Translate this page into:
Systematic review of drug utilization studies & the use of the drug classification system in the WHO-SEARO Region
Reprint requests: Dr Nilima A. Kshirsagar, National Chair Clinical Pharmacology (ICMR), National Institute for Research in Reproductive Health (ICMR), J.M. Street, Parel, Mumbai 400 012, Maharashtra, India e-mail: kshirsagarna@yahoo.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Information available on drug consumption is inadequate in most low and middle income countries. This systematic review was conducted to analyse published work on drug utilization research/studies (DUR) in the SEARO region of WHO for study objectives, methodology, results and recommendations and to identify the need for improving DUR and the use of the ATC/DDD system.
Methods:
A literature search for DUR was carried out in biomedical databases (PubMed, Scirus, Scopus and Google Scholar) up to May 2012. Publications were selected if those were in the English language, describing DUR or prescription practices, and study conducted in the WHO-SEARO countries.
Results:
A total of 318 publications were included in the review. Of these, 67 per cent were from India and 13 per cent were from Thailand. Majority of the publications were hospital based; only 16 per cent were community based. The ATC/DDD system was used in only 20 per cent of the publications, of which 73 per cent publications used DDD indicators. Several publications focused on antibiotics (31%). Publications that recommended the need for a policy or intervention to improve prescription practices/rational drug use amounted to 35 per cent.
Interpretation & conclusions:
Drug utilization studies using ATC/DDD system need to be promoted and carried out on an ongoing basis. DUR is important for rational use of drugs. Its relevance to policy making and resource allocation needs to be emphasized.
Keywords
Anatomical therapeutic chemical (ATC) classification
ATC/DDD
defined daily dose (DDD)
drug use
prescription monitoring
Pioneering work in drug utilization research carried out in Europe during 1966-1967 analyzed the differences in antibiotic use in six European countries1. It led to formation of the WHO European Drug Utilization Research Group (DURG) in 19692. Drug utilization Research (DUR), which includes “the marketing, distribution, prescription and use of drugs in a society, with special emphasis on the resulting medical, social and economic consequences”, was encouraged23. The Anatomical, Therapeutic, Chemical (ATC) classification system provides global standard for classifying drugs. A technical unit of measurement called the Defined Daily Dose (DDD) is defined as “the assumed average maintenance dose per day for a drug used for its main indication in adults”. The ATC/DDD system serves as tool for DUR and allows comparison of drug consumption statistics at international and other levels of healthcare2. As interest expanded beyond the Nordic countries, in 1982 the WHO Collaborating Centre for Drug Statistics Methodology was established4, with close integration of international drug utilization studies and supporting WHO's initiatives for achieving universal access to essential drugs and the rational use of drugs, particularly in middle or low income countries. Access to standardized and validated information on drug use is essential for assessing patterns of drug utilization, identification of problems, access and educational or other interventions and monitoring the outcomes of interventions for the rational use of drugs45. DUR studies are important for policy making at national level as well as for individual patient management. But, in most of the middle or low income countries the availability of information on drug consumption is inadequate. This information is often lacking on even the broadest measures of drug use like the overall volume of use and total spending on drugs4.
Hence, the present systematic review was conducted to analyse drug utilization studies conducted in the SEARO Region of WHO. It assesses the objectives, types of studies, methods used, drugs and healthcare settings covered and interventions investigated to improve prescription practices.
