Translate this page into:
Efficacy of leprosy vaccines across the globe: A systematic review & meta-analysis of randomized controlled trials
For correspondence: Prof. Chiranjib Bagchi, Department of Pharmacology, Tamralipto Government Medical College and Hospital, Tamluk, Purba Medinipur, 721 636, West Bengal, India e-mail: bchiranjib@yahoo.co.in
-
Received: ,
Accepted: ,
Abstract
Background & objectives
Although multi-drug therapy has decreased the burden of disease, leprosy is yet to be eliminated. Accelerating progress requires optimal use of existing tools, advanced diagnostic tests, newer drugs, and vaccines. The search for a vaccine with therapeutic and preventive potential is ongoing, but evidence on effectiveness and safety is lacking. This systematic review and meta-analysis will evaluate and compare the clinical efficacy, immunogenicity, and safety of leprosy vaccines in humans.
Methods
In June 2024, three databases were systematically searched with updated search keywords. Randomized controlled trials (RCTs) pertaining to leprosy vaccines for humans which evaluated either therapeutic or prophylactic vaccines in leprosy with a placebo or active comparator arm, with full-text access, were included in the study. There were no restrictions on language, country or date. For the risk of bias assessment in the studies included, the revised Cochrane risk-of-bias 2 tool for RCTs was used. A P value (two-sided) of <0.05 was considered as significant for all tests; however for heterogeneity, a one-sided P value of <0.1 was considered as statistically significant. The quality of generated evidence specific to the desired outcomes were assessed using the GRADE approach (Grading of Recommendations Assessment, Development and Evaluation). The study protocol was registered in PROSPERO (ID: CRD42024561651).
Results
A total of 2163 studies were retrieved from different databases. After removing duplicates and full text screening, 12 articles were finally selected. Out of these studies, eight used leprosy vaccines on prophylactic basis, while four used leprosy vaccines on therapeutic basis. In therapeutic use of leprosy vaccine, Ramu’s score was found to be significantly protective [-3.06 (95% confidence interval (CI): -3.96 to -2.16)] among the recipients of the therapeutic leprosy vaccine. Bacterial index was found to be insignificant [-0.26 (95% CI: -1.54 to 1.03)] among the recipients of therapeutic leprosy vaccine. In subgroup analysis among the eight prophylactic vaccine studies, pooled relative risk was found to be 0.61 (95% CI: 0.41 – 0.91).
Interpretation & conclusions
The findings of this meta-analysis suggest that both prophylactic and therapeutic leprosy vaccines were significantly better compared to the placebo. Leprosy vaccine in the form of Mw/Mycobacterium welchii/MIP along with combination of World Health Organization (WHO) multi-drug therapy (MDT) or Bacillus Calmette-Guerin (BCG) vaccine along with second line treatment with rifampicin were found to be protective among the recipients.
Keywords
BCG vaccine
leprosy
multi-drug therapy
Mw/Mycobacterium welchii/MIP
prophylactic
therapeutic vaccine
Leprosy is an ancient disease that has affected mankind for centuries, but its elimination has remained elusive. Caused by Mycobacterium leprae and Mycobacterium lepromatosis1, it is a chronic granulomatous disease that affects the skin, peripheral nerves, mucosa of the upper respiratory tract, and the eyes. Although leprosy can be cured with the currently available multi-drug therapy (MDT), it is still a disease associated with considerable social stigma and discrimination2,3.
The strength of cell-mediated immunity (CMI) mounted by the patient against M. leprae determines the clinical manifestations of the disease. Patients who develop strong cell-mediated immune reaction have low or undetectable mycobacteria and only a few lesions are classified as having tuberculoid form of leprosy. Patients anergic to M. leprae are found to have higher mycobacterial load, present with multiple lesions and are classified as lepromatous leprosy. Between these two extremes lies a mixed spectrum, varying from patients with moderate CMI (borderline tuberculoid) to patients with little lymphocytic cell response (borderline lepromatous). The cardinal features of leprosy include skin lesions, typically anaesthetic, at the tuberculoid end of the spectrum, with thickened peripheral nerves and acid-fast bacilli on skin smears or biopsy2.
Several therapeutic modalities have been used for eradication of leprosy but it was multi-drug therapy (dapsone, rifampicin and clofazimine), recommended by the World Health Organization (WHO) in the early 1970s4, that played a significant role in bringing down its prevalence. Following the introduction of MDT for leprosy in India in 1983, there has been a remarkable decline in the number of leprosy cases5. Within the first two decades of introduction of MDT in India, the total number of reported leprosy cases reduced by almost 97 per cent, from an overwhelming 40 lakhs in 1982 to less than two lakhs by 20056. In December 2005 India achieved the leprosy elimination goal, defined as a prevalence rate of less than one per 10,000 individuals7.
