Translate this page into:
Time dependent enhanced resistance against antibiotics & metal salts by planktonic & biofilm form of Acinetobacter haemolyticus MMC 8 clinical isolate
Reprint requests: Prof. B.A. Chopade, Department of Microbiology, University of Pune, Pune 411 007, Maharashtra, India e-mail: chopade@unipune.ac.in
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Available literature shows paucity of reports describing antibiotic and metal resistance profile of biofilm forming clinical isolates of Acinetobacter haemolyticus. The present study was undertaken to evaluate the antibiotic and metal resistance profile of Indian clinical isolate of A. haemolyticus MMC 8 isolated from human pus sample in planktonic and biofilm form.
Methods:
Antibiotic susceptibility and minimum inhibitory concentration were determined employing broth and agar dilution techniques. Biofilm formation was evaluated quantitatively by microtiter plate method and variation in complex architecture was determined by scanning electron microscopy. Minimum biofilm inhibiting concentration was checked by Calgary biofilm device.
Results:
Planktonic A. haemolyticus MMC 8 was sensitive to 14 antibiotics, AgNO3 and HgC12 resistant to streptomycin and intermediately resistant to netilmycin and kanamycin. MMC 8 exhibited temporal variation in amount and structure of biofilm. There was 32 – 4000 and 4 – 256 fold increase in antibiotic and metal salt concentration, respectively to inhibit biofilm over a period of 72 h as against susceptible planktonic counterparts. Total viable count in the range of 105 -106cfu / ml was observed on plating minimum biofilm inhibiting concentration on Muller-Hinton Agar plate without antimicrobial agents. Biofilm forming cells were several folds more resistant to antibiotics and metal salts in comparison to planktonic cells. Presence of unaffected residual cell population indicated presence of persister cells.
Interpretation & conclusions:
The results indicate that biofilm formation causes enhanced resistance against antibiotics and metal salts in otherwise susceptible planktonic A. haemolyticus MMC 8.
Keywords
Acinetobacter haemolyticus
antibiotic resistance
Calgary biofilm device
MBIC
MIC
persister cells
SEM
Members of genus Acinetobacter are considered as one of the most important nosocomial pathogens1 which exhibit multiple antibiotic and metal resistance and ability to form biofilm2. One of the members of the genus, A. haemolyticus is predominantly found on skin of tribal3 and urban4 Indian population. It has been reported to exhibit multidrug resistance against commonly used drugs for treatment5678 and production of haemolytic enzymes9.
Biofilm is an important mode of bacterial life fostering elevated resistance towards antibiotics and metal salts used in medicine10. Earlier, biofilm of Staphylococcus spp.11 and Escherichia coli12 have been reported to exhibit elevated resistance against antibiotics. Studies on Pseudomonas aeruginosa have also indicated planktonic and biofilm cells to be resistant to antimicrobial agents13. Mechanisms of resistance in biofilm vary significantly from that of planktonic forms14. Biofilm resistance mechanisms include (i) production of exopolymeric substances (EPS), (ii) slow growth rate, (iii) spatial and physiological heterogeneity, (iv) persister cells, and (v) small colony variants1516. However, none of these mechanisms have been reported to be involved in biofilm mediated resistance of genus Acinetobacter.
Information on biofilm formation and comparison of antibiotic and metal resistance profile in planktonic and biofilm form of A. haemolyticus is not available. Thus, the present study was aimed at studying sensitivity pattern of A. haemolyticus MMC 8 against 20 antibiotics belonging to five classes and metal salts commonly used in treatment. The study was further extended to understand temporal variation in biofilm formation and its effect on resistance in biofilm form of A. haemolyticus MMC 8 in comparison to planktonic counterparts.