Material & Methods
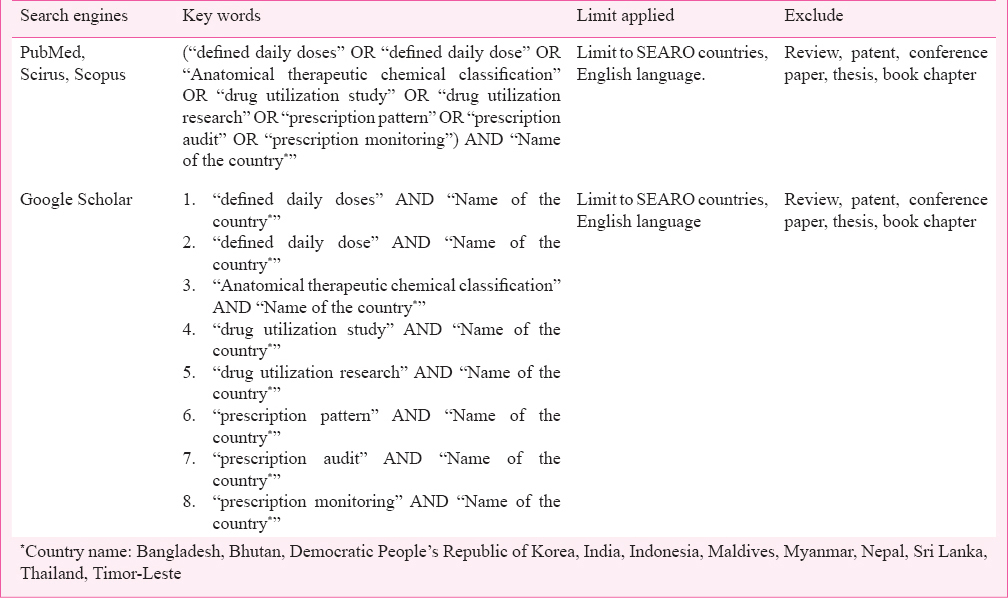
Search strategy: The inclusion criteria were, published articles, which are (i) in the English language, (ii) describing drug utilization studies, (iii) conducted in WHO-SEARO countries, and (iv) providing information about drug use or prescription practices or drug consumption or describing interventions to improve drug prescription practices, thereby improving rational drug use. The literature search was conducted till May 2012, and three major databases namely, PubMed, Scirus (ceased since 2014) and Scopus (since their inception to May 2012) were searched. The recent PRISMA guidelines and Cochrane handbook for systematic reviews provided a framework for the reporting structure of this systematic review67. The search was focused on retrieving publications on drug utilization research and the use of the WHO ATC/DDD code in WHO-SEARO countries. Searches performed with combinations of key words are shown in Table I. The references lists of the retrieved publications were searched manually for other publications that might meet the study eligibility criteria. The authors (whose emails were available in publication or on internet) of the shortlisted publications were contacted by email to seek their feedback on other publications relevant to our research question giving one month time to respond.
Screening process for inclusion: We limited our results to studies on human subjects. Publications identified from search strategy were first subjected to review of title. If it was not possible to decide on inclusion/exclusion on title review, abstracts were reviewed. If the title or abstract left room for doubt, the full text of the publication was obtained and reviewed. At each stage, two independent reviewers (SSB and NAK) assessed the publications against the eligibility criteria. There were no differences in eligibility assessment among the two reviewers.
Assessment of published reports/studies: Publications from SEARO countries obtained from the refined search were reviewed to analyse and capture information on the variables of interest in a spreadsheet. The data extraction in the spreadsheet was done by one of the authors (SSB) and these entries were then checked by another author (NAK).
Results
Our search strategy identified 818 publications from WHO-SEARO countries, of which 43 were repeated within the database. The number of publications identified for further screening for eligibility was 775. A total of 510 publications were eliminated after screening for eligibility criteria. Sixty nine publications were identified as duplicates across the databases (Fig. 1). A total of 196 publications were identified that met the inclusion criteria.
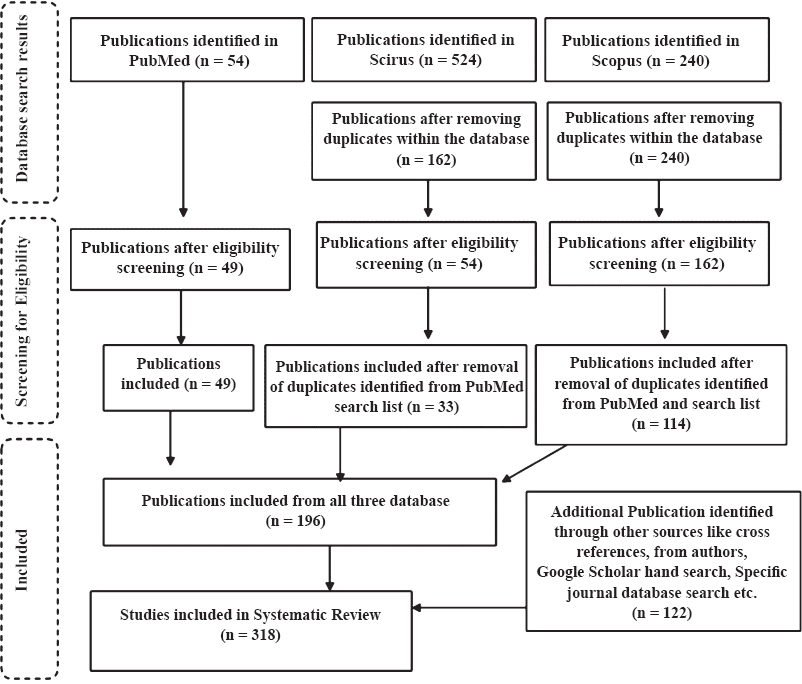
- Search strategy and screening process.