Although it has been 18 year since this announcement, the country is still far away from eradicating leprosy. A national sample survey in 20178 estimated new cases of leprosy to be 3,30,346 with disabilities reported in 2.05 per cent per 100,000 population and 13.9 per cent in new cases. As reported by the WHO2, during 2023, 1,82,815 new cases were reported globally, of which 1,31,425 cases were reported from Southeast Asia alone. Brazil, India and Indonesia reported more than 10,000 new cases each, together accounting for 78.1 per cent of global new cases. As per the Weekly Epidemiological Record (WER), WHO2, September 2023, 7,218 reports were those of new infection in children of which almost 77 per cent (n=5586) were reported from India. This data is also concerning considering the fact that occurrence of new leprosy cases among children is an indicator of recent transmission.
In view of the current stagnation in leprosy eradication, the Global leprosy strategy 2021–20302 calls for accelerating action to reach the goal of zero leprosy (zero disease, zero disability and zero stigma and discrimination) and is part of the road map for neglected tropical diseases (NTDs) 2021–2030. The Leprosy Elimination Framework is a blueprint for countries to move beyond leprosy control to interruption of transmission and elimination of leprosy disease. Significant acceleration of the current disease status requires optimal use of existing tools and also use of advanced diagnostic tests, newer drugs and vaccines9.
At the national level, India has implemented the National Strategic Plan and Roadmap for Leprosy 2023-202710 which aims to interrupt transmission (zero new child cases) followed by elimination of leprosy as a disease (zero new cases). Disease prevention and immune-prophylaxis are important components of the strategic pillars formulated for attainment of these dual goals10.
Although both chemoprophylaxis and immune-prophylaxis have been studied for leprosy prevention, the use of cross-reacting mycobacterial species has been the mainstay of vaccination efforts. Utilizing Bacillus Calmette-Guerin (BCG) to immunize humans against leprosy has been the most widely employed vaccination method. In order to determine the effectiveness of combining single-dose rifampicin with BCG vaccination in infancy, Schuring et al11 conducted a secondary analysis of data from the COLEP study, which demonstrated that BCG vaccination, in addition to single-dose rifampicin cut the risk of getting leprosy by almost 80 per cent. Cunha et al12 observed, in a cluster-randomized community trial, that a second dose of BCG vaccine was ineffective in preventing leprosy. This was in contrast to a randomized clinical trial (RCT) conducted by the Karonga prevention trial group13, which showed that a second dose of BCG vaccine protected against leprosy. In India, although BCG vaccination is offered to all infants at birth, as part of the Universal Immunization Programme, leprosy still remains endemic to our country. In addition to BCG, other non-pathogenic vaccine candidates have emerged, such as Indian Cancer Research Centre bacilli (ICRC bacilli)14, Mycobacterium vaccae15, Mycobacterium indicus pranii (MIP)16, and Mycobacterium habana17, which are intended to elicit cross-reactivity. Claiming an advantage over BCG in alleviating or delaying neurological disruptions caused by leprosy, adjuvanted recombinant protein vaccines targeting specific immune response, such as LEP-F1 + GLA-SE (LepVax), have also entered the picture18,19. The MIP vaccine, in addition to having demonstrable protective efficacy at five yr, lasting upto 8-10 yr, also has the added advantage of being a cost-effective alternative20.
Although various immunoprophylactic strategies have been studied alone and also in combination with chemoprophylaxis, due to varying study designs, evidence generated from many such studies has proven inconclusive. Data from various RCTs have yielded contrasting results regarding the effectiveness of these vaccination strategies. Comprehensive evidence on the effectiveness and safety of potential leprosy vaccines is glaringly lacking. This systematic review and meta-analysis (SR/MA) aimed to evaluate and compare the clinical efficacy, immunogenicity, and safety of leprosy vaccines for which well-designed RCTs have been conducted.
Materials & Methods
The study protocol was registered on the PROSPERO International Prospective Register (ID: CRD42024561651).
Eligibility criteria
RCTs related to leprosy vaccines for humans, with full-text access, were included in the study. RCTs that evaluated either therapeutic or prophylactic vaccines in leprosy with a placebo or active comparator arm were included in our review.
There were no restrictions on language, country or date. Articles with any other study designs like abstract-only articles (conferences, letters, commentaries), theses, books, reviews, editorials, author responses, previous systematic reviews and meta-analyses and animal studies were excluded.
The primary outcome of this study was to determine the clinical efficacy of leprosy vaccines tested in human compared to placebo or other vaccines. Secondary outcomes included local or systemic adverse effects or abnormal changes in laboratory parameters due to test leprosy vaccines.
