Translate this page into:
Effect of intermittent hypobaric hypoxia on efficacy & clearance of drugs
Reprint requests: Dr Krishna Kishore, Neurochemistry & Hematology, Defence Institute of Physiology & Allied Sciences, Lucknow Road, Timarpur, Delhi 110 054, India e-mail: krishnakishore_dipas@yahoo.co.in
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
People travelling to high altitude for occupational, recreational or religious purposes are mostly healthy and fit but sometimes they use drugs for common ailments like influenza, acute mountain sickness or chronic disease like diabetes. Limitation of oxygen at high altitude may compromise metabolism of drugs. Hence, we undertook this study to assess the effect of hypobaric hypoxia on some commonly used drugs in rats and rabbits.
Methods:
Effect of intermittent hypobaric hypoxia on phenotypic expression of anesthetic drugs pentabarbitone, thiopentone and zoxazolamine (sleeping time) was assessed in rats exposed to 282.4 mm Hg equivalent to 25000 feet in a decompression chamber. Plasma clearance of some commonly used drugs was investigated in rabbits exposed to 429 mm Hg equivalent to 15000 feet. Pharmacokinetic parameters were computed by plotting drug concentration versus time curve on semi log scale.
Results:
A significant delay in regaining rightening reflex was observed in rats exposed to intermittent hypobaric hypoxia in response to zoxazolamine, pentobarbitone and thiopentone sodium. Pharmacokinetics of acetyl salicylic acid, gentamicin, phenobarbitone and acetazolamide showed increase in plasma half life (t1/2), decrease in elimination rate constant (kel) and hence prolonged residence of these drugs in hypoxic animals.
Interpretation & conclusions:
This experimental study showed that hypoxia altered therapeutic effectiveness and clearance of several drugs, in rats and rabbits exposed to intermittent hypobaric hypoxia. s0 uch studies need to be done in human volunteers to see the effect of hypoxia on pharmacokinetics of some common drugs.
Keywords
Drug clearance
hypobaric hypoxia
rats
rabbits
sleeping time
Hypoxia, a subnormal oxygen concentration in cells is an important consideration in pharmacology because (i) altered cellular function may affect the therapeutic effectiveness of an agent, (ii) therapeutic agents may potentiate or protect against hypoxic pathology, (iii) hypoxia may increase or decrease drug-induced toxicity, and (iv) may alter the rate of drug metabolism and thus the effective therapeutic dose1. The liver is the most important site for systemic drug metabolism. Phase I (oxidation, reduction and hydrolysis) and phase II (conjugation) reactions convert lipophilic drugs into more polar and hence more readily excreted metabolites. The rate of metabolism of lipophilic drugs is the most important factor affecting the intensity and duration of their action2.
The role of hypoxia in modulating drug metabolism has been largely investigated in vitro and in animal studies. A few studies conducted in men exposed to high altitude hypoxia are inconclusive. In one study3 healthy subjects who lived at sea level were exposed to altitude induced hypoxia for 7 days at 4559 m above sea level. Hepatic CYP enzyme activity was measured before departure, at 24 and 96 h after arrival to high altitude location and at 1 month after return to sea level. No clinically significant effect of acute hypoxia on CYP enzymes was observed. In another study in human patients of chronic hypoxemia (PaO2 < 55 mmHg), antipyrine half-life was increased by 20 per cent indicating slower biotransformation of the drug4.
An open-label, controlled, prospective study was conducted to investigate the pharmacokinetics of sulphamethoxazole in healthy Chinese male volunteers at low and high altitudes5.
Significant changes were reported in the disposition of sulphamethoxazole in these subjects after either acute or chronic exposure to an altitude of ~3780 m in comparison to those residing at an altitude of ~400 m.
It has been reported that no substantial change occurs in cytochrome P450 and b5 in mice subjected to acute hypoxia6. Oxygen-requiring processes of hepatic heme and drug metabolism remain well maintained during hypoxia7. A decrease in hepatic cytochrome P450 content in rats submitted to 5,500 m simulated altitude for 35 days has been reported but no change in rats subjected to 4400 m for 6 to 8 months was observed8.
