Translate this page into:
The grand challenge of regulating health foods in India
For correspondence: Dr Sesikeran Boindala, Res Apt #105, Office Apt #305, Sumeda Apartments, Street #2, Kakathiya Nagar, Habsiguda, Hyderabad 500 007, Telangana, India e-mail: sesikeran@gmail.com
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Food is the primary source of nutrients to keep us nourished and healthy. Poor and unhealthy diets implicated with the increase of several non-communicable diseases (NCDs) require a food-based approach to reduce the ongoing rise. Traditional knowledge and science behind food-related health benefits became evident in the last three decades. Active ingredients, bioactive molecules and conventionally used herbs were clinically researched and proven to have beneficial outcomes. In the Indian scenario, the multiplicity of food products, including medicinal type formats, such as health supplements, containing plant, herbs or novel ingredients, brings in a new complexity to regulations. Several of these ingredients are pharmacologically active substances and could overlap with drug regulations. The data generated on the nutritional and health benefit of a supplement should be reproducible, outcomes measurable and disease risk reduction shown by well-designed research studies. Regulatory challenges occur at several levels, namely, harmonization of law, fair trade practice, population exposures to chemicals and contaminants, food borne illness, rise in NCD's, novel ingredients, new technologies and a legacy of regulatory practice. While regulatory and legal challenges will always exist, reliance on the role of scientific research in the regulatory context becomes significant.
Keywords
Food safety
health supplements
India
nutraceuticals
regulation
Introduction
In the current era, modern lifestyles built around automation, reduced physical activity and other socio-economic factors have contributed to the rising trend in non-communicable diseases (NCDs). Consumers now recognize the need to improve their diets with additional nutrition and healthy options. Supplements are increasingly being used to maintain an active lifestyle and to address specific health concerns. Advice by regulatory agencies to improve micronutrient intakes through a well-balanced diet may be inadequate1. In addition to fortified foods, alternative options of non-prescribed vitamin and mineral (and botanicals) supplement offered in pills and capsules augment nutrient intakes. The emergence of this new food format poses a regulatory challenge to distinguish them from drugs. Several factors make this differentiation possible.
Categorization
Conceptually, two factors guide regulatory jurisprudence - categorization and definitions. Categorization is a facet-based classification whereby all foods are aggregated by their purpose of use or as marketed. The Codex Categorization System2, adopted by India3 categorizes all foods and products into 16 categories in a hierarchical order4.
Foods consumed by the general population and eaten for enjoyment are placed in categories 1-16, except 13.0; foods under 13.0 are marketed to groups that require particular nutrition4. Category 13.0 is further divided into several sub-categories 13.1-13.52, for foods especially prepared and/or formulated 'to satisfy particular dietary requirements which exist because of a particular physical or physiological condition'. Included in these sub-categories are infant foods, infant formulae and foods for special dietary use (FSDU) or foods for special medical purpose2.
Food products are generally marketed in conventional food forms (biscuits, bread, soups, shakes, etc.) including fortified foods, oils, milk, flour, with added vitamins A and D or folic acid or minerals5. Only health supplements (category 13.6), intended to supplement the diet, are offered in the physical forms of pills, capsules, etc. Categorization of foods is a key structural regulatory principle for the differential regulatory treatment of food products and harmonizing them globally.
Definition
Supplements are defined internationally and reveal a high level of harmonization. The Dietary Supplement Health Education Act (DSHEA, 1994)6 is perhaps the first Act that deems supplements to be foods though these are marketed in formats typically associated with medicinal products, namely pills, tablets or capsules. Thereafter, several regulations have emerged17. The legal framework for health supplements provided in the Food Safety and Standards Act8 is based on these pre-existing Acts and regulations.
An extract from Section 228 provides a description of health supplements well harmonized with the international regulations: Health supplement is “a dietary substance, such as vitamins, minerals, proteins, amino acids, enzymes, plant or botanicals or their parts in the form of powder, concentrate or extract in water, alcohol or hydro-alcoholic extract, or substances from animal sources, for use by human beings to supplement the diet by increasing the total dietary intake and whereby such products may be formulated in the form of tablets, capsules, powders, granules, liquids and other dosage forms and is not represented for use as conventional foods. They shall not contain drugs, hormones, steroids or psychotropic substances”4.
In the Association of Southeast Asian Nations (ASEAN), the definition reads 'Health supplements mean any product that is used to supplement a diet and to maintain, enhance and improve the healthy function of a human body and may contain one or more or combinations of nutrients or substances including botanicals, in various forms such as extracts or concentrates, presented in dosage forms'9.