Search strategy
PubMed, Embase and Scopus were searched for studies published from inception till June 24, 2024 (for Embase) and till June 25, 2024 (for PubMed and Scopus). Supplementary Table I describes the search strategy in detail. We adapted the search terms in accordance with the bibliographic databases to search for relevant studies. Database-specific filters were put in place to refine the search results. Using the pre-defined search strategy, three independent authors searched the databases and incorporated the titles and abstracts of relevant studies for screening.
Study selection
The web-based Rayyan software (https://www.rayyan.ai/) was used for the screening process. The titles and abstracts of the studies were screened to select studies meeting our inclusion and exclusion criteria. Three reviewers initially screened the articles for inclusion or exclusion in a blinded manner; any disagreements were resolved by discussion and consensus between these reviewers. In the next phase, full text of the shortlisted articles was screened by two independent reviewers to assess suitability for inclusion. Any conflicts were resolved by discussion with a third senior author. We attempted to contact the corresponding authors by email to access missing information.
Data extraction and statistical analysis
A standard data extraction spreadsheet was prepared which included elements like general characteristics of the articles, population, intervention, comparison group, and outcome of interest (according to the study objectives) for pooling. Two independent reviewers extracted data from the included studies and populated the spreadsheet. During data extraction, no simplifications or assumptions were made. Attritions such as subject withdrawals, lost to follow up and dropout cases were investigated. Other issues of missing data and data imputation were critically appraised21. The revised Cochrane risk-of-bias 2 tool for RCTs was used to assess the risk of bias in various studies22. The assessment was independently validated by two authors – resolution of any conflicts of opinion, arising thereof, was done in consultation with a third author. When the data of interest was present in more than three studies, the efficacy outcomes between the leprosy vaccine arm and the comparator arms were directly compared and pooled. For dichotomous variables, we calculated the risk ratio (RR) for each study and then, using the DerSimonian-Laird random-effect model, pooled the RR across studies. Similarly, for continuous variables, the mean differences [with 95% confidence intervals (CI)] were also pooled using the same model. Sub-group analyses were conducted depending on the population age (children and young adults vs. all age groups) and the type of comparator (placebo vs. active vaccines).
Forest plot, the Cochrane Q test, and i2 statistics were used to explore heterogeneity between studies23. If P value obtained from the Cochrane Q test was <0.10 or i2 was >25 per cent, heterogeneity was considered to be present. Sensitivity analysis using the leave-one-out method was performed to explore the source of heterogeneity. Meta-regression was then performed for the duration of follow-up. The Stata software version 16.0 (Stata Corp, Texas, USA) was used to perform the statistical analyses. Barring heterogeneity, for which a one-sided P value <0.1 was considered, for all other tests, a two-sided P value <0.05 was considered statistically significant. The GRADE approach (Grading of Recommendations Assessment, Development and Evaluation) was used to assess the quality of evidence generated by the pooled analyses24,25.
The Preferred Reporting Items for Systematic Review and Meta-analysis Statement 2020 (PRISMA) was used to report this SR/MA.
Results
Altogether 2163 studies were retrieved from three different databases. Initially, 1556 articles were screened after removing 607 duplicate articles. Finally, after full text screening, out of 102 screened articles, 12 articles of RCTs designs were selected12,26-35 (Figure).
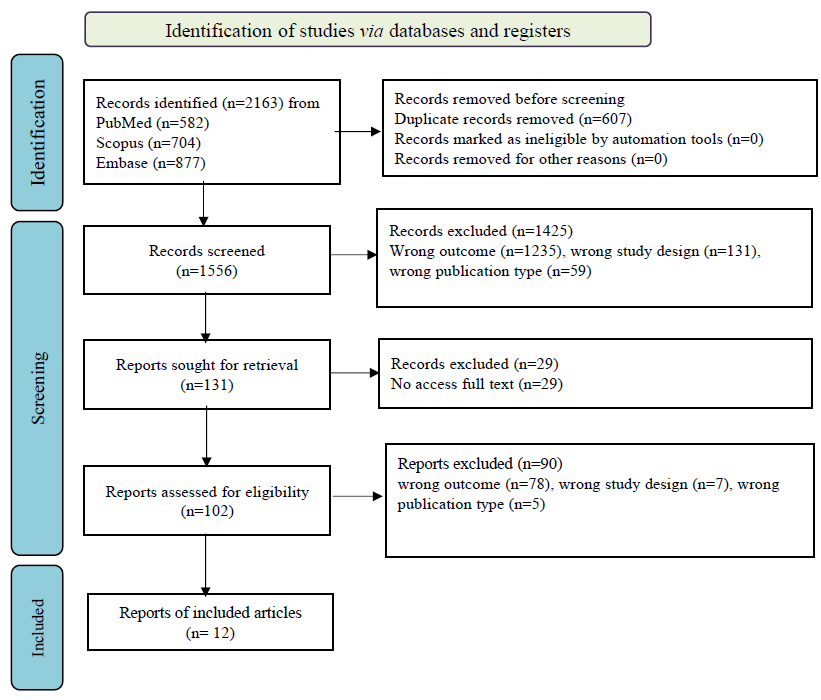
- Flowchart of literature search and study selection according to the PRISMA standard.
