Translate this page into:
Temporal course of late rectal toxicity & impact of intervention in patients undergoing radiation for cervical cancer
For correspondence: Dr Supriya Chopra, Department of Radiation Oncology, ACTREC, Tata Memorial Centre, Homi Bhabha National Institute, Navi Mumbai 410 210, Maharashtra, India e-mail: schopra@actrec.gov.in
-
Received: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
There is limited information available on the temporal course of late stage radiotherapy adverse effects. The present study reports on the temporal course of late toxicities after chemoradiation and brachytherapy.
Methods:
Women with cervical cancer who presented with late toxicity after (chemo) radiation were included in the study. Grade of toxicity (Clinical Toxicity Criteria for Adverse Events version 4.03) and type of intervention were recorded at three-monthly interval for the first year and then six monthly until 24 months. Direct cost for the management of toxicity was calculated. Univariate analysis was performed to understand the impact of various factors on persistence of toxicity.
Results:
Ninety two patients were included in this study. Grades I, II, III and IV toxicities were observed in 50 (54%), 33 (36%), 7 (8%) and 2 (2%) patients, respectively, at first reporting. Patients spent a median of 12 (3-27) months with toxicity. At 12 months, 48/92 (52.2%) patients had a complete resolution of toxicity, whereas 27/92 (29.3%) patients had low grade (I-II) persistent toxicity. Only 6/92 (6.5%) patients who had grade III−IV toxicity had resolution to a lower grade. Four (4.3%) patients died due to toxicity. At 24 months, 9 (10%) patients continued to have grade ≥ III toxicity. On an average, 7 (2-24) interventions were required for the clinical management of late toxicity and median direct cost incurred was ₹ 50,625 (1,125-303,750).
Interpretation & conclusions:
In this study late radiation toxicity resolved within 12 months in more than half of patients. However, others are likely to have had persistent lower grade toxicity or progression to higher grade. Structured strategies are hence needed for the effective management of late toxicities.
Keywords
Cervical cancer
late rectal toxicity
proctitis
radiotherapy
rectal ulcer
temporal course
Cervical cancer is the fourth common cancer worldwide1. Chemoradiotherapy (CRT) and high-dose rate brachytherapy (BT) is the recommended treatment for women with early and locally advanced cervical cancer and is associated with good local control and disease-free survival even in the advanced stage23456. More recently, data have become available for dose escalation through BT7. In recent years, increased emphasis is being laid to implement image-guided dose escalated BT with an aim to deliver >85 Gy to high risk clinical target volume (HRCTV). More recent data also suggest an increase in the rectal and bladder late effects at 2 cm3 doses >65 and >80 Gy89. Hence, recommendations within ongoing clinical protocols have been revised to preferably reduce bladder and recto-sigmoid 2 cm3 doses to <80 and <65 Gy respectively to minimize long-term morbidity7. This goal is often achieved in a vast majority of patients (good and poor responders) within the setting of magnetic resonance imaging-based image-guided intracavitary and/or interstitial BT7. However, the above aim is challenging in patients who have poor response or unfavourable anatomy or the centre lacks access to infrastructure or skill needed for combined intracavitary or interstitial BT. Therefore, this clinical aim outside the setting of image based BT may be associated with higher organs at risk doses in a proportion of the patients. An audit of patients receiving point A/CT based intracavitary-interstitial BT from our institution has reported a cumulative incidence of >19 per cent of grade ≥II late toxicity (three year rate of grade II, III and IV rectal toxicity of 14.4, 4.4 and 0.3%, respectively)10. While the lower grade toxicities may resolve over a period of time, higher grade late toxicity may be associated with need for additional interventions for symptom control in long-term survivors. The published clinical studies that report outcome of CRT and BT have suboptimal documentation of late events with little or no information on temporal course of events23456. Lack of structured information on the temporal course of late radiation toxicity and its response to intervention limit patients and physicians from having an informed evidence-based discussion regarding the expected duration of toxicity and possible burden on the patient following full course treatment. Hence, this present observational study was undertaken to understand and report temporal course of late toxicity in patients with cervical cancer treated with CRT and BT.