Of the 166 authors, we communicated, 22 responded, of whom five shared additional eight publications to be included in the review. Additional 114 publications were identified through other sources such as cross-references and Google Scholar searches of specific journal databases (Fig. 1). Finally, a total of 318 publications were included. Of the total 318 publications included, only abstract was available for 72 publications. The earliest publication on DUR from SEARO countries, was on antimicrobial agents, published in 1981 by Viswanathan and co-workers8.
Geographic distribution of publications: Of the 318 publications included, 214 studies were from India (67 %), 43 were from Thailand (13%), and 42 from Nepal (13%). No publications from North Korea, Myanmar, the Maldives or Timor-Leste were identified.
Aim and objectives of the studies: The aims of the studies were the following: prescription monitoring/DUR; comparisons between different groups (e.g. different hospital set-ups, private and government hospitals, rural and urban practices, pre and post policy or intervention and year-wise comparison); developing methods; studying the impacts of intervention; and correlation of antibiotic use and antibiotic susceptibility patterns. Table II gives some of interventions used to improve rational drug use. Some of the studies had more than one aim. Eleven per cent of the studies were performed to evaluate the impact of interventions, and the majority of these were from Thailand. Only eight (3%) publications focused on developing method to improve/regulate drug utilization practices and rational drug use (Table III).

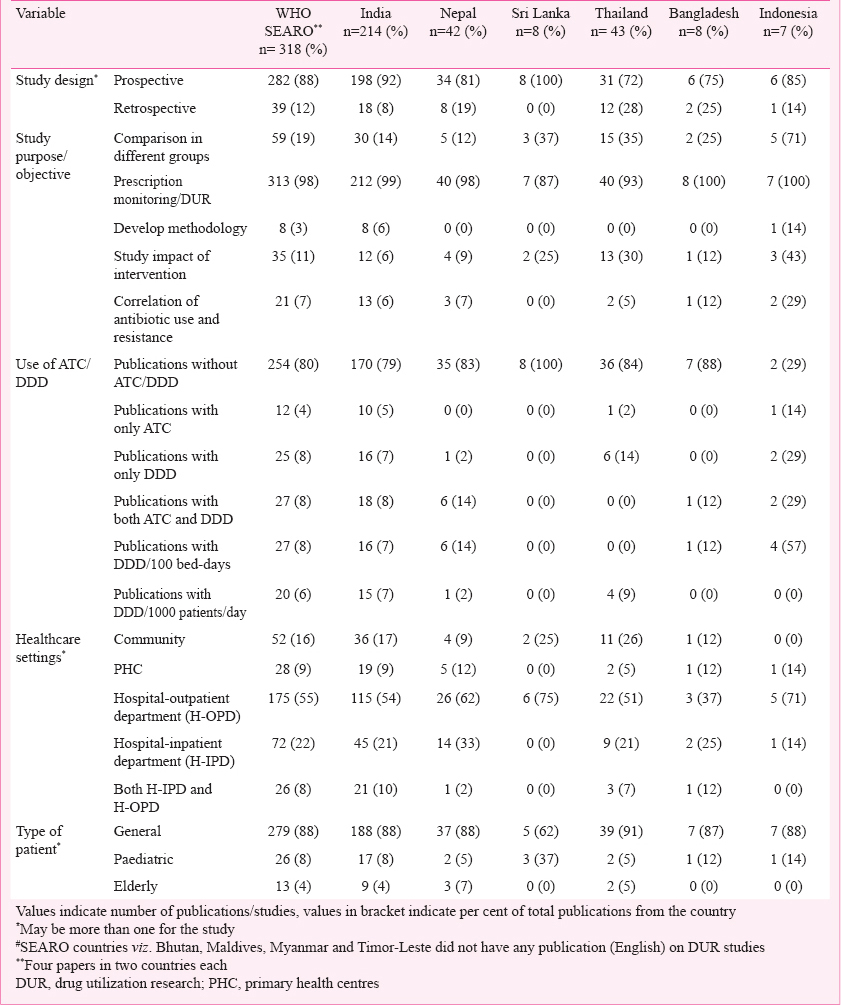
Use of ATC/DDD in DUR: The ATC/DDD system was used in 64 (20%) publications Table III, of which 47 (73%) publications used WHO-DDD indicators, viz. DDD/(100 bed-days) and DDD/(1000 population) Table III. In one study from India, the authors calculated DDD as the total quantity of drug administered divided by the number of patient-days the drug was given and expressed it in grams or milligrams18. None of the publications from Sri Lanka used ATC/DDD indicators. The trend of DUR and use of ATC/DDD methodology over the years in WHO-SEARO countries is given in Fig. 2.