The influence of moderate hypoxia or hypercapnia on salbutamol kinetics and its hypokaliaemic effect, following its administration through the intravenous, intra-tracheal, and oral routes was studied9, concluding that salbutamol kinetics and dynamics can be altered by hypoxia and hypercapnia. Thallium kinetics was studied during normoxia and hypoxia in cultured chick ventricular cells10. The results showed that cellular accumulation of thallium and the rate of washout of thallium were minimally decreased by hypoxia independent of blood flow. The effect of hypoxia and hyperoxia on the pharmacokinetics of propofol emulsion, hepatic blood flow and arterial ketone body ratio in the rabbit has been studied11, indicating that hypoxia produced an accumulation of propofol in blood and reduced its clearance which could be due to decreased hepatic blood flow and low energy change in the liver.
In this study, an attempt was made to assess effect of hypobaric hypoxia on several commonly used drugs in rats and rabbits exposed to chronic intermittent hypoxia.
Material & Methods
Animals and experimental design: Male Sprague Dawley albino rats were used to study the effect of hypoxia on Rightening Reflex (ability to turn spontaneously to the “on quarters” position when placed on the back) and male New Zealand White Rabbits were used to study the plasma clearance of drugs under hypoxic conditions. The study was conducted in n0 eurochemistry and h0 aematology l0 aboratories of Defence Institute of Physiology and Allied Sciences (DIPAS), Delhi.
Thirty six male Sprague Dawley albino rats weighing 200-250 g and 48 male New Zealand White rabbits weighing 1.5 to 3.0 kg, bred at Institute's animal facility in room maintained at 24 ± 0.5°C with 12 h light and dark cycle were used for the study. The rats were fed on standard pelleted diet supplied by Lipton India Ltd., and had free access to food and water. Rabbits were given green vegetables and pelleted diets. All the experiments were approved by Institutional Animal Ethics Committee (IAEC).
Exposure protocol: Exposure was carried out in a decompression chamber (Seven Star, India) maintained at 10 ± 2.5°C temperature and 65 ± 10 per cent relative humidity. Airflow rate was kept at 5.0 ± 0.5 lit/min.
For sleeping time study, hypoxia group rats were exposed to 282.4 mm Hg (376 mbar) pressure equivalent to 25000 feet for 3 h/day for 7 days. Control group was exposed to normal room air. For plasma clearance study rabbits were exposed to hypobaric hypoxia at 429 mm Hg (555 mbar) pressure equivalent to 15000 feet altitude for 5 h/day for 7 days. In control group rabbits were exposed to room air (normoxia).
Drug induced paralysis/sleeping time assessment (Experiment I): Drug induced sleeping time was studied immediately after termination of the last exposure. Rats (n=6) exposed to intermittent hypoxia and normoxia (n=6) were injected intra-peritoneally either zoxazolamine (60 mg/kg bw), pentobarbitone (50 mg/kg bw) or thiopentone sodium (40 mg/kg bw). Separate groups of rats were used for testing of each drug. The time of injection, time of loss and time of gain rightening reflex were recorded with the help of a stopwatch. Duration of sleeping time was computed as the time interval between loss and gain of rightening reflex.
Plasma clearance of drugs (Experiment II): In the present study, acetyl salicylic acid (25 mg/kg bw), gentamicin (10 mg/kg bw), phenobarbitone (30 mg/kg bw) and acetazolamide (25 mg/kg bw) clearance were assessed to study in vivo drug metabolizing status of hypoxia exposed animals.
Rabbits divided in two groups - hypoxia and normoxia (n=6 each) were injected either of the above mentioned drug in the amount specified by intravenous route in a peripheral ear vein, immediately after the last exposure. Blood sample (0.5 ml) was drawn from a vein in opposite ear and taken as zero hour sample. Thereafter blood samples were drawn after 30 min, 1, 2, 3, 5 and 24 h. Amount of the injected drug remaining in plasma was measured. Acetyl salicylic acid was measured spectrophotometerically on Beckman DU 50 spectrophotometer (Beckman Coulter Inc. USA) by trinder reagent12. Gentamicin and phenobarbitone were measured by Enzyme Immuno Assay kits (Dade Behring Ltd, UK). Acetazolamide concentration in plasma samples was measured by HPLC using UV detector13.
Separate sets of animals were used for testing of each drug. Pharmacokinetic parameters were computed by plotting drug concentration versus time curve on semi log scale. Regression analysis was done by GraphPad Prism (GraphPad Software Inc., San Diego, CA). Zero time concentrations were determined by extrapolating the regression line to Y axis. The values were depicted as mean ± SD. Data were analysed by s0 tudent's t test.