Material & Methods
Patient population: After clearance from the Institutional Ethics Committee of Tata Memorial Centre, Tata Memorial Hospital, Navi Mumbai, Maharashtra, India, eligible patients were identified from gynaecological cancers clinical follow up database, radiation oncology inpatient and institutional endoscopy database. The study inclusion necessitated primary diagnosis of cervical cancer and treatment with either definitive or adjuvant external-beam radiation therapy with or without chemotherapy and BT. Patients who presented with late rectal and bladder toxicities (>90 days after treatment completion) between January 2014 and June 2017 were included in this study. The time interval between the completion of treatment and first appearance of late toxicity was noted. As co-existence of disease relapse could interfere with the study of the temporal course of late radiation toxicity; those patients who had a relapse were excluded. Furthermore, patients developing late toxicity after pelvic re-irradiation were excluded.
Late toxicity management policies: The details of symptoms of the study cohort were obtained from the hospital electronic medical records, endoscopy online records, online prescription and admission modules. As per the general management algorithm in patients with late rectal or bladder toxicity, following pelvic radiation, if a patient presented with blood in stools or urine during a routine follow up visit, a blood test was carried out to check for anaemia secondary to blood loss and also to indirectly understand the duration of blood loss. For mild-to-moderate symptoms, haematinics, stool softeners and dietary modification were prescribed for 2-3 wk. If a clinically significant drop in haemoglobin was further noted or per rectal bleeding was persistent after initial clinical care then further investigations such as endoscopy, blood transfusion and endoscopic coagulation were considered. For patients whose symptoms did not resolve after these interventions, either a repeat session of endoscopic coagulation or referral for hyperbaric oxygen therapy (HBOT) were considered. If a patient, however, presented with rectal or anal ulcer that was identified on endoscopy then steroid enemas and HBOT were prescribed after the endoscopy results were available. In patients with refractory rectal symptoms, diversion stoma was considered.
In patients with mild bladder symptoms, patients were advised to increase the fluid intake along with medications given for urinary irritation symptoms. However, if bladder symptoms such as haematuria persisted, cold saline irrigation was initiated. Supplementation with hematanics and transfusion was performed, focal coagulation of bleeding telangiectatic spots was considered when feasible and HBOT was considered if symptoms were unresolved. In refractory cases, super-selective angio-embolization was considered; however, if symptoms remained unresolved then cystectomy was considered.
Data collection: For patients presenting with late radiation toxicity, information on Karnofsky’s performance status at diagnosis, haemoglobin and albumin levels, pre-existing comorbidities (such as diabetes and hypertension) and history of smoking were obtained. The treatment details such as external radiation technique and dose, use of concurrent chemotherapy and BT doses were obtained. This information was used to compute equivalent doses in 2 Gy (EQD2) to point A or HRCTV using EQD2 calculator worksheets11. Information regarding cumulative rectal and bladder doses to 2 cm3 volume was also calculated. The tumour dose and normal tissue calculations assumed α/β=10 and 3, respectively.
For the purpose of this study, records were reviewed to look for the following symptoms: bleeding per rectum, diarrhoea, pain while defecation, tenesmus, and incomplete evacuation, passing of blood in urine or stools, urinary urgency or frequency and history of faecal material or blood through vaginal orifice. Endoscopic assessments and abdomino-pelvic imaging records (ultrasound and computerised tomography imaging) were evaluated for any reference for rectal, bladder, sigmoid or bowel late effects. Records were also evaluated to monitor complete blood counts and history of any transfusions. Intervention performed during endoscopy or cystoscopy, i.e., argon plasma coagulation (APC) (with reference to area coagulated and multiplicity of procedure) clot evacuation laser or cautery coagulation were recorded. Surgical records were also assessed to note if any diversion procedures or super-selective angio-embolization procedures were needed. Note was also made if the patient was referred to another hospital for HBOT. For patients who required in patient care, number of days needed for inpatient care was also recorded. On the basis of information available on the electronic medical records system and clinical database grade of toxicity was allocated using Clinical Toxicity Criteria for Adverse Events (CTCAE) version 4.03. Toxicity grade was allocated at the first presentation of late event and subsequently at each three monthly follow up for total follow up duration. When a patient presented with two different grades of toxicity for different pelvic organ systems then toxicity of organ system with maximum grade was noted, e.g., if a patient presented with grade III rectal toxicity and grade II bladder toxicity then for purpose of the study the worst grade was recorded as it is likely to have maximum impact on the patient.
As the indirect costs associated with the management of toxicity could be complex, only direct procedural, admission and medicine costs for the management of toxicity were calculated. Hospital billing modules were also used to calculate the direct costs of interventions used for the management of late toxicity.