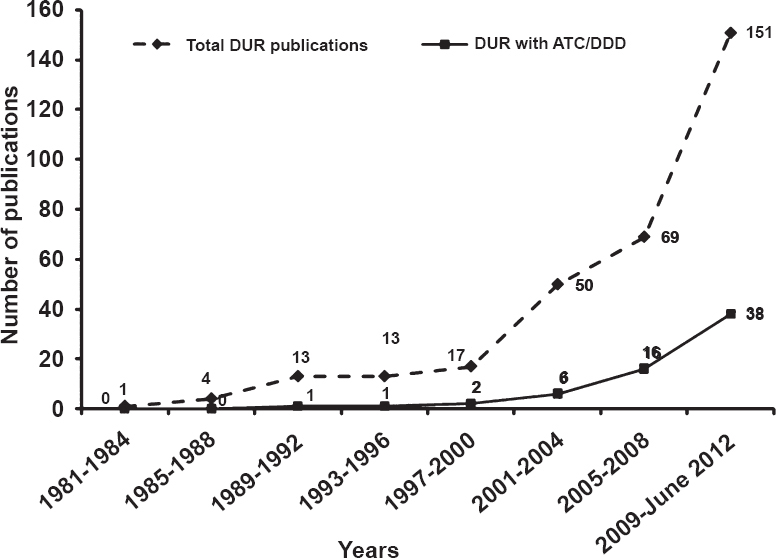
- Trend in drug utilization research (DUR) and use of ATC/DDD methodology over the years in WHO-SEARO countries.
Methods and design followed: Majority of the publications were prospective while only 39 (12%) were retrospective (Table III). Majority of the publications followed prescription monitoring at hospital outpatient departments (OPDs) or pharmacy stores. Besides prescription monitoring, a few publications analysed patient records and conducted questionnaire based surveys of doctors and patients. Sample sizes in the range 101-500 prescriptions/patients/doctors were seen in 148 (49%) publications while only 50 (16%) publications had sample sizes of more than 2000 prescriptions (Fig. 3).
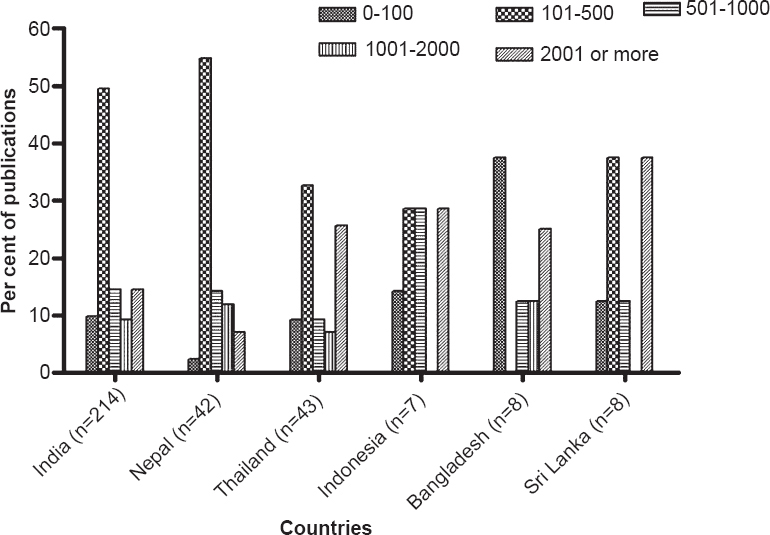
- Sample size used in drug utilization research (DUR) study publications from WHO-SEARO. (Other SEARO countries viz. Bhutan, Maldives, Myanmar and Timor-Leste did not have any publication on DUR studies).
