Translate this page into:
Survivin: A molecular biomarker in cancer
Reprint requests: Dr Rama Devi Mittal, Department of Urology, Sanjay Gandhi Post Graduate Institute of Medical Sciences Raebareli Road, Lucknow 226 014, Uttar Pradesh, India e-mail: ramamittal@gmail.com, rdm_pgi@rediffmail.com
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Survivin, a member of the inhibitor of apoptosis (IAP) protein family that inhibits caspases and blocks cell death, is highly expressed in most cancers and is associated with a poor clinical outcome. Survivin has consistently been identified by molecular profiling analysis to be associated with high tumour grade cancers, different disease survival and recurrence. Polymorphisms in the survivin gene are emerging as powerful tools to study the biology of the disease and have the potential to be used in disease prognosis and diagnosis. The survivin gene polymorphisms have also been reported to influence tumour aggressiveness as well as survival of cancer patients. The differential expression of survivin in cancer cells compared to normal tissues and its role as a nodal protein in a number of cellular pathways make it a high target for different therapeutics. This review discusses the complex circuitry of survivin in human cancers and gene variants of survivin, and highlights novel therapy that targets this important protein.
Keywords
Cancer
cancer therapy
gene polymorphism
survivin
Introduction
Cancer remains the second leading cause of death after cardiovascular diseases globally. Increasing evidence indicates that the unique member of the inhibitor of apoptosis (IAP) protein family, survivin, is not only an essential protein molecule for the regulation of mitosis and apoptotic inhibition but it also plays a role in certain physiological processes as well as in pathological conditions such as carcinogenesis in many human organs/cells. Eight members of the family of inhibitors of apoptosis proteins (IAPs) are reported, including X-linked inhibitor of apoptosis (XIAP), cIAP1, cIAP2, NAIP (NLR family, apoptosis inhibitory protein), livin, ILP2 (IAP-like protein 2), BRUCE and survivin123.
Survivin is an inhibitor of apoptosis protein and is expressed in a large number of malignancies4. Its expression levels correlate with more aggressive disease and poor clinical outcome. Survivin expression is minimal in normal tissues, therefore, it has become a lead target for both as a tumour diagnostic, prognostic and as well as for anti-cancer therapies. Overexpression of survivin in cancer may overcome cell cycle checkpoints to facilitate aberrant progression of transformed cells through mitosis5.
Survivin is expressed highly at G2/M phase and declines rapidly in G1 phase of cell cycle. This is largely transcriptionally controlled and involves cell cycle-dependent elements (CDEs) and cell cycle homology regions (CHRs) located in survivin gene promoter6. several single nucleotide polymorphisms (SNPs) have been identified in survivin gene, such as -31G/C, -1547A/G, -625G/C and -644C/T. Polymorphism at -31G/C in survivin is a common mutation in cancer cell lines leading to overexpression of survivin due to functional disruption of binding at the CDE/CHR repressor motifs7.
This review provides a brief description of the structure of survivin gene/protein, survivin expression and function in apoptosis, and gene variants of survivin.
Overview of survivin structure and functions
The gene encoding human survivin was cloned by Ambrosini et al8 in 1997. Survivin spans 14.7 kb at the telomeric end of chromosome 17 and encodes the 16.5 kD wild-type survivin protein of 142 amino acids in length9.
As the smallest member of the IAP family, all survivin isoforms characterized so far contain only one of the characteristic N-terminal BIR (Baculovirus IAP Repeats) domains; and an alpha-helix replaces the IAP characteristic RING finger domain involved in zinc ion-binding with the BIR domain (Fig. 1)8. The BIR domain is supposed to be important for anti-apoptotic function, whereas the coiled domain probably interacts with tubulin structures9. The survivin gene locus encodes multiple genetic splice variants with unique properties and functions. These isoforms include survivin, survivin-2B, survivin-ΔEx-3, survivin-3B, and survivin-2-alpha. Transcription and translation of these isoforms have been demonstrated by several groups of investigators101112. In malignant cells, all these isoforms are expressed at a very high rate as compared with normal tissues. Survivin has a dual function, playing both a role in cell death regulation and mitotic progression.