Results
Experiment I: Rats exposed to intermittent hypobaric hypoxia for 7 days showed quicker loss of rightening reflex in response to zoxazolamine (144.9 ± 17.5 in hypoxia vs 160.6 ± 39.3 sec in control rats), pentobarbitone (130.6 ± 8.2 in hypoxia vs 139.6 ± 2.1 sec in control rats) and thiopentone sodium (124.6 ± 7.7 in hypoxia vs 107.0 ± 4.0 sec in control rats) as compared to rats exposed to normoxia, but differences were not significant (Table). There was a significant (P<0.05) delay in regaining rightening reflex in response to pentobarbitone and thiopentone sodium indicating prolonged duration of anaesthetic effect of these drugs in hypoxic animals.

Experiment II: The half life (t1/2) is the time taken for the concentration of the drug in plasma to decline to half the starting concentration. Removal of acetyl salicylic acid from the plasma was computed as the first- order kinetics. Mean ± SD of half life (t1/2) of acetyl salicylic acid computed from log linear curve of concentration versus sampling time were 5.69 ± 1.02 h for control rabbits and 7.63 ± 0.84 h for hypoxia exposed rabbits (Fig. 1). Elimination rate constant (kel), can be determined from the slope of the log concentration- time curve and volume of distribution (Vd), is the volume of body fluid in which the drug is uniformly distributed at the concentration found in the plasma. Elimination rate constant (0.095 ± 0.01 in hypoxic vs 0.144 ± 0.032 in control animals; P<0.01) (Fig. 2) and volume of distribution (806.1 ± 122.1 in hypoxic vs 1071.1 ± 125.1 ml/ kg bw in control animals; P<0.01) (Fig. 3) of acetyl salicylic acid were 34 and 24.7 per cent lower in hypoxic animals compared to the control group, respectively. Total plasma clearance, the volume of plasma from which the drug is totally eliminated in a unit time was significantly (P<0.001) lower for acetyl salicylic acid in hypoxia (72.3 ± 7.0 ml/kg/h) exposed rabbits than in control (155.2 ± 39.4 ml/kg/h) group (Fig. 4).
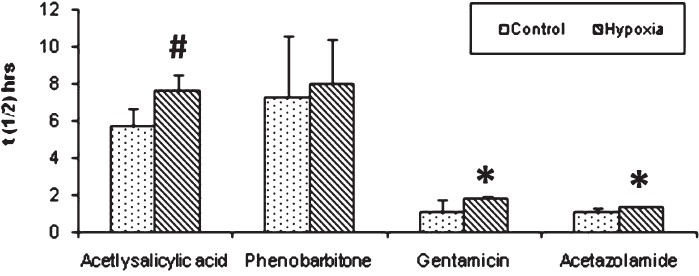
- Half life (t1/2). A significant increase in half life of acetylsalicylic acid, gentamicin and acetazolamide was observed in hypoxia group than control. Significance level set at P<0.05. *P<0.05; # P<0.01 compared to respective control. Values are mean ± SD (n=6).
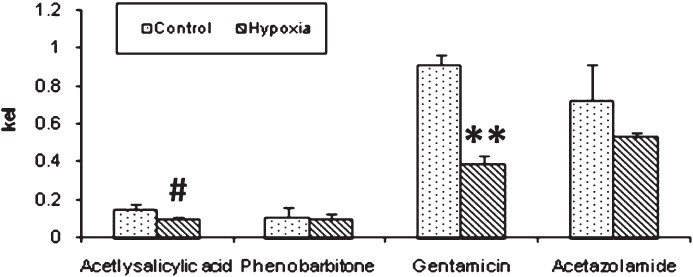
- Elimination rate constant (kel). The bar diagram shows a significant decrease in elimination rate constant of acetylsalicylic acid and gentamicin in hypoxia exposed rabbits. Significance level set at P<0.05. #P<0.01; **P<0.001 compared to respective control.