The EU directive defines food supplements as 'food stuffs the purpose of which is to supplement the normal diet and which are concentrated sources of nutrients or other substances with a nutritional or physiological effect, alone or in combination, marketed in dose form, namely forms such as capsules, pastilles, tablets, pills and other similar forms, sachets of powder, ampoules of liquids, drop dispensing bottles, and other similar forms of liquids and powders designed to be taken in measured small unit quantities'1.
In the US, the term 'dietary supplement' means a product (other than tobacco) intended to supplement the diet that bears or contains one or more of the following dietary ingredients; vitamin, mineral, herb or other botanical, amino acid, a dietary substance for use by human, to supplement the diet by increasing the total dietary intake or a concentrate, metabolite, constituent, extract or combination of any ingredient described above and enzymes. It is not represented for use as a conventional food or as a sole item of a meal or the diet6.
Codex7 provides guidelines for vitamin and mineral food supplements. 'Vitamin and mineral food supplements are sources in concentrated forms of those nutrients alone or in combination, marketed in forms such as capsules, tablets, powders, solutions etc., that are designed to be taken in measured small-unit quantities but are not in a conventional food form and whose purpose is to supplement the intake of vitamin and/or minerals for the normal diet'7. The terms health supplements used in ASEAN countries or food supplements in the EU countries and dietary supplements in the US are all placed under food category 13.64 and have the same regulatory meaning. The term 'nutraceuticals' while not recognized in international regulations, to describe a similar food category, is an additional term in the Indian regulations9.
FSDUs are specially prepared for persons with specific dietary needs due to an existing physiological condition or disorder and when normal foods are incapable of meeting their needs. Low- and very low-calorie diets (400 or 800-1200 kcal) are formulated for weight control and provide vitamins and minerals at recommended dietary allowance (RDA) levels10. These foods are presented in conventional food forms (biscuits, shakes and soups) and provide daily dietary needs for energy and macronutrients. Hence, FSDUs are not produced and marketed in the form of tablets, capsules, etc9.
Several regulatory declarations further distinguish health supplements from drugs; these do not provide or possess the properties to diagnose, mitigate or prevent a disease in humans and prohibited from making such claims. Mandatory labelling declarations such as 'HEALTH SUPPLEMENTS' and 'NOT FOR MEDICINAL USE' are required on product labels10.
Safety and efficacy of ingredients
Foods and their components or any substance added to food are subject to safety assessment using a scientific methodology of risk assessment, comprising hazard identification, characterization, exposure assessment and finally risk characterization8. Appropriate regulatory controls are applied taking into account risk assessments done through another methodology namely risk management; e.g., establishing maximum limits and/or a permitted list of foods to which these may be added, and/or label declarations on advisories or warnings, if required. Risk assessment and risk management processes rely on the scientific evidence and relevant data before rulemaking. Both processes are separate and performed by relevant subject matter experts.
Health supplements with combinations of vitamins and/or minerals are a major segment by demand globally. The World Health Organization (WHO) estimates that worldwide, 'more than 2 billion people experience deficiencies in essential vitamins and mineral intakes'11. The maximum amount of vitamins and minerals permitted in health supplements under the Act8 is the RDA, which may imply that intakes above are unsafe. Concerns arise from overconsumption on one hand while also a realization that sub-optimal intakes continue due to an unwillingness to adopt a balanced diet. Codex guidelines7 require maximum amounts of vitamins and minerals in food supplements per daily portion of consumption to be set, taking into account 'upper safe levels of vitamins and minerals established by scientific risk assessment' and considering the 'daily intake from other dietary sources'7.
Several countries including the EU and ASEAN have used a risk management approach required by Codex. Indian regulations will move into a safety-based approach based on a report on safe upper level limits of vitamins/minerals and the setting the maximum limits for health/dietary supplements and nutraceuticals by the Expert Committee of the Indian Council of Medical Research12.