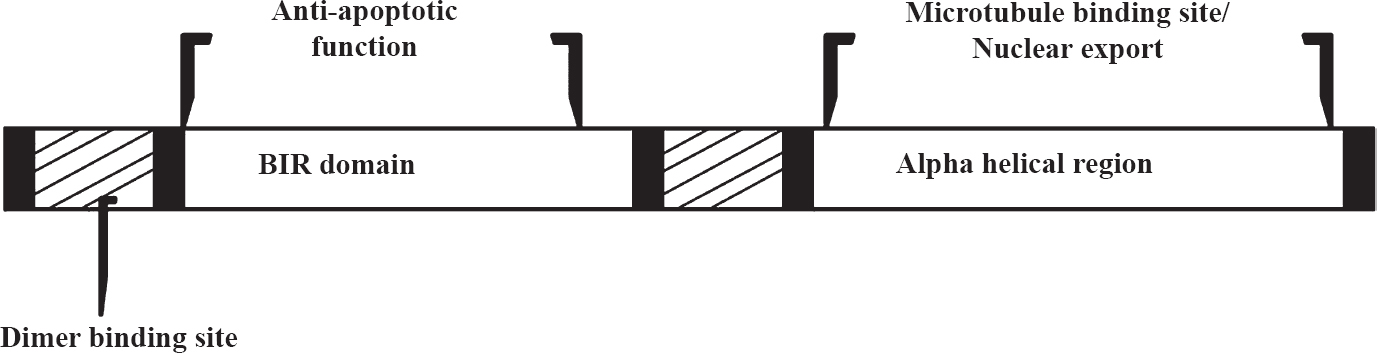
- Structure and function of survivin protein.
Survivin can be co-immunoprecipitated with caspases-3, -7, and -9 and it suppresses apoptosis induced by overexpression of these caspases, implying that survivin also is a caspase inhibitor13. It also inhibits cell death by interfering with caspase-9 processing, the main inhibitor in intrinsic pathway of apoptosis14. Activation of cell death pathways can be initiated through different mechanisms, including through ligand binding (FasL, TNF) to a death receptor on the cell surface (extrinsic pathway) or via direct mitochondrial signaling (intrinsic pathway). The mitochondrial pathway is initiated by activation of the Bax/Bcl-2 pathway leading to the release of apoptotic factors such as cytochrome c (cyt c) and apoptosis-inducing factor (AIF) from the mitochondrial intermembrane space into the cytoplasm. The release of cytochrome c from mitochondria results in caspase-3 activation through formation of the cytochrome c/Apaf-1/caspase-9 apoptosome complex. Caspase-3 cleaves a number of substrates including cytoskeletal proteins and DNA. Caspase-activated DNase (CAD) and inhibitor of CAD (ICAD) initiate cleavage and fragmentation of DNA. The IAPs inhibit cell death by physically interacting with caspases. Survivin has been shown to inhibit apoptosis through caspase-dependent and independent pathways (Fig. 2).
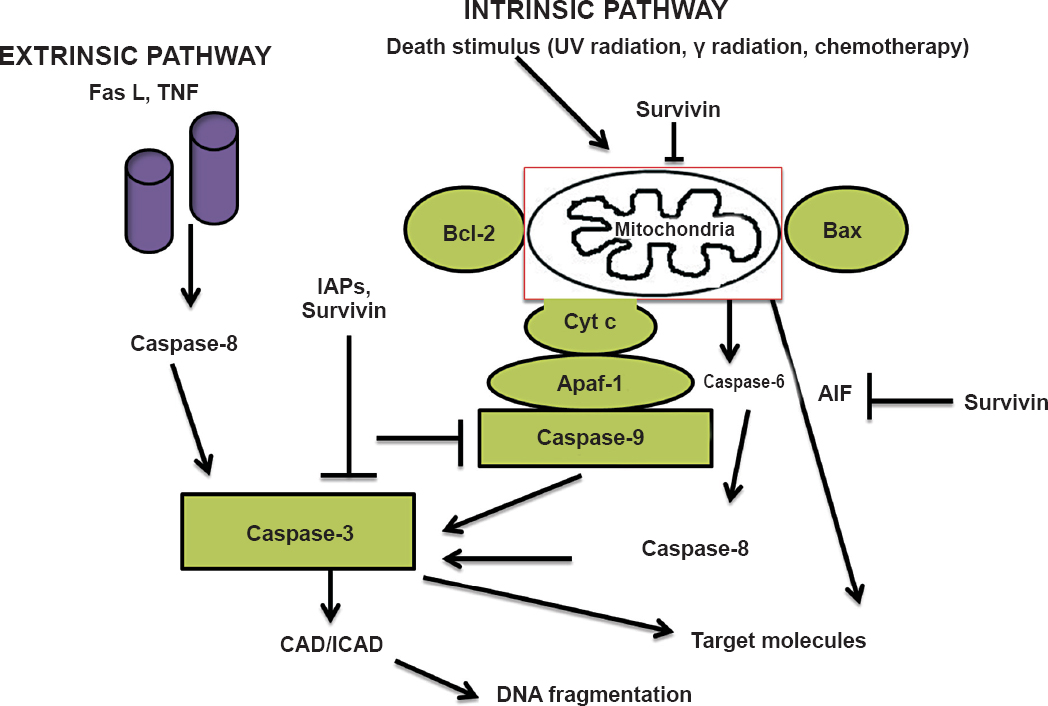
- Survivin (SVN) pathways to apoptosis. Activation of cell death pathways can be initiated through different mechanisms, including through ligand binding (FasL, TNF) to a death receptor on the cell surface (extrinsic pathway) or via direct mitochondrial signaling (intrinsic pathway). The mitochondrial pathway is initiated by activation of the Bax/Bcl-2 pathway leading to the release of apoptotic factors such as cytochrome c (cyt c) and apoptosis-inducing factor (AIF) from the mitochondrial intermembrane space into the cytoplasm. The release of cytochrome c from mitochondria results in caspase-3 activation through formation of the cytochrome c/Apaf-1(apoptotic peptidase activating factor-1)/caspase-9 apoptosome complex. Caspase-3 cleaves a number of substrates including cytoskeletal proteins and DNA. Caspase-activated DNase (CAD) and inhibitor of CAD (ICAD) initiate cleavage and fragmentation of DNA. The inhibitors of apoptosis (IAPs) inhibit cell death by physically interacting with caspases. Survivin has been shown to inhibit apoptosis through caspase-dependent and independent pathways.