Statistical analysis: Baseline characteristics of the patient such as age, haemoglobin and albumin were summarized as median and FIGO (International Federation of Gynaecology and Obstetrics) stage was summarized as percentage of patients in each clinical stage. Treatment parameters such as EQD2 (Gy) were summarized as median and categorized above and below median dose to point A for the purpose of statistical analysis. Similarly, rectal 2 cm3/max doses were categorized across known thresholds for late rectal toxicity (75 Gy EQD2). Chemotherapy compliance was reported as a proportion of patients receiving ≥4 cycles. CTCAE grades were summarized as proportion of patients with grade I, II, III, IV for each follow up at three monthly intervals. Time trends and impact of intervention were assessed as a change in the CTCAE grade over each follow up. Temporal change in each grade was assessed over the follow up period and response to each of interventions (such as APC and HBOT) was summarized as a proportional change in the grade of toxicity. Finally, the proportion of patients with resolving toxicity, low grade persistent and worsening toxicity was calculated along with the time spent with toxicity. Impact of known predictive factors on late and persistent toxicity (such as age, albumin, pre-existing co-morbidities, total dose, organ at risk and dose and grade at presentation) were assessed using univariate and multivariate analysis. SPSS software version 21 (SPSS, Chicago, IL, USA) was used for the statistical analysis.
Results
A total of 92 patients were evaluated in our institutional outpatient clinic with late radiation toxicity within the three-year study period. The median age of the study cohort was 53 yr (29-84 yr). Baseline diabetes and hypertension or both were recorded in 10.2, 12.5 and 2.2 per cent patients, respectively. These cohorts of women were treated with a combination of external radiation and BT. Broadly, the treatment policies during the study period involved treatment with pelvic external radiation to a dose of 40-50 Gy/20-25 fractions over 4-5 wk with the choice of technique and dose determined by the clinical stage or a clinical protocol. All patients were evaluated for concurrent doses of weekly cisplatin. The planning aim was to deliver a dose equivalent of 80-84 Gy to point A or HRCTV. The details of patients’ baseline characteristics and treatment are summarized in Table I. The median follow up period was 31 months (10-144 months) from completion of all treatment. Median EQD2 to point A (or HRCTV as applicable) was 80 Gy (62-99 Gy). Within the study cohort, 66 per cent patients received rectal 2 cm3/max doses >75 Gy EQD2 Gy3. Overall, 94 per cent (86/92) patients received concurrent chemotherapy of which 74 per cent patients received ≥4 cycles of weekly cisplatin 40 mg/m2.
| Variables | Frequency (%) |
|---|---|
| Age (yr) | 53 (29-84) |
| FIGO stage | |
| lB | 1 (1.1) |
| llA | 2 (2.2) |
| llB | 25 (27.2) |
| lllA | 11 (12) |
| lllB | 33 (35.9) |
| lVA | 9 (9.8) |
| Post-surgery | 11 (11.5) |
| Histology | |
| Squamous cell carcinoma | 76 (82.6) |
| Adenocarcinoma | 14 (15.2) |
| Adenosquamous carcinoma | 2 (2.2) |
| Comorbidities | |
| Diabetes mellitus | 9 (10.2) |
| Hypertension | 11 (12.5) |
| Both diabetes and hypertension | 2 (2.2) |
| HIV | 3 (3.4) |
| Tuberculosis (history) | 3 (3.4) |
| EBRT dose | |
| 45-46 Gy/23-25# | 50 (54.3) |
| 50-50.4 Gy/25-28# | 42 (45.7) |
| EBRT technique | |
| AP-PA parallel opposed | 5 (5.4) |
| 4 field box | 27 (29.3) |
| 3DCRT | 24 (26.1) |
| IG-IMRT | 20 (21.7) |
| Technique not specified | 16 (17.4) |
| Brachytherapy | |
| Intracavitary | 59 (64.1) |
| Intracavitary with interstitial | 4 (4.3) |
| Interstitial | 16 (17.4) |
| Technique not specified | 13 (15.2) |
| EQD2 to point A/Target | |
| <80 Gy | 44 (47.2) |
| >80 Gy | 46 (50) |
| Not known | 2 (2.3) |
| EQD2 rectum 2 cm3 | |
| <75 Gy | 30 (32.6) |
| >75 Gy | 61 (66.3) |
| Not known | 1 (1.1) |
| Chemotherapy - cisplatin | |
| Yes | 86 (93.54) |
| No | 5 (5.4) |
| Not known | 1 (1.1) |
EBRT, external-beam radiation therapy; 3DCRT: Three- dimensional chemo-radiation; IG-IMRT, image-guided intensity-modulated radiation therapy; FIGO, International Federation of Gynecology and Obstetrics