Site of studies: Majority of the studies were hospital based, and only 52 (16%) were community based; even fewer, 28 (9%) were carried out in primary health centres (PHCs) (Table III). About 175 (55%) of the studies were carried out at hospital outpatient departments (OPDs).
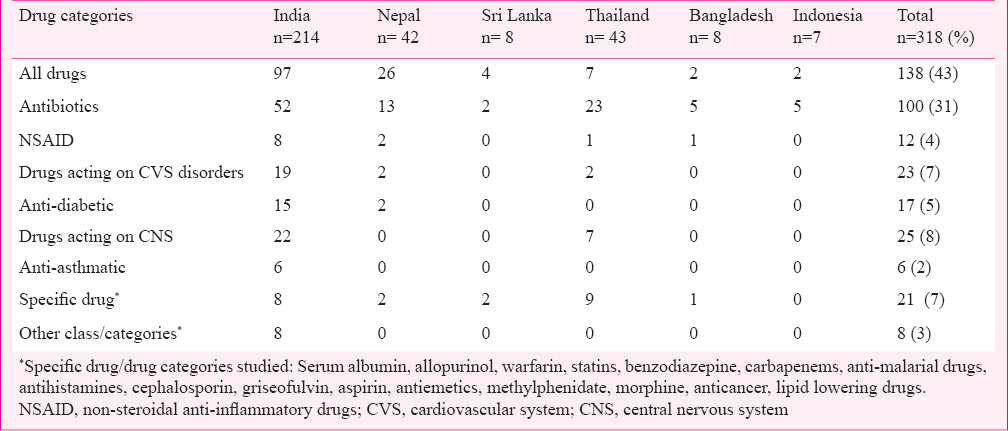
Types of patients, drugs and disease conditions studied: A total of 138 (43%) publications included drug utilization data of all drugs while 100 (31%) of the publications were on antimicrobial use in different diseases. Most of the publications (279, 88%) focused on the general patient population of all ages, while 26 (8%) and 13 (4%) focused on paediatric and elderly populations, respectively (Table III). Only 21 (7%) of the publications evaluated the drug use pattern of specific drugs. Table IV lists drugs/drug categories investigated.
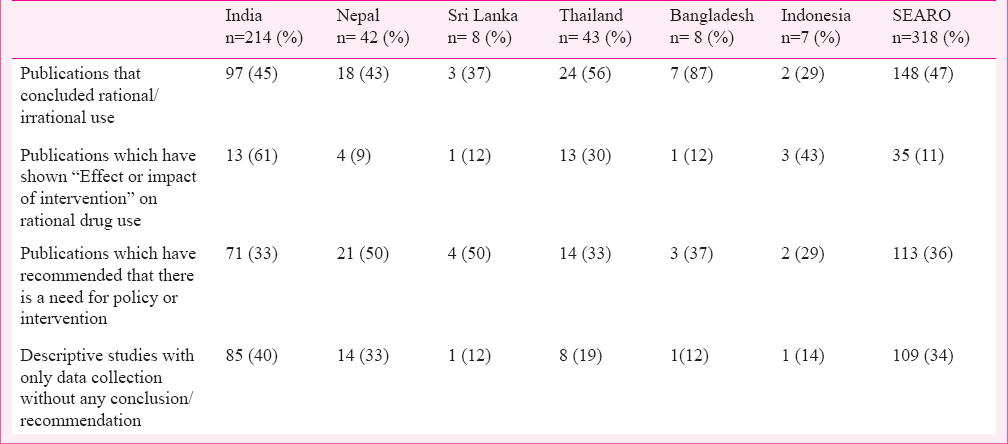
Outcomes of the studies: About 34 per cent (109) of the publications were descriptive studies conducted based on prescription monitoring at the hospital or community level, providing data only on prescribing and dispensing practices. Publications concluding on rational/irrational drug use were 148 (47%), while 35 (11%) have shown the impact of some intervention on drug use or prescription practice (Table V) and 113 (36%) of the publications recommended the need for a policy or intervention to improve prescription practices/rational drug use.