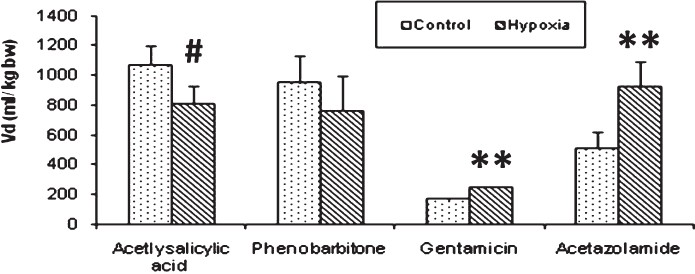
- Volume of distribution (Vd). The volume of distribution significantly decreased in case of acetylsalicylic acid in hypoxia exposed group whereas, a significant increment was observed for gentamicin and acetazolamide drugs. Significance level set at P<0.05. #P<0.01; **P<0.001 compared to respective control.
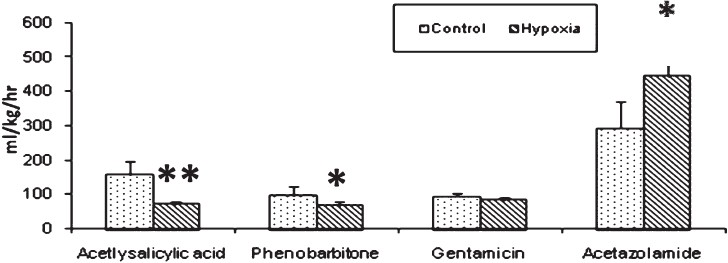
- Plasma clearance (ml/kg/h). A significant decrease in plasma clearance of acetylsalicylic acid and phenobarbitone in hypoxia group was observed in comparison to control group whereas, acetazolamide plasma clearance significantly increased in hypoxia group. Significance level set at P<0.05. *P<0.05; **P<0.001 compared to respective control.
Elimination of aminoglycosides like gentamicin occurs almost entirely by glomerular filtration. The half-life (t1/2) for gentamicin in control rabbits (1.11 ± 0.65 h) was significantly lower (P<0.05) than that of hypoxia exposed rabbits (1.80 ± 0.17 h) (Fig. 1). Elimination rate constant for gentamicin was also significantly (P<0.001) lower in hypoxic rabbits (0.389 ± 0.041) than that of control (0.909 ± 0.051) rabbits (Fig. 2). In hypoxia exposed (241.4 ± 12.4 ml/ kg bw) animals apparent volume of distribution for gentamicin was significantly more (P<0.001) than in control (163.9 ± 13.9 ml/ kg bw) rabbits (Fig. 3) and total plasma clearance rate in hypoxic rabbits (83.9 ± 6.2 ml/kg/h) was 11% lower than control (94.3 ± 10.9 ml/kg/h) rabbits (Fig. 4).
Acetazolamide is excreted unchanged and its clearance is affected by renal function. The pharmacokinetic profile of this drug was similar to gentamicin. Elimination rate constant for acetazolamide in hypoxia-exposed (0.527 ± 0.028) animals was lower (27%) than control (0.720 ± 0.188) animals (Fig. 2). Median plasma clearance time (t1/2) of acetazolamide in hypoxia-exposed rabbits was significantly (P<0.05) prolonged (28%), as compared to control rabbits (Fig. 1). Volume of distribution showed significant (P<0.001) increase as hypoxic animals as compared to control (Fig. 3).
Prolonged plasma half-life (8.01 ± 2.40 in hypoxia vs 7.28 ± 3.33 h in control) (Fig. 1), decreased rate of clearance (66.6 ± 10.9 in hypoxia vs 96.7 ± 27.2 ml/kg/h in control) (Fig. 4) and decreased volume of distribution (760.8 ± 238.4 in hypoxia Vs 950.1 ± 178.5 ml/kg bw in control) (Fig. 3), pointed out slowed metabolism and increased binding of the drug to plasma proteins and its entry to tissue compartments as well.
Discussion
Zoxazolamine is a potent muscle relaxant, which is metabolically inactivated by CYP1A1/2 enzyme activity (and to a lesser extent CYP2E1). It has also been used as a functional test to evaluate chemicals for their ability to inhibit CYP1A1/2 enzyme activity in vivo1415. Pentobarbital is a short acting barbiturate approved as a short-term hypnotic and veterinary use as an anesthetic16. Therefore, sleeping time test was used for preliminary investigations on whether hypoxic stress interferes with metabolism of drugs.
Significantly prolonged sleeping time with pentobarbitone and thiopentone sodium in hypoxia-exposed rats suggests slowed elimination of these drugs. Similar results have been reported earlier17 in rabbits. Also reduced rate of pentobarbital disappearance in mice during exposure to acute hypoxia suggesting depressed in vivo metabolism of pentobarbital and enhanced CNS sensitivity to the barbiturates have been repoprted18.