Out of these 12 articles, eight studies tested the use of leprosy vaccines for prophylaxis12,26-31. The earliest and the latest study of leprosy vaccine for prophylaxis were published in the year 1973 and 2008, respectively. Three studies were published from Asia (India, Burma, Vietnam)27,29,31, while two studies each were published from Africa (Malawi, Uganda)13,30 and South America (Brazil, Venezuela)12,28. One study was published from Oceania (Papua New Guinea) continent26. The combined number of participants in these eight studies was 2,41,905, ranging from 565 (Vietnam)31 to 92,770 (Brazil) participants12. Five out of these eight studies were conducted among close contacts of leprosy patients, out of which two studies considered household contacts, including children as well as adult participants28,29. Three studies included only household contacts who were children, where the age of participants ranged from newborn to 20 yr. These studies were published from Burma, Uganda, and Vietnam27,30,31. One study each from Malawi13 and Papua New Guinea26 included community dwelling individuals from all age groups; while another study conducted in Brazil12 included school children aged 7-14 yr. Fifty per cent (4/8) of these trials studied intradermal single BCG vaccine in the intervention arm, out of which three studies included unvaccinated participants in the corresponding control arm12,27,30. Normal saline was used as control against single dose of BCG vaccine in the study conducted in Papua New Guinea, where community-dwelling individuals from all age groups participated in the trial26. The other half (4/8) of the studies included BCG as well as an adjunct therapy in the form of M. leprae bacilli / killed Mycobacterium vaccae/ KML BCG13,28,29,31. Three of these studies included a placebo arm which consisted of tetanus toxoid injection (in addition to MDT) conducted in India29, 0.2 mg or 0.04 mg BCG vaccine, conducted in Venezuela28 and undefined placebo13. The remaining study, conducted in Vietnam among children living in close contact with patients of leprosy, had three study arms where each control arm consisted of unvaccinated children31. The total period of follow up in these eight studies ranged from 5-(Venezuela, Malawi)13,29 to 16 yr (Papua New Guinea)26 (Table I).
| Author, yr | Country (continent) | Study population | Age (yr) | Leprosy vaccine dosing protocol | Comparator arm protocol | Duration of follow up | Total (n) |
|---|---|---|---|---|---|---|---|
| Cunha et al12, 2008 | Brazil (South America) | School children | 7–14 | Revaccination with 0.1 ml of lyophilized BCG ID | Unvaccinated | 6 yr and 8 months | 92770 |
| Bagshawe et al26, 1989 | Papua New Guinea (Oceania) | Community dwelling individuals | All age groups | Intradermal injection of 0.1 ml BCG | Normal saline | 16 yr | 5356 |
| Stanley et al30, 1981 | Uganda (East Africa) | Contacts or relatives of known leprosy patients | Children (0-15) | Single dose of freeze-dried BCG vaccine | Unvaccinated | 8 yr | 16150 |
| Bechelli et al27, 1973 |
Burma (Asia) |
Household contacts or children | Children (0-14) | BCG vaccine | Unvaccinated | 7 yr | 22630 |
| Sharma et al29, 2005 |
India (Asia) |
Household contacts | 1-65 | MDT + killed Mycobacterium w 2 doses at 6-month intervals per ml | MDT + Tetanus toxoid | 8-10 yr | 20456 |
| Convit et al28, 1992 | Venezuela (South America) | Household contacts | All age groups | BCG plus 6×108 M leprae bacilli | BCG Contacts with a skin-test response to PPD of less than 10 mm (negative) received 0.2 mg & those with larger indurations (positive) received 0.04 mg | 5 yr | 29113 |
| Karonga Prevention Trial Group13, 1996 |
Malawi (East Africa) |
Community dwelling individuals | All age groups | BCG alone or BCG + KML BCG (Glaxo): 0.1 ml + 6 * 109 per ml | Placebo | 5-9 yr | 54865 |
| Truoc et al31, 2001 | Vietnam (Asia) | Children living in close contact | 3-20 | BCG alone | Unvaccinated | 8 yr | 174 |
| BCG+107 killed Mycobacterium vaccae | Unvaccinated | 246 | |||||
| 108 killed M. vaccae alone | Unvaccinated | 145 | |||||
BCG, Bacillus Calmette-Guerin