Material & Methods
Bacterial cultures: Sixteen Acinetobacter strains isolated from clinical samples in the departments of Microbiology at three different hospitals namely Madurai Medical College (MMC), Madurai, Sri Ramchandra Medical College (SRMC), Chennai. All India Institute of Medical Sciences (AIIMS), New Delhi, India, were procured and identified by 16S rRNA gene sequencing and were used for biofilm screening at Institute of Bioinformatics and Biotechnology, University of Pune, Pune (data not shown). On the basis of biofilm formation ability A. haemolyticus MMC 8 (GenBank EU779836) was used for further studies. For antibiotic and metal susceptibility, Escherichia coli NCIM 2931 (ATCC 25922) was used as per recommendations of CLSI Document M07-A917. Cultures were maintained in 50 per cent (v/v) glycerol at - 80°C. Planktonic and biofilm cultures were grown at 37°C with 150 rpm and static conditions, respectively.
Susceptibility testing against antibiotics and metal salts: Susceptibility of A. haemolyticus MMC 8 was checked against 20 antibiotics, by Kirbey Bauer disc diffusion assay4. The antibiotics include amikacin, gentamicin, tobramycin, kanamycin, netilmycin, streptomycin, ampicillin, amoxycillin, amoxicillin/clavulanic acid combination, penicillin-G, ceftazidime, ceftriaxone, cefepime, meropenem, imipenem, lomefloxacin, chloramphenicol, polymyxin B, rifampicin and tetracycline. Overnight broth culture (100 μl, 105 cfu/ml) was spread on Muller-Hinton agar (MHA) plates and antibiotic discs (Hi-Media Ltd, Mumbai) were placed aseptically. After 24 h of incubation, zone diameters were measured. Metal susceptibility was determined by agar dilution technique as described previously18. MHA plates containing concentrations of 0.01, 0.1, 1, 10 mM of AgNO3 and HgCl2 were prepared, spot inoculated (5 μl) with 105 cfu / ml and incubated. After 24 h, plates were observed for presence or absence of growth.
Determination of minimum inhibitory concentration (MIC): MIC of antibiotics was determined by broth dilution technique as described by CLSI17. Briefly, gentamycin, tobramycin, amoxycillin/clavualnic acid, rifampicin and polymyxin B in range of 2 - 2048 μg/ml were prepared in Muller-Hinton broth (MHB). Each tube was inoculated to maintain final density at 105 cfu/ml and incubated. For metal salts, MHA plates containing metal concentrations in the range of 0.1 to 1 mM (AgNO3) and 0.01 - 0.1 mM (HgCl2) were prepared. Culture was spotted (5 μl) and the plates were incubated. After 24 h lowest concentration of antibiotic and metal salt which inhibited growth was recorded as MIC.
Biofilm formation: Biofilm formation was scored in microtiter plate as described earlier19 with a few modifications. Briefly, culture (5 μl) was inoculated in 195 μl Luria Bertani (LB) broth maintaining final cell density of 105 cfu/ml. After every 24 h broth was aspirated and wells were washed with PBS (200 μl). To the dried plate, aqueous 0.1 per cent (w/v) gentian violet (200 μl) was added and allowed to stand for 15 min. After careful aspiration bound stain was solubilized in alcohol (200 μl) and absorbance was measured at 570 nm on multiplate reader (Molecular devices, USA). Sterile uninoculated L-B broth was used as negative control.
Scanning electron microscopic (SEM) analysis of biofilm: SEM analysis of challenged and unchallenged pegs was performed as described earlier19.
Determination of minimum biofilm inhibiting concentration (MBIC): MBIC was determined employing Calgary Biofilm Device (CBD) (Innovotech, Canada). The plate trough was filled with 24.375 ml of MHB and inoculated with 0.625 μl culture (final cell density = 105 cfu/ml). These plates were incubated for a period of 24, 48 and 72 h in static conditions. After the respective period of incubation CBD lid was washed with PBS and transferred to microtiter plate containing gentamycin, tobramycin, amoxycillin/clavualnic acid, rifampicin, polymyxin B, AgNO3 and HgCl2 dilutions. After incubation for 24 h, pegs were washed with PBS and transferred to a fresh plate containing 200 μl MHB in each well. This pegs, were sonicated in Ultrasonic cleaner, (Equitron, India) at 40 per cent power for 10 min and incubated for 24 h. The well which did not allow growth was recorded as MBIC and spotted on MHA plate. After 24 h of incubation, the spots which did not grow were recorded as minimum biofilm eradicating concentration (MBEC).