India and China, among several other countries, have a rich traditional practice in the administration of plant and herbal remedies for health and medicinal purposes. While traditional medicine is well recognized and regulated, health supplements are yet to achieve a similar acceptance in the regulatory space particularly for claims. The WHO acknowledges traditional knowledge in its definition 'traditional and complementary medicine is the sum total of the knowledge, skills, and practices based on the theories, beliefs, and experiences indigenous to different cultures, whether explicable or not, used in the maintenance of health as well as in the prevention, diagnosis, improvement or treatment of physical and mental illness'13. While the classical methodology of clinical evaluations and dose-response studies form the core of evaluating safety of substances added to foods (e.g., food additives), a regulatory approach of 'history of safe use'14 and 'qualified presumption of safety' are also relied upon where traditional knowledge exists about the food or food component (botanicals and their extracts), in the country or elsewhere. The Scientific Committee of European Food Safety Authority (EFSA)15 used 'qualified presumption of safety approach for microorganisms in food to propose criteria presuming a botanical or a botanical preparation to be safe'1415.
Nutrition and health claims
Food claims are messages that convert scientific knowledge into consumer benefits and applicable to all foods with a few exceptions. They should be truthful, well supported by scientific substantiation and not mislead the consumer through false and exaggerated language. Globally, the claims framework is well harmonized16171820 (Fig. 1).
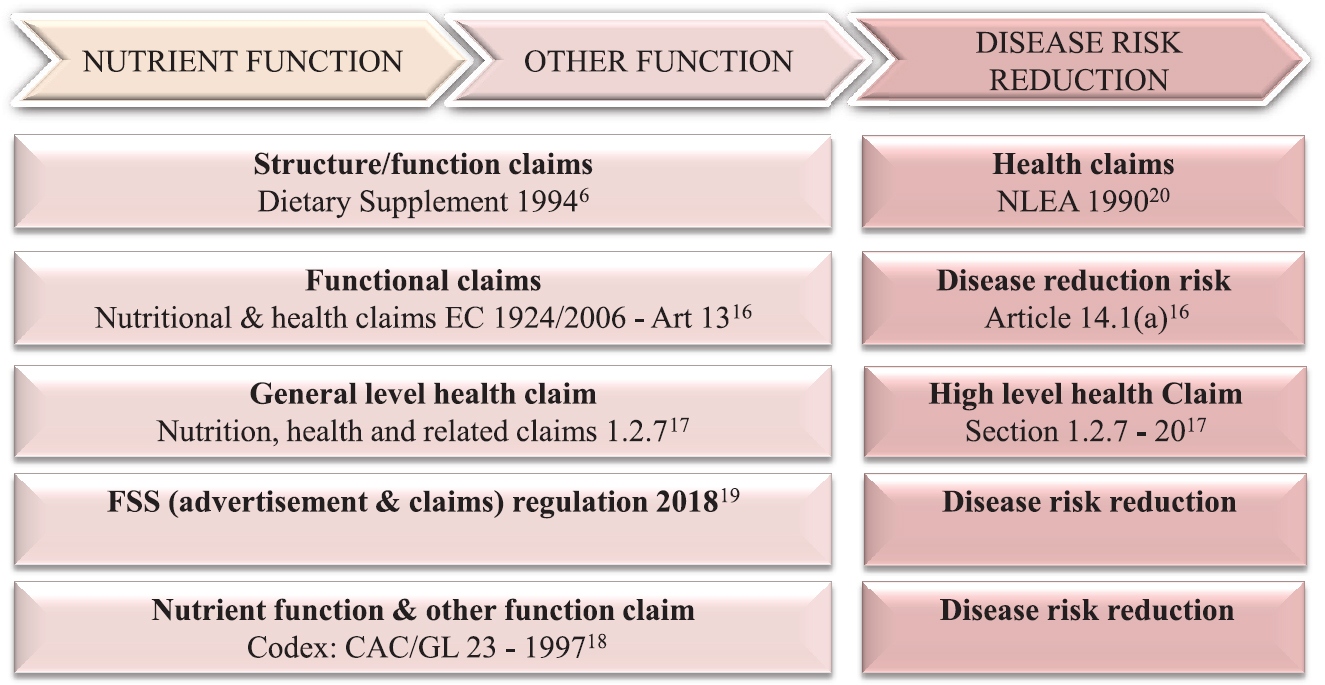
- Harmonization of claims framework. NLEA, Nutrition Labeling and Education Act; FSS, Food Safety and Standards; CAC/GL, Codex Alimentarius Commission/Guidelines.
Broadly, claims may be described as 'functional' (non-disease) and reduction of risk factors (disease related) (Fig. 1). Functional claims comprise nutrient function claims, other function claims18 and structure or function claims620 and the reduction of disease risk claims18. Two Indian regulations1019 on claims address provisions with some overlap.