We were able to identify four studies where leprosy vaccines were tested for therapeutic benefit32-35. All these RCTs were from India dating from 1992 to 2004. The combined number of study participants in these studies was 291, with numbers ranging from 40 to 90 participants in the specific studies. The study population comprised multibacillary leprosy patients (all age groups), untreated leprosy patients (> 12 yr), paucibacillary leprosy patients (15-60 yr) and untreated bacteriologically positive multibacillary leprosy patients (>18 yr). In the intervention arm, Mycobacterium w was administered intradermally, eight doses at three-month intervals in one study31. In the study conducted by Narang et al33, in addition to 12 months of MDT-MBR, one intervention arm received WHO-recommended BCG vaccine (live bacilli count 105/dose) intradermally while the other intervention arm received killed Mw/MIP bacilli (first dose: 1×108, and subsequent dose: 0.5×108). Majumder et al34 conducted their study among paucibacillary leprosy patients where the intervention consisted of intradermal injection of low-dose of Convit vaccine (containing 1.6×107 heat-killed M. leprae in 0.1 ml saline) followed by BCG vaccination (1.5×107 BCG in 0.1 ml saline) after three months interval. Both the study arms also received single dose of 600 mg rifampin, 100 mg of minocycline, and 400 mg of ofloxacin. De Sarkar et al35 recruited untreated bacteriologically positive multibacillary leprosy patients where the intervention consisted of WHO/MDT for 12 months plus four doses of intradermal Mw/MIP vaccine, 0.1 ml each, administered at three monthly intervals35. Placebo was used in two studies - micronized starch dissolved in distilled water was used as placebo in one study32 while in the other study, participants in the placebo arm received 0.1 ml of normal saline along with MDT-MBR33. Single dose of 600 mg rifampin, 100 mg of minocycline, and 400 mg of ofloxacin and MDT were used as comparators in two studies. The total duration of study was one yr in two of these studies while the other two studies lasted for two year (Table II).
| Author, yr | Country | Study population | Age (yr) | Leprosy vaccine dosing protocol | Comparator arm protocol | Follow up period (yr) | Total (n) |
Intervention arm (n) |
Comparator arm (n) |
|---|---|---|---|---|---|---|---|---|---|
| Zaheer et al32, 1993 | India | Patients with Multi-bacillary Leprosy | All age groups | Mycobacterium w id eight doses at 3-month intervals | Placebo: 1g micronized starch dissolved in 100 mL distilled water id eight doses at 3-month intervals | 2 | 81 | 45 | 36 |
| Narang et al33, 2005 | India | Untreated leprosy patients | >12 | WHO 12 months MDT-MBR & BCG intradermally (105 live bacilli/per dose) | 12 months M.D.T. MBR with 0.1 ml of normal saline as placebo | 2 | 40 | 20 | 20 |
| 12 months MDT-MBR and Myco bacterium w (1×108) killed bacilli as first dose and 0.5×108/dose in subsequent doses | 12 months M.D.T. MBR with 0.1 ml of normal saline as placebo | 2 | 40 | 20 | 20 | ||||
| Majumder et al34, 2000 | India | Pauci-bacillary leprosy patients | 15-60 | Low-dose Convit vaccine containing 1.6×107 heat-killed M. leprae in 0.1 ml saline and 1.5×107 BCG (Japan) in 0.1 ml saline - two injections, one initially and another after 3 months plus single dose of Rifampicin 600 mg, ofloxacin 400 mg and minocycline 100 mg | Single dose of Rifampicin (600 mg), ofloxacin (400 mg) and minocycline (100 mg) | 1 | 90 | 60 | 30 |
| De Sarkar et al35, 2001 | India | Untreated bacteriologically +ve MB | >18 | WHO/MDT for 12 months plus four doses each of 0.1 ml M. w vaccine intra-dermally at 3 monthly intervals | WHO/MDT only for 12 month | 1 | 40 | 20 | 20 |
Ramu’s score was found to be significantly reduced [-3.06 (95% CI: -3.96 to -2.16)] among the recipients of the therapeutic leprosy vaccine (Supplementary Fig. 1). Bacterial index was found to be insignificant [-0.26 (95% CI: -1.54 to 1.03)] among the recipients of therapeutic leprosy vaccine (Supplementary Fig. 2). Additionally, in the study by Zaheer et al32, 13/31 (41.9%) patients in the leprosy vaccine arm and 5/25 (20%) patients in the comparator arm were bacteriologically negative. In the study by Majumder et al34, 20/60 (33.3%) patients in the leprosy vaccine arm and 4/30 (13.3%) patients in the comparator arm reported resolution of healing.
Overall certainty of generated evidence in outcomes i.e. infection rate, Ramu’s score and bacteriological index were found to be of moderate grade. Mean difference in anticipated absolute effects in four RCTs in Ramu’s score and bacteriological index were 2.93 lower (3.94 lower to 1.93 lower) and 0.48 lower (1.66 lower to 0.71 higher) respectively (Supplementary Table II). About 50 per cent (6/12) of the studies were found to have both medium and high risk of overall bias (Supplementary Table III).