Results
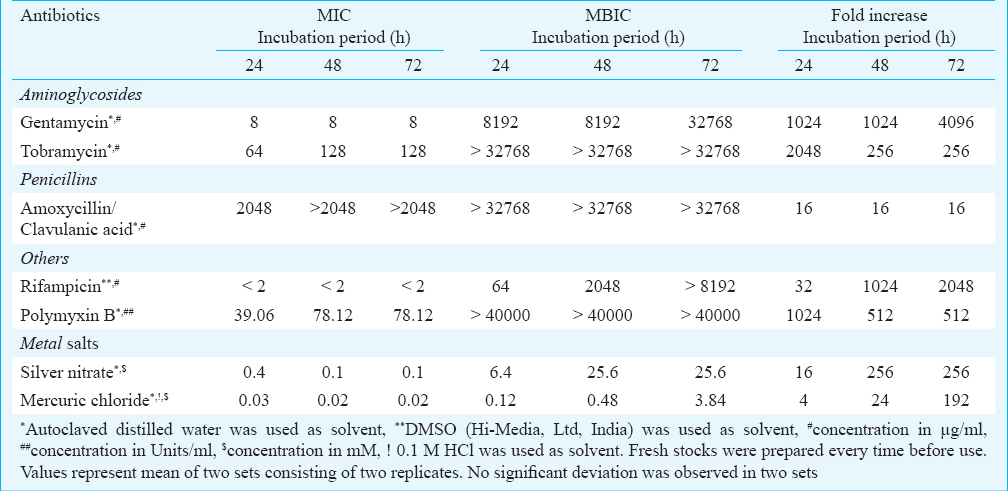
Planktonic A. haemolyticus MMC 8 is sensitive to antibiotics and metal salts: A. haemolyticus MMC 8 was sensitive to 14 antibiotics (amikacin, gentamicin, tobramycin, ceftazidime, ceftriaxone, cefepime, lomefloxacin, ampicilin, amoxicillin, amoxicillin, clavulanic acid combination, penicillin chloramphenicol, rifampicin, tetracycline). However, it was resistant to streptomycin and intermediately resistant to kanamycin, netilmycin. In case of metal salts it was sensitive to both AgNO3 and HgCl2. MIC of antibiotics which are commonly used for treatment suggested A. haemolyticus MMC 8 to be resistant to amoxicillin/clavulanic acid with highest MIC of 2048 μg/ml. For other antibiotics like gentamicin, tobramycin and rifampicin, MIC was 8, 64 and 2 μg/ml, respectively while for polmyxin B it was 39.06 U/ml. MIC values of AgNO3 and HgCl2 were 0.4 and 0.03 mM, respectively (Fig. 1 a, b, c, e). Moreover, the MIC values for tobramycin and polymyxin B increased with culture age (Fig. 1c, d), while for AgNO3 and HgCl2 these decreased to 0.1 and 0.02 mM, respectively (Fig. 1f, g).