Pharmacokinetics of some of the commonly used drugs has been investigated in this study. Acetyl salicylic acid has been reported to have neuroprotective action against hypoxic hypoxia and chemical hypoxia19. Acetazolamide is a carbonic anhydrase inhibitor and is the mainstay for prevention and treatment of acute mountain sickness20. Gentamicin is an aminoglycoside antibiotic, used to treat many types of bacterial infections, particularly those caused by Gram-negative bacteria, and phenobarbitone is used for treatment of epileptic seizures.
Elimination of aminoglycosides after parenteral administration occurs almost entirely by glomerular filtration. We observed delay in half-life of gentamicin as well as acetazolamide. Both the drugs do not require any metabolic transformation before elimination by kidneys. The elimination half-life for gentamicin has been reported to be 1 h in rabbits which is quite similar to the values found in control rabbits in the present study. Elimination rates can be highly variable with the aminoglycoside antibi-otics in humans with normal renal function. Patients with decreased renal function can have significantly prolonged half-lives for gentamicin clearance. A significant increase in the pharmacokinetics of drugs like aminoglycosides eliminated through the kidneys may be impaired and require a different than usual dosage regimen under various physiological, pathological and environmental conditions. A decrease in body temperature is associated with a decrease in glomerular filtration rate in rats21, in other species and in humans are temperature requiring processes and hence temperature sensitive22 and may, therefore, impair the elimination of aminoglycosides. Hypoxia-ischaemia altered renal function and gentamicin pharmacokinetics. Therefore, altered renal function during hypoxia could be the reason for prolonged stay of these drugs in plasma.
Acetazolamide is distributed throughout body tissues where it may bind certain proteins such as carbonic anhydrase. Therefore, high concentrations of acetazolamide may accumulate in tissues rich in carbonic anhydrase such as erythrocytes, renal cortex23. Slowed blood flow in some of the tissues in splanchnic region during hypoxia can also hamper clearance of these drugs. Our results did not confirm to a earlier study24, where the pharmacokinetics of acetazolamide in volunteers at sea level, on the day of arrival at high altitude (4360 m) and after 10 months of stay at this altitude was investigated. Clearance of acetazolamide was found to be faster at high altitude. The causal factors were increased uptake of the drug by erythrocytes and decreased protein binding.
Slowed clearance of acetyl salicylic acid and phenobarbitone in rabbits exposed to hypoxia in our study suggests compromised hepatic drug metabolizing capacity.
Another study carried out in rabbits subjected to acute moderate hypoxia and serum mediators such as interferon-γ, interleukin-2 and interleukin 1β, demonstrated downregulation of CYP1A1, 1A2, 2B4, 2C9 and 2C19 and up-regulation of CYP3A625. Ex vivo and in vitro experiments26–27, demonstrated that hypoxia downregulates CYP1A1, CYP1A2, CYP2B6, CYP2C9 and CYP2C19 and upregulates CYP3A6, and change in hepatic CYP P450 is mediated by interferon-γ, interleukin-1β and interleukin-2. Decreased disposition of acetaminophen in rats has been reported which was due to impaired glucuronidation and sulphation reactions28. These changes could also be induced through reduced hepatic blood flow and a lowered energy state of liver mitochondria211. Therefore, decreased blood flow in liver could also be responsible for inhibition of drug metabolism in our study.
In conclusion, our results suggest that intermittent hypobaric hypoxia impairs in vivo drug metabolizing ability in animals. These results can have serious implications in the dosage regimen, design and continuation of therapeutic measures in sojourners going to high altitude for occupational, recreational or religious purposes. Hence, care must be taken while prescribing medication to high altitude sojourners (with or without pre-existing medical conditions) as the ascent to high altitude has several deleterious effects on physiological functions and deficient metabolic activity and expression of enzymes along with factors like renal insufficiency hepatic insufficiency could lead to alteration in drug metabolism.
Acknowledgment
The authors acknowledge Director, DIPAS and Defence Research & Development Organization, India, for support to facilitate this research work.
References
- The effect of hypoxia on drug metabolizing enzymes. Drug Metab Rev. 1995;27:471-95.
- [Google Scholar]
- Acute hypoxia and cytochrome P450-mediated hepatic drug metabolism in humans. Clin Pharmacol Ther. 2002;71:214-20.