Late toxicity: The median time to late toxicity was 12 months (3-11 months) after completing treatment. At the time of clinical presentation with late toxicity, grades I, II, III and IV toxicity were reported in 54, 36, 7.7 and 2.3 per cent patients, respectively. Most of the patients presented with bleeding per rectum as a major complaint. All patients were offered treatment on the basis of severity of their symptoms as described under methods. The need for interventions across various grades at different time points is summarized in Table II. It is noteworthy that after three months of first intervention, only five and nine per cent of those with grade I and II toxicity had complete or partial relief of symptoms and only 3.3 per cent with grade III-IV responded to therapeutic management. After the second intervention at six months follow up, 38 per cent patients became symptom free (grade 0). However, 33.7 and 20.7 per cent persisted to have grade I and II toxicity. The incidence of grade III-IV toxicity remained stable over the first six months. Although the overall symptom burden seemed to decrease at 9-12 month time period with 42 per cent patients becoming symptom free, there was an increase in proportion of patients with grade III-V toxicity at nine-month time period (>8%), while in other patient’s toxicity persisted at a lower grade. At nine months from first documentation of late toxicity, a total of three patients died (grade V toxicity). One patient died because of severe radiation proctitis and associated medical complications at home; other two patients died because of coexisting radiation cystitis, one developed obstructive uropathy and septicaemia while the other patient died at home due to complications related to haematuria.
| CTCAE max grade | Number of patients undergoing intervention for the management of late toxicity | ||||
|---|---|---|---|---|---|
| Three months | Three months | Nine months | 12 months | >18 months | |
| Grade I | 7 | 5 | 1 | 0 | 0 |
| Grade II | 0 | 27 | 13 | 2 | 0 |
| Grade III | 0 | 5 | 7 | 8 | 2 |
| Grade V | 0 | 0 | 1 | 2 | 1 |
| Total undergoing intervention (%) | 7 (7.6) | 37 (40.2) | 22 (23.9) | 12 (13.4) | 3 (8.1) |
| Total attending follow up | 92 | 92 | 92 | 89 | 37 |
CTCAE, clinical toxicity criteria for adverse event
At 12 months, proportion of patients with grade III-IV toxicity increased to 14.1 per cent (from baseline proportion of 9%) and one patient died because of toxicity (1.1%). At 24 months, 7.6 per cent patients had unresolved grade III toxicity and 4.3 per cent patients had death due to toxicity. Close to one third patients continued to have persistent grade I-II toxicity. On analyzing time spent with toxicity, an average patient spent a median of 12 months in toxicity (3-27 months) with 86 per cent patients spending greater than six months with late toxicity.
Impact of medical or surgical therapeutic interventions: Within this observational cohort, patients underwent multiple interventions (medical, surgical or endoscopic). The median number of interventions was seven (2-24). The number of interventions used at different time points as a function of baseline grade of toxicity is summarized in Table III. Overall 40/92 patients (43.4%) required blood transfusions that lead to transient changes in allocation of the toxicity grade (grade III). A median of three (1-10) transfusions were needed during the follow up period. In this study cohort, 66 per cent patients underwent APC (Argon plasma coagulation) with 34 per cent patients needing multiple sessions of APC (2-5). In patients wherein APC was not feasible or those who were refractory to APC and other conservative procedures, HBOT was advised. HBOT was recommended to 20 (21.7%) patients. This was recommended at 12-18 months after the diagnosis of toxicity. Upto 40 sessions of HBOT were used in 15/20 patients. Of the patients who were recommended HBOT 18/20 (90%) had either complete resolution of symptoms or persistence of toxicity as a grade I event. Overall the number of interventions per patient and time spent with toxicity (>12 months) increased with increasing grade of toxicity and this correlation was statistically significant (P<0.001 and P<0.01 respectively). None of the patients underwent surgical intervention such as fistula repair or bowel resection for late toxicity.
| Late toxicity status | Frequency (%) |
|---|---|
| Resolved completely | 48 (52.2) |
| Persistent at same Grade I/II | 27 (29.3) |
| Grade III-IV initially persistent at Grade I-II | 6 (6.5) |
| Persistent at same Grade III-IV | 7 (7.6) |
| Evolved to Grade V | 4 (4.3) |
| Total | 92 (100.0) |
In this study cohort, 60 per cent patients required inpatient admissions. The median time spent in hospital was seven days (7-56 days). The median direct cost of interventions and medical support for management of toxicity was comparable to that of primary treatment within our subsidized healthcare set up. The median direct cost of managing late toxicity was ₹50,625 (₹ 1,125-303,750). As expected, patients with higher grade of toxicity incurred higher financial burden (P<0.001). A summary of the evolution and resolution of various grades of toxicity is summarised in Table III and Figure.