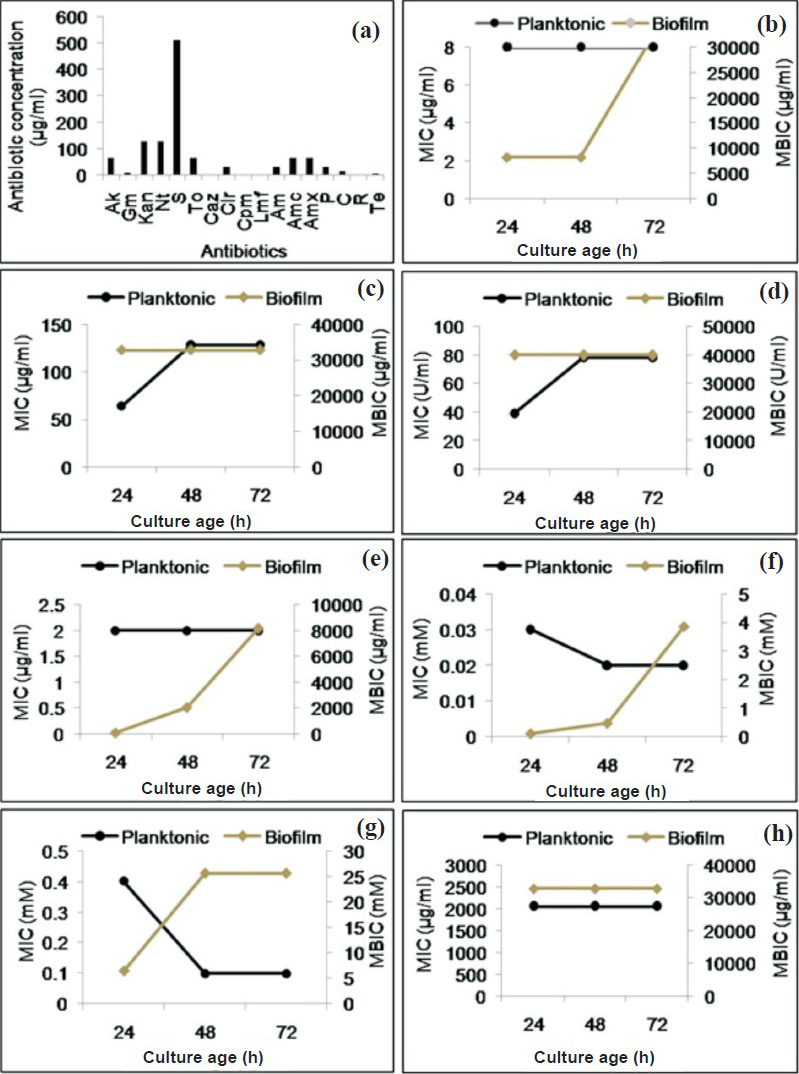
- MIC and MBIC of antibiotics and metal salts against A. haemolyticus MMC 8 (a) MIC of antibiotics (Ak, amikacin; Gm, gentamicin; Kan, kanamycin; Nt, netilmycin; S, streptomycin; To, tobramycin; Caz, ceftazidime; Cir, ceftriaxone; Cpm, cefepime; Lmf, lomfloxacin; Am, ampicillin; Amc, amoxycillin/clavuanic acid combination; Amx, amoxycillin; P, penicillin G; C, chloramphenicol; R, rifampicin; Te, tetracycline), (b-h) Comparison of MIC and MBIC of antibiotics and metal salts against A. haemolyticus MMC 8 over a period of 72 h; (b) gentamicin, (c) tobramycin, (d) polymyxin B, (e) rifampicin, (f) HgCl2, (g) AgNO3, (h) amoxicillin/clavulanic acid. Each experiment was repeated twice and average value is plotted.
Biofilm formation of A. haemolyticus MMC 8 increases with incubation period: Biofilm formation by microtiter plate method demonstrated that A. haemolyticus MMC 8 established its biofilm over a period of time. The O.D. value of 24 h old biofilm was 0.192±0.0003, while for 48 and 72 h old biofilm these were 0.302 ± 0.006 and 0.85 ± 0.01, respectively. This indicated that the amount of biofilm formed by A. haemolyticus MMC 8 increased with increase in period of incubation (Fig. 2a). Further, SEM analysis showed structural variation in compactness and architecture of biofilm. One day old biofilm (Fig. 2b) comprised cellular aggregates while two days old (Fig. 2c) biofilm was organized forming cellular heaps embedded within exopolymeric matrix. The three days old (Fig. 2d) biofilm appeared like an intrinsically entangled aggregates of cells.