In subgroup analysis, among the eight prophylactic vaccine studies, pooled relative risk was found to be 0.61 (95% CI: 0.41 – 0.91) and was statistically significant (P=0.016). Country-wise, all the studies had significant protective effect among the recipients except the studies from Burma, Brazil and Venezuela12,27,28. Studies on all age groups had significant protective effect (RR=0.63, 95% CI: 0.51 – 0.77) compared to collective studies on children and young adults (RR=0.59, 95% CI: 0.23 – 1.52). In the control arm, the studies having combined MDT + TT, normal saline, and unnamed placebo had significant protection among the recipients compared to studies having unvaccinated and only BCG in the control arm (Supplementary Fig. 3).
In sensitivity analysis, five studies out of eight studies of prophylactic vaccine were found to be statistically significant (Supplementary Fig. 4)12,13,27,28,30. In therapeutic leprosy vaccine study, all the three studies on Ramu’s score32,33,35 and one study on bacteriological index32 were statistically significant.
Discussion
Leprosy is one of the oldest diseases of mankind. Despite availability of MDT against leprosy, it continues to occur in more than 120 countries in the globe. India, Brazil and Indonesia contributed more than 10,000 new cases as per WHO estimate on 2019. As a primary level of prevention, administration of single dose of rifampicin among the close household contacts is recommended by WHO36. Moreover, treatment with MDT has also led to several complications like drug resistance, side effects, etc. The effectiveness of MDT in controlling leprosy has hit a plateau, with mathematical models indicating that the disease will continue to be a significant public health issue for several more decades31,35. Again, mass BCG vaccination against tuberculosis has also significantly contributed to the reduction of leprosy, though this beneficial effect is frequently overlooked in contemporary leprosy control strategies37,38. A few studies have proved the beneficial role of leprosy vaccine in preventing infection. Leprosy vaccines have been studied both in a prophylactic role (among the non-diseased) and in therapeutic role (among the diseased) separately in some parts of the globe. Of late, greater emphasis has been given on the role of BCG vaccination in both leprosy control and research efforts39. In this background, the present SR/MA was performed to find out the beneficial role of leprosy vaccines among the recipients.
Total number of participants in the selected prophylactic and therapeutic studies were 2,41,905 and 291, respectively. Tawfik et al40 studied 3,26,264 participants in their systematic review and network meta-analysis. In all four RCTs of the therapeutic trial, Mw/MIP was administered in three studies either alone or in combination with 12 months WHO MDT. The study by Majumder et al34 administered low-dose Convit vaccine containing 1.6×107 heat-killed M. leprae in 0.1 ml saline and 1.5×107 BCG plus single dose of rifampin (600 mg), ofloxacin (400 mg) and minocycline (100 mg). In contrast, BCG vaccine along with M. leprae (heat killed/human) or single-dose rifampin were used in the intervention arm in the study conducted by Tawfik et al40. The present systematic review and meta-analysis included participants from all age group (up to 60 yr) that corroborated the study by Tawfik et al40 (0 – 70 yr). About 50 per cent (6/12) of the studies were found to have both medium and high risk of overall bias in the present SR/MA, while all the seven studies except two were found to have low to moderate risk by Tawfik et al40.
Our result showed that both prophylactic and therapeutic leprosy vaccine were significantly better compared to the placebo, which is consistent with similar studies11,37-41. Some studies even reported higher efficacy of the leprosy vaccine ranging from 34-80 per cent38. Among the prophylactic vaccine studies in subgroup analysis, pooled relative risk was found to be 0.61 (95% CI: 0.41 – 0.91) and was statistically significant (P=0.016). In contrast, pooled relative risk ranged from 0.48 – 1.08 in the study by Tawfik et al40 but none of them were statistically significant40. Present SR/MA showed that when all age groups were considered, leprosy vaccine had significant protective effect (RR=0.63, 95% CI: 0.51–0.77) compared to the collective studies done on children and young adults (RR=0.59, 95% CI: 0.23–1.52), which is contradictory to the finding of Schuring et al11, who found it to be beneficial among children. In contrast, Setia et al41 showed that the protective effect of BCG did not depend on the age of vaccination41. Present SR/MA found that the studies having combined MDT + TT, normal saline, and unnamed placebo in the control arm had significant protection among the recipients compared to studies having unvaccinated subjects or only BCG vaccinated subjects in the control arm. In therapeutic vaccine trials, the tested leprosy vaccines were found to be protective with reference to Ramu’s score, in our study, which was similar to the study by Setia et al41, where overall protective effect was found to be 26 per cent41. The present SR/MA included studies which exhibited high heterogeneity (i2 91.42%), which was also found in similar studies38,40.