- [Google Scholar]
- Antipyrine as a probe for human oxidative drug metabolism: Identification of the cytochrome P450 enzymes catalyzing 4-hydroxyantipyrine, 3-hydroxymethylantipyrine, and norantipyrine formation. Clin Pharmacol Ther. 1996;59:613-23.
- [Google Scholar]
- Comparison of the pharmacokinetics of sulfamethoxazole in male chinese volunteers at low altitude and acute exposure to high altitude versus subjects living chronically at high altitude: An open-label, controlled, prospective study. Clin Ther. 2009;31:2744-54.
- [Google Scholar]
- Metabolism of xenobiotics in the liver in acute hypoxia in intact mice and mice adapted to oxygen deficiency. Farmakol Toksikol. 1984;47:98-101.
- [Google Scholar]
- Hepatic heme and drug metabolism in rat with chronic mountain sickness. Am J Physiol. 1986;251:G467-74.
- [Google Scholar]
- Hepatic cytochrome P-450 in rats submitted to chronic hypobaric hypoxia. Am J Physiol. 1990;259:C654-9.
- [Google Scholar]
- Influence of hypoxia and hypercapnia on the kinetics and hypokaliaemic effect of salbutamol in the rabbit. Xenobiotica. 1995;25:271-81.
- [Google Scholar]
- The effect of hypoxia on thallium kinetics in cultured chick myocardial cells. J Nucl Med. 1987;28:1453-60.
- [Google Scholar]
- The influence of hypoxia and hyperoxia on the kinetics of propofol emulsion. Can J Anesth. 1999;46:1150-5.
- [Google Scholar]
- Determination of methazolamide concentrations in human biological fluids using high performance liquid chromatography. Pharm Biomed Anal. 1998;16:1021-7.
- [Google Scholar]
- Biochemical basis of enhanced drug bioavailability by piperine: Evidence that piperine is a potent inhibitor of drug metabolism. J Pharmacol Exp Ther. 1985;232:258-62.
- [Google Scholar]
- Effects of ciprofloxicin and endrofloxicin onzoxaxolamine kinetics, plasma concentration and sleeping times in mice. Toxicol Lett. 1993;69:1-14.
- [Google Scholar]
- UBC Committee on Animal Care. In: Euthanasia-overdose of pentobarbital. Vancouver, British Columbia, Canada: The University of British Columbia; 2005.
- [Google Scholar]
- Effect of hypoxia alone or combined with inflammation and 3-methylcholanthrene on hepatic cytochrome P450 in conscious rabbits. Br J Pharmacol. 1999;128:365-73.
- [Google Scholar]
- Effect of acute hypoxia on brain-sensitivity and metabolism in barbiturates in mice. Psychopharmacology. 1970;17:193-7.
- [Google Scholar]
- Acetylsalicylic acid increases tolerance against hypoxic and chemical hypoxia. Stroke. 1997;28:2006-11.
- [Google Scholar]
- Acetazolamide in the treatment of acute mountain sickness: clinical efficacy and effect on gas exchange. Ann Intern Med. 1992;116:461-5.
- [Google Scholar]
- The effect of hypothermia on renal function haemodynamics in the rat. Acta Physiol Scand. 1995;153:179-84.
- [Google Scholar]
- Reynolds JEF, ed. Martindale: The extra pharmacopoeia. London: The Pharmaceutical Press; 1993.
- Pharmacokinetics of acetazolamide in healthy volunteers after short- and long-term exposure to high altitude. J Clin Pharmacol. 1998;38:533-9.
- [Google Scholar]
- Hypoxia-inducible factor-1 and activator protein-1 modulate the upregulation of CYP3A6 induced by hypoxia. Br J Pharmacol. 2003;140:1146-54.
- [Google Scholar]
- Effect of hypoxia on cytochrome P450 activity and expression. Curr Drug Metab. 2004;5:257-71.
- [Google Scholar]
- Animal model of acute moderate hypoxia are associated with a down-regulation of CYP1A1, 1A2, 1B4, 2C5 and 2C16 and up-regulation of CYP3A6 and P-glycoprotein in liver. Drug Meta Dispos. 2007;35:765-71.
- [Google Scholar]
- Effect of chronic hypoxia on acetaminophen metabolism in rat. Biochem Pharmacol. 1991;42:1029-38.
- [Google Scholar]