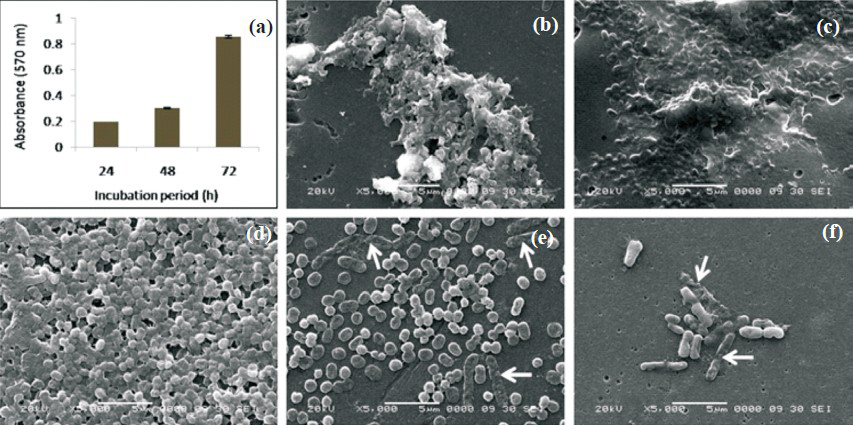
- Biofilm formation by A. haemolyticus MMC 8 (a) Time course by microtiter plate method, Bars and error bars indicate mean and standard deviation of six readings obtained in two sets Scanning electron micrographs (b) 24 h, (c) 48 h, (d) 72 h of unchallenged biofilm, (e) biofilm challenged with gentamicin, (f) AgNO3. White arrows indicate affected cells due to gentamycin (8192 μg/ml) and silver nitrate (6.4 mM).
Biofilm formation enhances resistance in A. haemolyticus MMC 8 against antibiotics and metals salts: A. haemolyticus MMC 8 sensitive to four antibiotics (gentamycin, tobramycin, polymyxin B, rifamficin) and two metal salts (AgNO3, HgCl2) became resistant to all after forming biofilm. This resistance increased with age of biofilm. The minimum antibiotic concentration required for inhibiting biofilm growth was about 1000 fold higher for 24 and 48 h biofilm and 4000 fold for 72 h old biofilm (Table, Fig. 1b-h). This was observed for all antibiotics. Further, on spot inoculating the well containing MBIC a few viable cells in the range of 105 - 106 cfu/ml survived the antibiotic and metal treatment. Presence of this unaffected cell population was confirmed by SEM analysis (Fig. 2 d, e). This indicated presence of metabolically inactive cells known as persister cells thriving even at high concentration of antibiotic and metals salts.
Discussion
In the present study, it was observed that A. haemolyticus MMC 8 produced and stabilized its biofilm over a period of 72 h. Concurrent with our results, Nancharaiah et al20 have reported increasing biofilm formation of P. putida over a period of time.
It is important to note that one day old biofilm formed by A. haemolyticus MMC 8 was resistant to almost all the antibiotics and metal salts. However, three days old biofilm required relatively much higher concentrations of antibiotics and metal salts for inhibition. Earlier reports on P. aeruginosa have demonstrated five days old biofilm susceptible, while seven days old biofilm resistant towards tobramycin and pipercillin21. This indicates the severity of the infection caused by biofilm of A. haemolyticus and the necessity to treat at the earliest.
It was we observed that 32 - 4000 and 4 - 256 fold increase in antibiotic and metal salt concentration, respectively was required to inhibit biofilm even after 24 h of exposure as compared to planktonic counterparts. Earlier studies on S. aureus, E.coli and P. aeruginosa have shown it to be 2–64 times more resistant in biofilm form than in logarithmically growing planktonic cells against antibiotics and metal ions after 24 h of exposure22. Our results are concurrent with those of Teitzel and Parsek23 who reported P. aeruginosa biofilm to be more resistant than planktonic free floating cells.