There are certain limitations of the present SR/MA such as non-availability of some of the full text articles and presence of heterogeneity among the included studies. Literature review yielded several observational studies, which were conducted to evaluate the efficacy of leprosy vaccines including combined chemotherapy and immunotherapy i.e., with Mw/MIP or BCG, some in areas with mandatory BCG vaccination policies, but such studies were not included as our SR/MA focused only on RCTs. Additionally, as the initial focus of the study, was to evaluate the clinical efficacy, immunogenicity, and safety of leprosy vaccines, immunotherapy was not considered as a search term at screening. The strength of the present SR/MA lies in the fact that it represents an up-to date search of the relevant articles with search string in three major databases, conducted as per Cochrane guide and PRISMA flowchart.
A subgroup analysis showcasing the benefit of the combined chemotherapy and immunotherapy (Mw/MIP or BCG) towards achieving quicker therapeutic improvement in comparison to immunotherapy only, would definitely add value. But it has not been performed due to the limited number of available studies on therapeutic vaccines (n=3), which significantly reduces the statistical power necessary for meaningful subgroup evaluations. Given these constraints, the focus of this meta-analysis is on estimating the overall therapeutic effect of combining chemotherapy and immunotherapy. Conducting a subgroup analysis in this context could produce unreliable conclusions. Therefore, the decision of not to include subgroup analysis highlights the need for further research to enable more robust and refined analyses in the future.
Conclusion
Our study found that leprosy vaccines are effective when used both as prophylaxis and for therapeutic benefit. Leprosy vaccine in the form of Mw/MIP along with combination of WHO MDT or BCG vaccine along with second-line treatment with rifampicin was found to be protective among the recipients. When all age groups were considered, leprosy vaccines were found to provide greater protective benefit compared to when only children and younger age groups were considered. Concurrent use of MDT with prophylactic vaccines provided better protective effect than vaccine alone. When used with therapeutic intent, leprosy vaccines significantly improved clinical scores but their effect on bacteriological index remained inconclusive. Of the 12 RCTs included in our study, six were of moderate to high risk of overall bias. Therefore well designed RCTs for leprosy vaccines are needed to generate stronger evidence for such vaccines.
Financial support & sponsorship
None.
Conflicts of Interest
None.
Use of Artificial Intelligence (AI)-Assisted Technology for manuscript preparation
The authors confirm that there was no use of AI-assisted technology for assisting in the writing of the manuscript and no images were manipulated using AI.
References
- A new Mycobacterium species causing diffuse lepromatous leprosy. Am J Clin Pathol. 2008;130:856-64.
- [Google Scholar]
- Global leprosy (Hansen disease) update, 2022: new paradigm – control to elimination. Available from: https://iris.who.int/handle/10665/372992, accessed on August 24, 2024.
- Davidson’s Principles and Practice of Medicine (24th ed). Edinburgh: Elsevier; 2023.
- Guidelines for diagnosis, treatment, and prevention of leprosy. Available from: https:// www.who.int/publications/i/item/9789290226383, accessed on December 28, 2023.
- Kumar B, Kar HK, Dogra S, eds. IAL textbook of leprosy (3rd ed). New Delhi and London: Jaypee Brothers Medical Publishers (P) Ltd; 2023.
- Eradication of leprosy from India: Reflections on past, present & future. Indian J Med Res. 2024;159:1-5.
- [Google Scholar]
- National Leprosy Eradication Programme. Available from: https://dghs.gov.in/content/1349_3_NationalLeprosyEradicationProgramme.aspx, accessed August 20, 2024.
- National sample survey to assess the new case disease burden of leprosy in India. Indian J Med Res. 2017;146:585-605.
- [Google Scholar]
- Leprosy Factsheet. Available from: https://www.who.int/news-room/fact-sheets/detail/leprosy, accessed on August 20, 2024.
- National Strategic Plan and Roadmap for Leprosy 2023-2027. Available from: https://dghs.gov.in/WriteReadData/userfiles/file/Leprosy%20New/NSP%20%20Roadmap%2 for%20Leprosy%202023-2027.pdf, accessed on August 20, 2024.
- Protective effect of the combination BCG vaccination and rifampicin prophylaxis in leprosy prevention. Vaccine. 2009;27:7125-8.
- [Google Scholar]
- BCG revaccination does not protect against leprosy in the Brazilian Amazon: A cluster randomised trial. PLoS Negl Trop Dis. 2008;2:e167.
- [Google Scholar]
- Randomised controlled trial of single BCG, repeated BCG, or combined BCG and killed Mycobacterium leprae vaccine for prevention of leprosy and tuberculosis in Malawi. Lancet. 1996;348:17e24.
- [Google Scholar]
- Immunological properties of M. leprae culture isolates ICRC bacilli: Hypothesis on relationship between M. leprae and ML-culture isolates. Acta Leprol. 1984;2:175-94.
- [Google Scholar]
- The use of the name Mycobacterium w for the leprosy immunotherapeutic bacillus creates confusion with M. tuberculosis-W (Beijing strain): A suggestion. Infect Genet Evol. 2008;8:100-1.