Consistent with the lack of a structural caspase activation and recruitment domain (CARD) motif, survivin does not directly bind to and inhibit caspases15. Instead, it interacts with several adaptor or cofactor molecules, one example being X-linked IAP (XIAP). By interacting with XIAP, survivin enhances XIAP stability. It can further act by sequestering SMAC/DIABLO (second mitochondria-derived activator of caspases/direct IAP binding protein with low pI), preventing XIAP's inhibition16. In each case, survivin enhances XIAP's inhibitor activity of caspase-9 dependent cell death17 and of death receptor mediated apoptosis18.
Regulation of survivin and p53
The p53 protein is a transcription factor, which can induce apoptosis by regulating the apoptotic genes. Survivin is a target of p53 for its action and downregulation, and p53 may induce apoptosis by antagonizing the anti-apoptotic activity of survivin. Survivin may also influence p53 activity through regulation of mouse double minute 2 homolog (mdm2) and proteosome19. However, the negative regulation of survivin by p53 is poorly understood. Survivin promoter has a p53 binding element. It may be possible that p53 directly binds survivin promoter alone or in combination with other protein(s) to repress survivin. E2F (a transcriptional activator) may also bind survivin promoter20. Since p53 has affinity with E2F, it is possible that both form a (p53-E2F) complex that represses survivin gene expression212223. It also interacts with transcriptional repressor (sin3) and histone deacetylases (HDAC) that together can form a p53-sin3-HDAC complex and binds survivin promoter to repress it2124. p53 represses survivin through a cascade, which involves protein p21 complex p21/cip1, which is a p53-induced gene that inhibits cyclin-dependent kinase 2 (cdk2) to prevent phosphorylation of retinoblastoma (RB) proteins25. This results in the accumulation of hypophosphorylated pRB. This protein binds E2F family transcription factor and forms a pRB-E2F complex, which may repress survivin gene expression20212627.
Survivin expression and its role in different cancer
Regulatory mechanisms of survivin expression are yet not fully understood. At the transcriptional level, survivin expression has been demonstrated to involve cell-cycle-dependent element/cell cycle gene homology region (CDE/CHR) G1 repressor elements in the BIRC5 (baculoviral IAP repeat containing 5) promoter4.
Survivin is expressed in embryonic and foetal tissues, but is undetectable in normal adult tissues8. However, overexpression of survivin has been reported in almost all human malignancies including bladder cancer, lung cancer, breast cancer, stomach, oesophagus, liver, ovarian cancers and haematological cancers828. Based on detection of protein by immunohistochemistry and mRNA by polymerase chain reaction techniques, overexpression of survivin has been reported in various human malignancies (Table).