Biofilm formed by A. haemolyticus MMC 8 was found resistant to amoxicillin/calvulanic acid and positive for β-lactamse production. Prior studies on β-lactamse positive Klebsiella pneumoniae have demonstrated increased rate of antibiotic degradation than penetration in biofilm24. Similar mechanism may be exhibited by A. haemolyticus MMC 8. In our earlier studies on A. haemolyticus MMC 8 was found to produce exopolymeric substances (EPS) which increased with incubation period (unpublished data). EPS overproducing mutant of P. aeruginosa was found to produce thick biofilm which posed major problem in antibiotic diffusion leading to elevated resistance25. Moreover, EPS is known to be composed of charged polymers such as polypeptides26, nucleic acids27 and polysaccharides28. These charged molecules make EPS act as ionic resin, thereby reducing the number of antibiotic molecules and metal ions entering in the interiors of biofilm23. This may be the reason for elevated resistance against aminoglycosides and metals.
In the course of biofilm formation, physiological gradients are produced within the biofilm decreasing growth rate of bacteria in small areas of biofilm29 leading to development of subpopulation which remains unaffected by antibiotic and metal salts and known as persister cells30. Persister cells exhibit a typical phenotype of not growing in presence of high concentrations of antimicrobial agents or rather do not die and hence contribute to resistance31. In the present study, a small population (105-106 cfu/ml) of cells was seen growing on MHA plates after removing antibiotic and metal salt stress. This indicated development of persister cells within biofilm formed by A. haemolyticus MMC 8 leading to an increase in resistance. Our results are concurrent with those of Singh et al32 who have reported role of persister cells in antibiotic resistance in biofilm of S. aureus. Presence of metal ion resistant persister cell population has also been reported in E.coli33. Previously, higher concentrations of antibiotics have also been reported to be ineffective in eradicating biofilm of P. aeruginosa34.
In conclusion, the study describes antibiotic and metal resistant profile of planktonic and biofilm of A. haemolyticus MMC 8. Increasing complexity of biofilm, production of exopolymeric substances and presence of persister cells may be the reason leading to elevated resistance in biofilm formed over a period of 72 h.
Acknowledgment
Authors acknowledge financial assistance to UPE Phase I and II awarded to University of Pune. The study was partly supported by a major research project awarded by the University Grants Commission, New Delhi, India, to the last author (BAC). Authors acknowledge the technical assistance provided by the scanning electron microscopy facility of Department of Physics, University of Pune.
References
- A study on nosocomial pathogens in ICU with special reference to multiresistant Acinetobacter baumannii harbouring multiple plasmids. Indian J Med Res. 2008;128:178-87.
- [Google Scholar]
- An increasing threat in hospitals: multidrug resistant Acinetobacter baumannii. Nat Rev Microbiol. 2007;5:939-51.
- [Google Scholar]
- Species distribution and physiological characterization of Acinetobacter genospecies isolated from healthy human skin of tribal population of India. Indian J Med Microbiol. 2007;25:336-45.
- [Google Scholar]
- Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45:493-6.
- [Google Scholar]
- Occurrence of species from Acinetobacter genus in clinical material and other sources. Med Dosw Mikrobiol. 1992;44:41-8.
- [Google Scholar]
- Antimicrobial susceptibility of Acinetobacter strains identified by DNA-DNA hybridization. Acta Pathol Microbial Immunol Scand. 1990;98:320-6.
- [Google Scholar]
- Molecular epidemiology of imipenem-resistant Acinetobacter haemolyticus and Acinetobacter baumannii isolates carrying plasmid-mediated OXA-40 from a portuguese hospital. Antimicrob Agents Chemother. 2007;51:3465-6.
- [Google Scholar]
- Antimicrobial drug susceptibility of clinical isolates of Acinetobacter species (A. baumannii, A. haemolyticus, genospecies 3 and genospecies 6) Antimcrob Agents Chemother. 1989;33:1617-9.
- [Google Scholar]
- Shiga toxin 2-producing Acinetobacter haemolyticus associated with a case of bloody diarrhea. J Clin Microbiol. 2006;44:3838-41.