- [Google Scholar]
- Response of Mycobacterium habana vaccine in patients with lepromatous leprosy and their household contacts. A pilot clinical study. Lepr Rev. 2001;72:179-91.
- [Google Scholar]
- A phase 1 antigen dose escalation trial to evaluate safety, tolerability and immunogenicity of the leprosy vaccine candidate LepVax (LEP-F1 + GLA-SE) in healthy adults. Vaccine. 2020;38:1700-7.
- [Google Scholar]
- LepVax, a defined subunit vaccine that provides effective pre-exposure and post-exposure prophylaxis of M. leprae infection. NPJ Vaccines. 2018;3:12.
- [Google Scholar]
- Cost-effectiveness of incorporating Mycobacterium indicus pranii vaccine to multidrug therapy in newly diagnosed leprosy cases for better treatment outcomes & immunoprophylaxis in contacts as leprosy control measures for National Leprosy Eradication Programme in India. Indian J Med Res. 2021;154:121-31.
- [Google Scholar]
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, eds. Cochrane Handbook for Systematic Reviews of Interventions (2nd ed). Chichester (UK): John Wiley & Sons; 2019.
- RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
- [Google Scholar]
- GRADE guidelines: 14. Going from evidence to recommendations: The significance and presentation of recommendations. J Clin Epidemiol. 2013;66:719-25.
- [Google Scholar]
- GRADE guidelines: 15. Going from evidence to recommendation-determinants of a recommendation’s direction and strength. J Clin Epidemiol. 2013;66:726-35.
- [Google Scholar]
- BCG vaccination in leprosy: Final results of the trial in Karimui, Papua New Guinea, 1963-79. Bull World Health Organ. 1989;67:389-99.
- [Google Scholar]
- BCG vaccination of children against leprosy: Seven-year findings of the controlled WHO trial in Burma. Bull World Health Organ. 1973;48:323-34.
- [Google Scholar]
- Immunoprophylactic trial with combined Mycobacterium leprae/BCG vaccine against leprosy: Preliminary results. Lancet. 1992;339:446-50.
- [Google Scholar]
- Immunoprophylactic effects of the anti-leprosy Mw vaccine in household contacts of leprosy patients: Clinical field trials with a follow up of 8-10 years. Lepr Rev. 2005;76:127-43.
- [Google Scholar]
- BCG vaccination of children against leprosy in Uganda: Final results. J Hyg (Lond). 1981;87:233-48.
- [Google Scholar]
- Vaccination against leprosy at Ben San Leprosy Centre, Ho Chi Minh City, Vietnam. Vaccine. 2001;19:3451-8.
- [Google Scholar]
- Combined multidrug and Mycobacterium w vaccine therapy in patients with multibacillary leprosy. J Infect Dis. 1993;167:401-10.
- [Google Scholar]
- Comparative evaluation of immunotherapeutic efficacy of BCG and mw vaccines in patients of borderline lepromatous and lepromatous leprosy. Int J Lepr Other Mycobact Dis. 2005;73:105-14.
- [Google Scholar]
- Efficacy of single-dose ROM therapy plus low-dose convit vaccine as an adjuvant for treatment of paucibacillary leprosy patients with a single skin lesion. Int J Lepr Other Mycobact Dis. 2000;68:283-90.
- [Google Scholar]
- Impact of combined Mycobacterium w vaccine and 1 year of MDT on multibacillary leprosy patients. Int J Lepr Other Mycobact Dis. 2001;69:187-94.
- [Google Scholar]
- Leprosy. Available from: https://www.who.int/news-room/fact-sheets/detail/leprosy/?gad_source=1&gclid=CjwKCAjwlbu2BhA3EiwA3yXyu4Bclj3rcu9ldOj0TTroMkjgaw58fOc-HbTXeJZk0uoscvMzXN1hhRoCP3QQAvD_BwE, accessed on August 28, 2024.
- Advances and hurdles on the way toward a leprosy vaccine. Hum Vaccin. 2011;7:1172-83.
- [Google Scholar]
- Immunoprophylaxis of leprosy: Current status and future prospects. Indian J Dermatol Venereol Leprol. 2007;73:71-2.
- [Google Scholar]
- BCG vaccination and leprosy protection: Review of current evidence and status of BCG in leprosy control. Expert Rev Vaccines. 2010;9:209-22.
- [Google Scholar]
- Efficacy of chemoprophylaxis and immunoprophylaxis in leprosy prevention: A systematic review and network meta-analysis of randomized controlled trials. Clin Microbiol Infect. 2021;27:1754-61.
- [Google Scholar]
- The role of BCG in prevention of leprosy: A meta-analysis. Lancet Infect Dis. 2006;6:162-70.
- [Google Scholar]