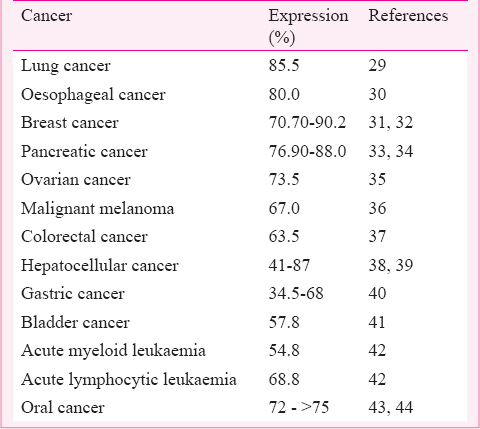
Almost all cancers have alternative survivin expression profile compared to normal tissues. Survivin is one of the important genes involved in tumour aggressiveness and therapy resistance. Salz et al45 showed that survivin expression induced transcriptional changes in the tissue microenvironment further promoting tumourigenesis in the bladder tissue. Khan et al46 reported higher expression of survivin as a critical factor for radioresistance in head and neck squamous cell carcinoma (HNSCC) cell lines. Dysregulation of oncoapoptotic genes, growth factors, receptors and their downstream signaling pathway components represent a central driving force in tumour development in different cancer47.
DNA polymorphisms with more than one variant (allele) having a frequency greater than 1 per cent in a human population have been estimated to occur on the average at one in every 1000 base pairs throughout the human genome48. The incidences of polymorphism in genomic DNA, their susceptibility to genetic alterations, and the risk of tumour progression in patients with cancer can vary substantially between different racial groups495051. Although most polymorphisms are functionally neutral, some affect regulation of gene expression or the function of the coded protein52.
The survivin gene codifies a multifunctional protein involved in the regulation of the cell cycle and inhibition of the apoptotic pathway, and a polymorphism located in its promoter region is associated with gene regulation. Most of the polymorphic studies are confined in promoter region among which the single nucleotide polymorphism (SNP) at -31G/C is most studied in all cancers in comparison to other polymorphic sites53545556. The following are some examples of the survivin gene polymorphisms that were found to be associated with cancer:
Breast cancer
Breast cancer is the most common malignancy among women, accounting for nearly one in three cancers diagnosed among women in the United States, and it is the second leading cause of cancer death among women57. A hospital-based case control study by Ulybina et al58 showed no risk for breast cancer with survivin gene variants at +9194A/G. However, Boidot et al59 showed that survivin expression might induce breast tumour proliferation by promoting genetic instability.
Bladder cancer
A case control study conducted in Japan showed two survivin polymorphisms associate with higher risk of bladder cancer60. A hospital-based study from north India53 showed that individuals with CC genotype of survivin -31G/C had 2.6 folds higher risk for bladder cancer. A study in Taiwanese population showed increased bladder cancer risk with G/C genotype61.
Colorectal cancer
Colorectal cancer is a major cause of morbidity and mortality worldwide. Alterations in pathways regulating important biological processes including cell survival, cell proliferation, epithelial to mesenchymal transition and stroma production have been shown to contribute to colorectal carcinogenesis and tumour progression. A real time PCR study showed association between survivin 31G/C and development of colorectal cancer62. Another study showed that the frequencies of the survivin -31C allele and CC genotype were significantly higher in colorectal cancer patients than in healthy subjects63. Variant CC genotype of -31G/C of survivin, expressed 1.6 fold higher mRNA levels compared to cases with the -31G/G and -31G/C genotypes63.
Endometrial cancer
There is only one study on endometrial cancer and survivin gene polymorphism till date. Survivin is upregulated in endometrial cancer (EC). The presence of allele C of -31G/C was found to be significantly increased in EC tissues compared to the healthy tissues in case of Iranian population54.
Oesophageal cancer
A case control study from north India showed that SNP -31G/C was found to be significantly associated with oesophageal cancer susceptibility at variant CC genotype64. A study in Chinese population suggested that survivin promoter polymorphisms -625G/C might influence the susceptibility to oesophageal cancer by influencing survivin expression65.
Gastric cancer
A study in Chinese population has demonstrated that survivin -31G/C polymorphism may be involved in distal gastric carcinogenesis and tumour differentiation65. Another study on Brazilian population suggests that presence of C allele of -31G/C gene polymorphism may be a risk factor for gastric cancer66.