Discussion
The present study is an analysis of the published literature from the WHO-SEARO Region on DUR and the use of WHO ATC/DDD system. Most of the studies were prospective, hospital-based using hospital patient records manually to calculate prescription monitoring indicators. The focus of DUR studies was on a rational use of drugs. To address irrational use of drugs prescribing, dispensing and use of the drugs need to be regularly monitored on an ongoing basis in terms of the type and the amount of irrational use, along with the reason for the same19. To improve rational use of drugs, several educational and guidelines-based interventions will be useful and the effectiveness of these interventions needs to be evaluated using DUR studies. It was found that such intervention based DUR studies were lacking in SEARO countries. Prescription monitoring alone will only be useful to know the irrational practice. Most of the studies have only commented on irrational or rational use along with need for intervention or policy without commenting or proposing any intervention which can be useful to prevent irrational drug use. Only a few studies have shown that the different interventions practiced at community or hospital level can be useful in improving rational drug use.
In 1993, WHO and INRUD (International Network for the Rational Use of Drugs) developed and published a standard method for selected drug use indicators in health facilities. These indicators were prescribing indicators, patient care indicators, facility indicators and complementary drug use indicators2021. The core prescribing indicators consist of the average number of drugs per prescription, percentage of patients prescribed antibiotics, percentage of patients prescribed injections, percentage of medicines prescribed by generic names, percentage of prescriptions for essential medicines, etc22. These can be used to identify problems in general prescribing and the quality of care. Results from the use of these indicators can help identify the motives of irrational use and other problems in the use of medicines. Some of the authors have studied interventions for rational use of drugs and found these effective and useful. These interventions were based on providing guidelines or educational activity. Drug utilization research provides highly useful information about the availability and use of medicines. Indices such as DDD/(1000 population)/year or DDD/(100 bed-days) are useful for comparing various regions within the country and various countries within a region and for correlation with gross domestic product (GDP) and health indices. Information from drug utilization research can be very useful for policy makers to allocate resources4. Most of the DUR studies from SEARO Region lack the use of ATC/DDD which is also observed by Irwin & Sharland23 in their systematic review on antibiotic prescribing in resource poor countries. The major difficulty in using the ATC/DDD system may be the wide range of branded generic drugs and fixed dose combinations which makes it time consuming and difficult to assign ATC code and calculate DDDs. Researchers need to be educated on how to use the ATC/DDD system. For National use, ATC codes need to be allocated to National drug lists and products used, and this will require resources and competency24. The WHO Collaborating Centre for Drug Statistics Methodology, besides reviewing and revising the classification of drugs and establishing DDDs for drugs, provides support to building capacity in the use of information related to the consumption of medicines and setting up national medicine classification systems5.
The ATC/DDD system was developed and used for presenting drug consumption data4. Unlike high income countries, data on production, expenditure and consumption of pharmaceuticals are not readily available in middle or low income countries, and if available, may be incomplete as the data come from different sources. In addition, rather than being an indicator of volume, the use of monetary values gives a distorted picture of production and consumption since it fails to reflect the scale of consumption of traditional medicines and low priced generics (branded and non-branded)25. In some high income countries research on use of medicine is routine in healthcare facilities, and numerous studies have demonstrated its effectiveness4. SEARO countries do not have comprehensive drug utilization data on the use of medicines at the National level. Besides, large databases such as those used in high income countries are generally unavailable in middle or low income countries. In the SEARO countries many of the studies were carried out in individual hospitals and/or healthcare settings. The sampling method was used especially while studying drug utilization in community settings26. The health seeking behaviour of the population is different in the private sector and public sector. Likewise, the prescription patterns also vary. Hence, attention was paid to both types of healthcare providers. Holloway et al27 have commented that in community studies while analyzing prescriptions, it is easier to calculate percentage of prescriptions containing antimicrobials but for calculating DDD much more data collection is required, thus allowing more room for error.
In hospitals where computerized record systems exist, reporting of the use of medicines can occur using the ATC/DDD system. However, in hospitals without such computerized systems, very little such information can be obtained. Most computerized record systems where available have ICD (International Classification of Diseases) code, but not ATC/DDD.