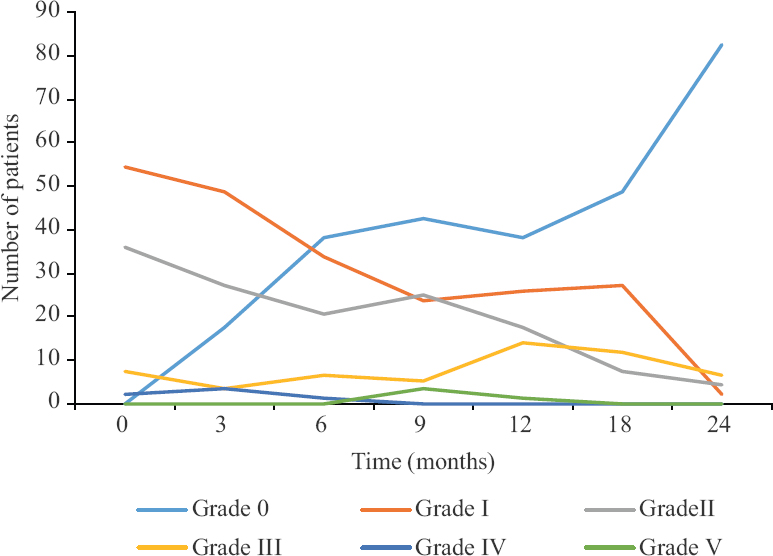
- Temporal evolution and resolution of late rectal toxicity.
Factors impacting temporal course of toxicity: Age at diagnosis of toxicity, pre-existing diabetes mellitus or baseline albumin levels had no statistical impact on resolution of toxicity. (P=0.10, P=0.54 and P=0.19, respectively). No statistically significant correlation was observed between grade ≥ II late toxicity and EQD2 ≥ 80 Gy10 (43.4% vs. 40% P=0.50). Similarly, no correlation was observed between organ at risk (rectum) 2 cm3/max doses (≥75 Gy3) and incidence or resolution of grade ≥II toxicity. Higher grade of toxicity (grade III-IV) at the time of first clinical presentation was associated with lower probability of resolution of toxicity (33% vs. 66% P<0.001). As most of the factors did not correlate with evolution or resolution of toxicity multivariate analysis was not performed.
Discussion
The randomized and prospective phase II studies on CRT and BT capture acute and late toxicity using maximum grade method as recommended by Radiotherapy and Oncology Group (RTOG)234561213 or more recently using CTCAE criteria7. While there may be inherent differences related to the two toxicity scoring methods14 neither of these methods have a mechanism to capture the temporal trends of toxicity. While the available information allows physicians to communicate the expected risk of late effects with the patients, at present there is a gap in knowledge on temporal trends. This often leads to incomplete information both with the physician as well as the patient regarding ‘what to expect’ once a patient is diagnosed with late radiation toxicity. Also, there is limited evidence-based information regarding impact of various medical and endoscopic interventions used for management of late toxicity. The present study investigated not only the temporal trends of toxicity but also focused on comprehensively reporting the need for therapeutic interventions, response and direct financial impact of toxicity on consecutively recruited patients.
It was observed that approximately half of the patients (52%) had complete resolution of symptoms of toxicity and approximately one third of the patients (36%) continued to have low grade persistent symptoms. Close to 12 per cent of patients either had persistent grade III or higher toxicity or further increase in severity of toxicity. Furthermore, this transition time of toxicity (worsening to a higher or resolution to a lower grade) was generally observed at 9-12 month period. Also, patients who had persisting toxicity at 12 months were likely to have persistent symptoms of toxicity or progress to a higher grade. This is also a time period where patients with refractory severe toxicity can begin to develop potentially fatal complications and sequelae. The temporal course of late rectal and bladder effects is also described by Georg et al15. The authors reported that late rectal adverse events after pelvic radiotherapy (bleeding, urgency, incontinence, frequency) persisted for a median of 19±17 months and within three years the late adverse effects resolved in upto 81 per cent of patients with a three and five year prevalence rate of nine and two per cent respectively. However, the time course of resolution of bladder events could be much lower (61%) with 18 and 21 per cent prevalence rates at three and five years respectively16. While the rectal time patterns between these two studies (Georg et al15 and the present study) showed some similarity, lack of patients with bladder symptoms in the present study limits the comparison.
