common orthopaedic surgery procedures: knee arthroscopy and knee anterior cruciate ligament reconstruction. Arthroscopy. 2011;27:1317-22.
- [Google Scholar]
- Joint infection after knee arthroscopy: medicolegal aspects. Orthop Traumatol Surg Res. 2009;95:278-83.
- [Google Scholar]
- A systematic review of intra-articular local anaesthesia for postoperative pain relief after arthroscopic knee surgery. Reg Anesth Pain Med. 1999;24:430-7.
- [Google Scholar]
- Intra-articular bupivacaine: potentially chondrotoxic? Br J Anaesth. 2009;102:439-41.
- [Google Scholar]
- Comparison of the efficacy and safety of intravenously administered dexketoprofen trometamol and ketoprofen in the management of pain after orthopaedic surgery: A multicentre, double-blind, randomised, parallel-group clinical trial. Clin Drug Investig. 2006;26:517-28.
- [Google Scholar]
- Comparative study of analgesic efficacy and morphine-sparing effect of intramuscular dexketoprofen trometamol with ketoprofen or placebo after major orthopaedic surgery. Br J Clin Pharmacol. 2003;55:126-33.
- [Google Scholar]
- Intra-articular injection of ketorolac in the rat knee joint: effect on articular cartilage and synovium. Br J Anaesth. 1998;80:837-9.
- [Google Scholar]
- Primary culture and pheno typing of murine chondrocytes. Nat Protoc. 2008;3:1253-60.
- [Google Scholar]
- Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55-63.
- [Google Scholar]
- Local infiltration with NSAIDs for postoperative analgesia: evidence for a peripheral analgesic action. Acta Anaesthesiol Scand. 2000;44:672-83.
- [Google Scholar]
- Dexketoprofen trometamol in postoperative pain management. Acute Pain. 2003;5:57-62.
- [Google Scholar]
- Systematic review of dexketoprofen in acute and chronic pain. BMC Clin Pharmacol. 2008;8:11.
- [Google Scholar]
- Intra-articular analgesia for arthroscopic meniscectomy. Br J Anaesth. 1995;75:552-5.
- [Google Scholar]
- Intra-articular tenoxicam improves postoperative analgesia in knee arthroscopy. Can J Anesth. 1999;46:653-7.
- [Google Scholar]
- Analgesia after day-case knee arthroscopy: double-blind study of intra-articular tenoxicam, intra-articular bupivacaine and placebo. Br J Anaesth. 1997;78:163-8.
- [Google Scholar]
- The effect of local anaesthetics administered via pain pump on condrocyte viability. Am J Sports Med. 2008;36:1484-8.
- [Google Scholar]
- Effects of local anesthetics on articular cartilage. Am J Sports Med. 2011;39:2245-53.
- [Google Scholar]
- Effect of intraarticular injection of lornoxicam on the articular cartilage&synovium in rat. Indian J Med Res. 2008;127:362-5.
- [Google Scholar]
- The effects of ketorolac and morphine on articular cartilage and synovium in the rabbit knee joint. Can J Physiol Pharmacol. 2004;82:502-5.
- [Google Scholar]
- Intra-articular injection of tenoxicam in rats: assessment of the local effects on the articular cartilage and synovium. J Int Med Res. 2004;32:312-6.
- [Google Scholar]
- Disappearance kinetics of solutes from synovial fluid after intra-articular injection. Br J Clin Pharmacol. 1994;38:349-55.
- [Google Scholar]
- Anti-inflammatory drugs suppress proliferation and induce apoptosis through altering expressions of cell cycle regulators and pro-apoptotic factors in cultured human osteoblasts. Toxicology. 2009;258:148-56.
- [Google Scholar]
- Effects of non-steroidal anti-inflammatory drugs on cell proliferation and death in cultured epiphyseal-articular chondrocytes of fetal rats. Toxicology. 2006;228:111-23.
- [Google Scholar]
- Risk of hemorrhage in the knee joint following intraarticular injections. Z Rheumatol. 1987;46:317-21.
- [Google Scholar]