This study showed that there were very few publications from SEARO Region on DUR. In a study done by Teng et al28 in China, there were 2911 publications on DUR, of which 1268 have used DDD in their study. In addition, when checked for global DUR publications in Scopus database, around 4840 publications were found. The population of WHO-SEARO countries is approximately 1.8 biillion which is about 25 per cent of the global population29. But DUR publications, considering in Scopus search are only 6.6 per cent of the global DUR publications.
The health information systems in countries of the WHO-SEARO Region are at various stages of development. A high level preparatory meeting held at the Regional Office at New Delhi in July 2010 recommended a regional strategy for strengthening health information systems with the goal of improving the availability, quality and use of health information for enhanced efficiency and effectiveness of health programmes30. Promotion of data collection is important and includes developing data quality standards that specify the completeness of medical records. Achieving millennium development goals, access to medicines and rational use of medicines are healthcare priorities in SEARO Region countries31. DUR studies and use of ATC/DDD system will be useful for assessing and comparing medicines use across countries and can contribute to public health policy decisions and resource allocations. ATC/DDD system in healthcare/hospital information system will be useful to provide drug utilization information quickly, which is important for evaluating impact of new investments32. These methodologies and applications need to be considered for future DUR studies in SEARO Region countries.
Our study had a few limitations. Firstly, we were unable to get full text versions of publications retrieved. We tried contacting the corresponding authors/first authors of these publications requesting for a copy of the article. Despite our efforts, we were unable to get full text of 72 publications. We reviewed the abstract of such publications and included these in our study if these provided the information we needed. In the process, we may have missed information (viz. sample size, etc.) and could not evaluate the quality of the articles. Yet, we preferred to include such studies in order to avoid losing data. Secondly, we included only English language publications. This may be the reason that for a few countries viz. North Korea and Thailand, the number of publications on DUR is less, as publications in their languages are not included or searched.
In conclusion, our results show that DUR studies in SEARO region countries have been carried out mainly using hospital patient records for rational use of medicines. Studies in community setting and those evaluating intervention have been limited. DUR studies with ATC/DDD coding are useful for comparing drug use across healthcare settings and countries and help take policy decisions and resources allocations. In SEARO countries ATC /DDD code needs to be allocated to National lists for which resources and capacity building is needed. Considering the importance of DUR studies, this investment would be worthwhile.
References
- The consumption of drugs; report on a study, 1966-1967. Copenhagen: World Health Organization, Regional Office for Europe; 1968.
- [Google Scholar]
- Coding and classification in drug statistics- From national to global application. Norwegian J Epidemiol. 2001;11:37-40.
- [Google Scholar]
- WHO. What is drug utilization research and why is it needed? In: Introduction to drug utilization research. Geneva: World Health Organization; 2003. p. :8-12. Available from: http://apps.who.int/medicinedocs/pdf/s4876e/s4876e.pdf
- [Google Scholar]
- ATC/DDD classification: drug utilization data constrains in developing countries. 2002. WHO Drug Inf. 16:233-40. Available from: http://apps.who.int/medicinedocs/pdf/s4951e/s4951e.pdf
- [Google Scholar]
- Guidelines for ATC classification and DDD assignment 2011. 2011. (14th ed). Oslo, Norway: WHO Collaborating Centre for Drug Statistics methodology. Norwegian Institute of Public Health; Available from: http://www.whocc.no/filearchive/publications/2011guidelines.pdf
- [Google Scholar]
- PRISMA-Transparent reporting of systematic reviews and meta-analysis. Available from: http://www.prisma-statement.org
- [Google Scholar]
- The Cochrane Collaboration 2006. Cochran handbook for systematic reviews of interventions 4.2.6, updated September 2006. Available from: http://www.cochrane.org/sites/default/files/uploads/Handbook4.2.6Sep2006.pdf
- [Google Scholar]
- Effects of managerial intervention on drug utilization pattern at King Chulalongkorn Memorial Hospital. J Med Assoc Thai. 2002;85(Suppl 1):S336-43.
- [Google Scholar]
- Study Group Antimicrobial resistance in Indonesia: Prevalence and Prevention (AMRIN). Optimizing antibiotic usage in adults admitted with fever by a multifaceted intervention in an Indonesian governmental hospital. Trop Med Int Health. 2008;13:888-99.