Head and neck cancer
In a study conducted in Serbian population, -31G/C gene polymorphism in the promoter region of the survivin gene showed no association with risk for head and neck cancer67. Radiotherapy is the main therapy for head and neck squamous cell carcinoma (HNSCC). Survivin showed the most promising biomarker of radioresponse in case of head and neck cancer cell lines68.
Hepatocellular carcinoma
Early detection of hepatocellular carcinoma (HCC) is seldom available because of the lack of reliable markers. A study from Taiwanese population suggested that survivin T9809C SNP might contribute to the prediction of susceptibility and pathological development to HCC while other polymorphisms of survivin at -31G/C and -241C/T did not show any association with HCC69. Survivin -31G/C promoter polymorphism has not shown any major role in genetic susceptibility to hepatocellular carcinogenesis in Turkish population70. SNPs of survivin at -31G/C and -625G/C did not show any significant association with HCC in Chinese Han population55. Survivin+9194A/G polymorphism also did not show any significant association with hepatocellular carcinoma in Korean population71.
Lung cancer
A study conducted in Korean population showed that only the -31G/C genotype distribution was significantly different between the cases and controls. Individuals with at least one -31G allele were at a significantly decreased risk of lung cancer compared to those individuals with the -31CC genotype72. Polymorphism in survivin may be a genetic modifier for non-small cell lung cancer prognosis in Chinese population73. The BIRC5 promoter polymorphism at nucleotide -31 did not influence the expression of survivin mRNA and protein in non-small cell lung cancer cells and tumours in a study reported in Czech population74.
Oral cancer
A study conducted in Taiwanese population reported significantly higher risk for oral cancer with -31GG, +9194GG, and +9809 TT homozygotes of survivin gene variants in comparison to wild type genotype75. An expression study by Khan et al46 showed 72 per cent of survivin expression in tissue samples of oral cancer. Lauxen et al44 showed >75 per cent expression of survivin protein in oral squamous cell carcinoma (OSCC) tissue samples.
Pancreatic cancer
Only one study has been reported on pancreatic cancer and survivin gene polymorphism which showed significant association of survivin -31G/C with advanced tumour stage as well as the presence of lymph node metastasis56.
Renal cell carcinoma
There is only one study reported in renal cell carcinoma (RCC) and survivin gene polymorphism showing a significantly increased occurrence of RCC associated with the CC genotype76. The polymorphism was associated with risk of developing advanced stage and moderately differentiated RCC. Patients carrying the CC genotype had a significantly greater prevalence of high clinical stage disease76.
Role of survivin in cancer therapy
Evidences indicate that the expression of survivin is altered in cancer, and that certain changes may be directly implicated in the carcinogenic process. Due to its role as a cancer gene intersecting multiple cellular networks, survivin has been vigorously used as a cancer drug target. While comparing with other apoptosis-based cancer therapies77, survivin provides several advantages. Firstly, the disabling survivin is expected to compromise multiple signaling networks required for tumorous maintenance78. Second, survivin may be a unique target for molecular antagonists, cancer vaccine and gene therapy. Thirdly, expression of survivin is regulated by Wnt signaling pathway that has main role in stem cells and it is possible that survivin antagonists may affect cancer stem cells79. Fourth, survivin is important in tumour formation/progression, especially angiogenesis80, and survivin inhibitors have been shown to act on both the transformed population and endothelial cells in tumour. Fifth, although survivin expression has been shown in cytokine stimulated haematopoietic progenitors and in activated T cells, targeting this pathway does not affect the normal cells or tissues suggesting a favourable toxicity profile of survivin based therapeutics. Survivin-directed immunotherapy has been on the move and several phase I trials with administration of survivin peptides or survivin directed autologous cytotoxic T lymphocytes (CTL) generated ex vivo have been completed8182. Survivin-based vaccination was found to be safe, with no side effects and associated with antigen-specific immunologic responses8183. Different strategies have been targeted for inhibition of survivin in vivo such as identification of a specifically interacting peptide. This peptide can recognize survivin intracellularly and cause the degradation of the ligand survivin complex84. Further, survivin inhibition can be achieved by targeting with nano-based drug delivery devices coupled with biocompatible natural product derived therapeutics85. Other strategies under investigation to target survivin include antisense oligonucleotides, siRNA86, ribozymes, immunotherapy and small molecular weight molecules. These include use of the antisense oligonucleotide LY2181308, the low molecular weight molecule inhibitor YM155 and survivin-directed autologous CTL. The optimum use of survivin in the treatment of cancer is likely to be in combination with conventional cancer therapies for different cancer4486878990.