All claims must be scientifically substantiated to protect the consumer interest and health. Claims may be authorized and listed in regulations, on the strength of existing scientific evidence212223, described as significant scientific agreement (US, Canada); general-accepted scientific evidence of beneficial physiological effect in humans (EU); established food-health relationships based on the totality and weight of evidence (Australia, New Zealand). The need for a further substantiation is generally not required for functional claims (nutrient function or other function) when the level of evidence obtained is acceptable across the scientific community22. Structure or function claims used under the U.S. Food and Drug Administration (FDA)6 must bear a disclaimer 'This statement has not been evaluated by the FDA. This product is not intended to diagnose, treat, cure or prevent any disease'.
Often, the route to verify claims is a systematic review of scientific literature and where required a clinical verification. In case of botanicals and herbal remedies, a systematic review of traditional texts should be considered as equivalent to clinical verification for claim substantiation.
Characterization of food safety systems
Modern food control systems based on risk have replaced traditional frameworks. 'Risk analysis provides a means to strengthen the ability of traditional food safety systems to meet current challenges'24 (Fig. 2). India moved to a risk-based system of food control in 20068.
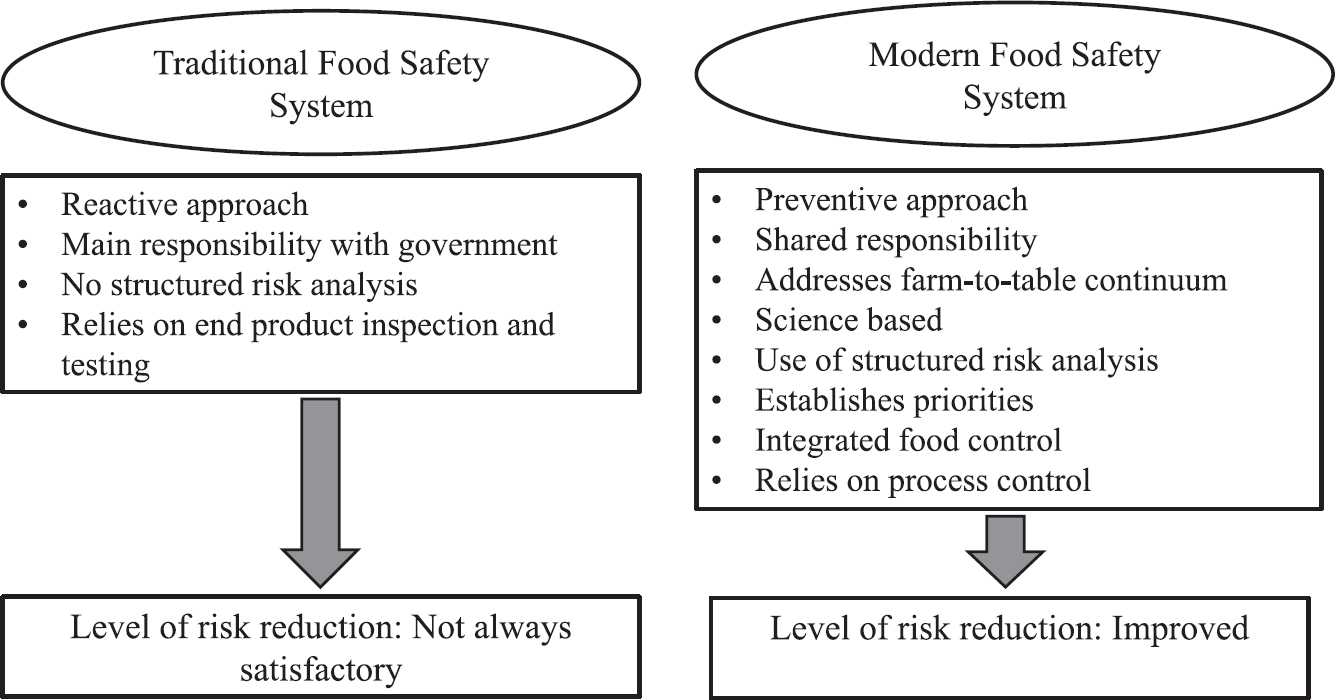
- Characterization of food safety systems. Source: Ref 24.
A regulatory challenge posed by the Act8 is institutionalizing functional competencies required for risk assessment and risk management. Second, for risk-based systems to emerge from an outdated predecessor - the Prevention of Food Adulteration Act 19548, the inherited architecture of 'standards making' - needs complete overhaul and revision. A simplified structural arrangement between regulations is critically required to remove ongoing complexities, arising from overlap and duplicity, poor cross-referencing and contextual misplacements of clauses. An illustrated example is the approval of plant sterols.