- [Google Scholar]
- Mechanisms of antibiotic resistance in bacterial biofilms. Int J Med Microbiol. 2002;292:107-13.
- [Google Scholar]
- Comparative assessment of antibiotic susceptibility of coagulase negative staphylococcal biofilm versus planktonic culture as assessed by bacterial enumeration or rapid XTT colorimetry. J Antimicrob Chemother. 2005;56:331-6.
- [Google Scholar]
- Increased antibiotic resistance of Escherichia coli mature biofilm. App Env Microbiol. 2009;75:4093-100.
- [Google Scholar]
- Biofilms and planktonic cells of Pseudomonas aeruginosa have similar resistance to killing by antimicrobials. J Bacteriol. 2001;183:6746-51.
- [Google Scholar]
- Antimicrobial susceptibility of Staphylococcus aureus and Staphylococcus epidermidis biofilms isolated from infected total hip arthroplasty cases. J Orthop Sci. 2006;11:46-50.
- [Google Scholar]
- Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents. 2010;35:322-32.
- [Google Scholar]
- Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standards. In: CLSI Document M07-A9 (9th ed). Wayne, PA, USA: CLSI; 2012.
- [Google Scholar]
- Plasmid mediated silver resistance in Acinetobacter baumannii BL54. BioMetals. 1994;7:49-56.
- [Google Scholar]
- Characterization of eDNA from the clinical strain Acinetobacter baumannii AIIMS 7 and its role in biofilm formation. Sci World J 2012 doi:10.1100/2012/973436
- [Google Scholar]
- Compatibility of the green fluorescent proteins and a general nucleic acid stain for quantitative description of a Pseudomonas putida biofilm. J Microbiol Methods. 2005;60:179-87.
- [Google Scholar]
- Enhanced activity of combination of tobramycin and piperacillin for eradication of sessile biofilm cells of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1990;34:1666-71.
- [Google Scholar]
- Heavy metal resistance of biofilm and planktonic Pseudomonas aeruginosa. App Environ Microbiol. 2003;69:2313-20.
- [Google Scholar]
- Role of antibiotic penetration limitation in Klebsiella pneumoniae biofilm resistant to ampicillin and ciprofloxacin. Antimicrob Agents Chemother. 2000;44:1818-24.
- [Google Scholar]
- Alginate overproduction affects Pseudomonas aeruginosa biofilm structure and function. J Bacteriol. 2001;183:5395-401.
- [Google Scholar]
- The biofilm matrix-an immobilized but dynamic microbial environment. Trends Microbiol. 2009;9:222-7.
- [Google Scholar]
- Extracellular DNA required for bacterial biofilm formation. Science. 2002;295:1487.
- [Google Scholar]
- Alginate is not a significant component of the extracellular polysaccharide matrix of PA14 and PAO1 Pseudomonas aeruginosa biofilms. Proc Natl Acad Sci USA. 2003;100:7907-12.
- [Google Scholar]
- Spatial patterns of DNA replication, protein synthesis and oxygen concentration within bacterial biofilms reveal diverse physiological states. J Bacteriol. 2007;189:4223-33.
- [Google Scholar]
- Antimicrobial resistance of Pseudomonas aeruginosa biofilms. Microb Infect. 2003;5:1213-9.
- [Google Scholar]
- Persister cells and tolerance to antimicrobials. FEMS Microbiol Lett. 2004;230:13-8.
- [Google Scholar]
- Role of persisters and small colony variants in antibiotic resistance of planktonic and biofilm associated Staphylococcus aureus: an in vitro study. J Med Microbiol. 2009;58:1067-73.
- [Google Scholar]
- Persister cells mediate tolerance to metal oxyanions in Escherichia coli. Microbiology. 2009;151:3181-95.
- [Google Scholar]
- A dose response study of antibiotic in Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother. 2000;44:640-6.
- [Google Scholar]