We could not demonstrate correlation between radiation doses and grade of toxicity at the time of first clinical presentation could not be demonstrated, it was observed that the severity of the grade at first diagnosis correlated with lower probability of resolution over 24 months (P<0.001) and higher number of interventions needed for management of late sequelae (P<0.001). The median numbers of interventions per patient for management of toxicity were seven (2-24) suggesting that patients often require multiple sequential or simultaneous interventions for management of toxicity. Overall 66 per cent of the patients underwent APC with some patients undergoing multiple sessions. These observations are similar to other published literature on response after APC in patients with radiation proctitis1617181920.
Out of 92 patients, 20 patients received HBOT. HBOT was used at a median of 12-18 months after diagnosis of toxicity. In the entire study sample, 18/20 (90%) patients had resolution of toxicity to either none or grade I with the use of HBOT. Similar results have been reported by other investigators20. This suggests that HBOT use may be associated with higher response rates. While a recent randomized study19 demonstrated a lack of efficacy of HBOT, our observation of response following HBOT was similar to that reported in earlier publications and systematic literature review21. In our clinical practice HBOT was used only when patients were refractory to APC or had rectal ulcers, or telangiectasia were located in the anal canal wherein APC was not the preferred approach. Whether early intervention with HBOT results in better symptom resolution however, remains to be investigated in future studies.
In the observational period patients underwent multiple interventions. While a formal cost analysis was not performed in this study, the summary of direct costs provided an estimate of the financial burden. Though the present study attempted to capture the temporal trends of late toxicity, the study was not without certain limitations. The follow up period was three years which is relatively short and does not provide complete outcome of patients who still had persistent toxicities. Secondly, the study sample did not have enough representative patients with bladder symptoms. While capturing temporal trends in toxicity in multiple organ systems within the pelvis, it is important to document temporal trends of each of the organ systems separately. Due to insufficient patients with toxicity of more than one organ system that persisted over a period of time, only those with one organ system toxicity with maximum grade per patient were used to document linear trends. Furthermore, due to the inherent limitations of a retrospective study design, we could not comprehensively capture the sequelae associated with the management of toxicity (i.e., ulcer and stricture related to coagulation, procedural injuries, HBOT related ear pain or barotrauma) as some of these procedures were performed in another treatment facility. Furthermore, the spectrum of other toxicities which are more subjective like bloating, sense of incomplete defecation, tenesmus and lower abdominal pain could not be adequately captured during this retrospective analysis. Also the calculation of financial impact could be limited to only direct costs and did not necessarily capture any other costs that patients may have incurred in-between follow up period.
The results of the present study highlight the need to strengthen the late toxicity reporting mechanisms following radiotherapy as the late events are a continuing event for a substantial time period before they resolve or evolve. While maximum incidence method may capture the severity of event, better methods to capture prevalence and persistence of toxicity are needed. Traditionally, a higher-grade event in CTCAE is allocated more importance.
However, a protracted persistence of grade II event is likely to have more impact than a single episode of a grade III event. Modifications from the traditional ways of summarizing toxicity in trials have been attempted and seem to be promising; however, most of these attempts have been made in studying post-treatment toxicity in head and neck malignancies22. More recently within cervical cancer studies a methodological approach has been developed that helps in identifying patients with late, persistent and substantial treatment-related symptoms (LAPERS)23. Furthermore, efforts are underway from our group to develop and report month and severity scoring system (MOSES)24 that provides a better correlation with patients’ quality of life. Designed to be used in combination with CTCAE reporting, it presently does not include low grade persistent symptoms or less frequent occurrence of a severe event or a method for accounting cumulative impact of multiplicity of a symptom complex arising from different organ systems22. Therefore, further research is needed to develop toxicity scoring systems that can better represent burden of late morbidity in long-term survivors.
Overall, the results of this observational study suggest that late radiation toxicity with a lower grade resolves within 12 months in a vast majority of patients. Toxicity symptoms that persist at the same grade until 9-12 months are likely to progress to a higher grade and are occasionally fatal. Hence, more structured systems and evidence-based recommendations are needed for reporting and managing late radiation toxicities.
Financial support & sponsorship: None.
Conflicts of Interest: None.