Indian regulations approved phytosterols (an ingredient) under two regulations; the first under the heading 3.1 Food Additives3. In another regulation, phytosterols are placed under the heading 'nutraceutical ingredients' (Schedule VIB)10. Plural regulatory treatments across regulations for the same ingredient create complexities and misrepresentation: whether the substance is a food additive, nutraceutical ingredient or novel food? International regulations may use different regulatory instruments to approve phytosterols, but these are made contextually clear to all involved in the standards-setting process25.
Conclusion
Well-established regulatory mechanisms exist for the control of food safety and effective communication of health benefits to the consumer. Regulatory methodologies of risk assessment and risk management provide a scientific basis for effective laws and regulations for public health outcomes. A spinoff from these methodologies is the developmental need of specific skill sets required for regulatory sciences and governance. The Food Safety and Standards Act through its mandates ensures the capability to face the emerging challenges of food safety and health of its people.
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002. Available from: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32002L0046&from=EN
- General standards for food additives. Available from: http://www.fao.org/gsfaonline/docs/CXS_192e.pdf
- 2016. Food Safety and Standards (Food product standards and food additives) Seventh Amendment Regulations. Available from: http://www.fssai.gov.in
- 2017. Good regulatory practice: Health supplements & nutraceuticals: PFNDAI Bulletin. :8. 10-12 Available from: http://www.pfndai.org/Document/BulletIn /2017/03.Mar2017_Bulletin_Web.pdf
- Food Safety and Standards (Fortification of Foods) Regulations 2018
- Public law 103-417; 103rd congress. National Institutes of Health, Office of Dietary Supplements. Available from: https://ods.od.nih.gov/About/DSHEA_Wording.aspx
- Guidelines for vitamin and mineral food supplements: CAC/GL 55-2005
- Food Safety and Standards Act 2006. Available from: http://www.fssai.gov.in
- 2018. Resource Center for Health Supplements and Nutraceuticals, Compliance Guidance. Available from: http://rechan.in/pdf/Compliance_Document.PDF
- Food Safety and Standards (Health supplements, nutraceuticals, foods for special dietary uses, foods for special medical purpose, functional foods, novel foods) Regulations 2016
- Community-based management of severe acute malnutrition: A joint statement by the World Health Organization, the World Food Programme, the United Nations System Standing Committee on Nutrition and the United Nations Children's Fund. WHO; 2007.
- 2017. Policy and economic issues, traditional and complementary medicine policy. Part I. Ch. 5. WHO; :17. Available from: http://www.apps.who.int/medicinedocs/documents/s19582en/s19582en.pdf
- The role of the concept of “history of safe use” in the safety assessment of novel foods and novel food ingredients. Opinion of the Senate Commission on food safety (SKLM) of the German research foundation (DFG) Mol Nutr Food Res. 2011;55:957-63.
- [Google Scholar]
- Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on nutrition and health claims made on foods. Available from: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:404:0009:0025:EN:PDF
- Australia New Zealand Food Standards Code - Standard 1.2.7; Nutrition, health and related claims. Available from: https://www.legislation.gov.au/Details/F2017C01048
- Guidelines for use of nutrition and health claims. CAC/GL 23-1997
- 2018. Draft Notification on Food Safety and Standards (Advertisements and claims) Regulation. Available from: http://www.fssai.gov.in
- Public Law 101-535 101st Congress. Available from: https://www.govinfo.gov/content/pkg/STATUTE-104/pdf/STATUTE-104-Pg2353.pdf
- Recommendations for successful substantiation of new health claims in the European Union. Trends Food Sci Technol. 2018;71:259-63.
- [Google Scholar]
- Preparing dossiers: Strength of the evidence and problems of proof. Proc Nutr Soc. 2012;71:127-40.
- [Google Scholar]
- EU Commission Regulation. EC/432/2012. Available from: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2012:136:0001:0040:EN:PDF
- Food safety risk analysis. Part I. An overview and framework manual (Provisional edition). Rome: Food and Agriculture Organization of the United Nations; 2005.
- 2008. Phytosterols, phytostanols and their esters. World Health Organization; Available from: http://www.fao.org/fileadmin/templates/agns/pdf/jecfa/cta/69/Phytosterols.pdf