- [Google Scholar]
- Antimicrobial consumption and impact of “Reserve antibiotic indent form” in an intensive care unit. Indian J Pharmacol. 2010;42:297-300.
- [Google Scholar]
- Surveillance of antibiotic consumption using the “focus of infection” approach in 2 hospitals in Ujjain, India. PLoS One. 2012;7:e38641.
- [Google Scholar]
- Drug utilization evaluation of third generation cephalosporins in a tertiary care hospital in South India. Indian J Pharm Educ Res. 2008;42:295-300.
- [Google Scholar]
- Thammasat University Antimicrobial Stewardship Team. Impact of education and an antifungal stewardship program for candidiasis at a Thai tertiary care center. Infect Control Hosp Epidemiol. 2010;31:722-7.
- [Google Scholar]
- Small Group intervention vs formal seminar for improving appropriate drug use. Soc Sci Med. 1996;42:1163-8.
- [Google Scholar]
- A cross-sectional prescription audit database for anti-anginal drugs with impact of essential drug list and standard treatment guidelines on prescription pattern in Nasik city. Res J Pharm Technol. 2011;4:1111-4.
- [Google Scholar]
- Changing prescribing behaviour: early low dose aspirin in suspected acute myocardial infarction. Int J Cardiol. 1998;67:237-40.
- [Google Scholar]
- Drug utilization pattern in the intensive care unit of a tertiary care hospital. J Clin Pharmacol. 2006;46:945-51.
- [Google Scholar]
- WHO. WHO policy perspectives on medicines. Promoting rational use of medicines: core components. 2002. Geneva: World Health Organization; Available from: http://apps.who.int/medicinedocs/pdf/h3011e/h3011e.pdf
- [Google Scholar]
- WHO. How to investigate drug use in health facilities. Selected drug use indicators- EDM Research Series No. 007. 1993. Geneva: World Health Organization; Available from: http://apps.who.int/medicinedocs/en/d/Js2289e/8.6.html
- [Google Scholar]
- Ten recommendations to improve use of medicines in developing countries. Health Policy Plan. 2001;16:13-20.
- [Google Scholar]
- Field-tests for rational drugs use in twelve developing countries. Lancet. 1993;342:1408-10.
- [Google Scholar]
- Measuring antibiotic prescribing in hospitalised children in resource-poor countries: a systematic review. J Paediatr Child Health. 2013;49:185-92.
- [Google Scholar]
- WHO. Drug classification systems. In: Introduction to drug utilization research. Geneva: World Health Organization; 2003. p. :33-37. Available from: http://apps.who.int/medicinedocs/pdf/s4876e/s4876e.pdf
- [Google Scholar]
- WHO. The world medicines situation. 2004. Geneva: World Health organization; Available from: http://apps.who.int/medicinedocs/pdf/s6160e/s6160e.pdf
- [Google Scholar]
- Trends in antibiotic use among outpatients in New Delhi, India. BMC Infect Dis. 2011;11:99.
- [Google Scholar]
- Community-Based Surveillance of Antimicrobial Use and Resistance in Resource-Constrained Settings Project Group. Surveillance of community antimicrobial use in resource constrained settings-experience from five pilot projects. Trop Med Int Health. 2011;16:152-61.
- [Google Scholar]
- Review of the use of defined daily dose concept in drug utilisation research in China. Pharmacoepidemiol Drug Saf. 2012;21:1118-24.
- [Google Scholar]
- WHO. World malaria report 2012: South-East Asia Region. 2012. :84-5. Available from: http://who.int/entity/malaria/data/sear12.pdf
- [Google Scholar]
- WHO. Regional strategy on health information systems. 2010. New Delhi: World Health Organization, Regional Office for South-East Asia; Available from: http://www.searo.who.int/LinkFiles/RC_63_a-17-SEA-RC63-26.pdf
- [Google Scholar]
- Progress towards Millennium Development Goals 4 and 5 on maternal and child mortality: an updated systematic analysis. Lancet. 2011;378:1139-65.
- [Google Scholar]
- A strategy for promoting improved pharmaceutical use: the International Network for Rational Use of Drugs. Soc Sci Med. 1992;35:1329-41.
- [Google Scholar]