Conclusion
Since its discovery in 1997, survivin has provided unique opportunities for basic and translational studies. There are multiple strategies targeting the survivin network which have quickly passed proof of principle, and many have entered in the clinical trials in humans14. However, the results of these trials are still awaited. Further research in identifying the novel targets of survivin and understanding their biological functions will enhance our knowledge about the role of these novel regulators in tumour genesis and will facilitate the potential diagnosis and treatment of cancer.
Acknowledgment
The first author (PKJ) acknowledges the University Grants Commission (UGC), New Delhi, India, for providing Junior Research Fellowship (JRF).
References
- Survivin, cancer networks and pathway-directed drug discovery. Nat Rev Cancer. 2008;8:61-70.
- [Google Scholar]
- Inhibitor of apoptosis proteins: translating basic knowledge into clinical practice. Cancer Res. 2004;64:7183-90.
- [Google Scholar]
- Survivin, versatile modulation of cell division and apoptosis in cancer. Oncogene. 2003;22:8581-9.
- [Google Scholar]
- Structural, functional and therapeutic biology of survivin. Cancer Lett. 2006;244:164-71.
- [Google Scholar]
- Survivin apoptosis: an interloper between cell death and cell proliferation in cancer. Lab Invest. 1999;79:1327-33.
- [Google Scholar]
- A mutation found in the promoter region of the human survivin gene is correlated to overexpression of survivin in cancer cells. DNA Cell Biol. 2004;23:527-37.
- [Google Scholar]
- A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Mad. 1997;3:917-21.
- [Google Scholar]
- Survivin: a protein with dual roles in mitosis and apoptosis. Int Rev Cytol. 2005;247:35-88.
- [Google Scholar]
- Identification of a novel splice variant of the human anti-apoptopsis gene survivin. Biochem Biophys Res Commun. 2004;314:902-7.
- [Google Scholar]
- Survivin 2alpha: a novel survivin splice variant expressed in human malignancies. Mol Cancer. 2005;4:11.
- [Google Scholar]
- Transcriptional expression of survivin and its splice variants in brain tumors in humans. J Neurosurg. 2003;99:738-45.
- [Google Scholar]
- IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas(CD95), Bax, caspases, and anticancer drugs. Cancer Res. 1998;59:5315-20.
- [Google Scholar]
- Targeted therapy by disabling crossroad signaling networks: the survivin paradigm. Mol Cancer Ther. 2006;5:478-82.
- [Google Scholar]
- Chemically synthesized human survivin does not inhibit caspase-3. Protein Sci. 2008;17:1624-9.
- [Google Scholar]
- Solution structure of human survivin and its binding interface with Smac/Diablo. Biochemistry. 2005;44:11-7.
- [Google Scholar]
- XIAP is not required for human tumor cell survival in the absence of an exogenous death signal. BMC Cancer. 2010;10:11.
- [Google Scholar]
- Survivin regulates the p53 tumor suppressor gene family. Oncogene. 2004;23:8146-53.
- [Google Scholar]
- Aberrant regulation of survivin by the RB/E2F family of proteins. J Biol Chem. 2004;279:40511-20.
- [Google Scholar]
- Transcriptional repression of the anti-apoptotic survivin gene by wild type p53. J Biol Chem. 2002;277:3247-57.
- [Google Scholar]
- Physical and functional interactions between p53 and cell cycle co-operating transcription factors, E2F1 and DP1. EMBO J. 1995;14:6184-92.
- [Google Scholar]
- Novel function of the cyclin A binding site of E2F in regulating p53-induced apoptosis in response to DNA damage. Mol Cell Biol. 2002;22:78-93.
- [Google Scholar]
- Human survivin is negatively regulated by wild-type p53 and participates in p53-dependent apoptotic pathway. Oncogene. 2002;21:2613-22.
- [Google Scholar]
- p21/CDKN1A mediates negative regulation of transcription by p53. J Biol Chem. 2003;278:32507-16.
- [Google Scholar]
- Selective sensitization of retinoblastoma protein-deficient sarcoma cells to doxorubicin by flavopiridol-mediated inhibition of cyclin-dependent kinase 2 kinase activity. Cancer Res. 2001;61:2579-82.
- [Google Scholar]
- p53 regulation of G(2) checkpoint is retinoblastoma protein dependent. Mol Cell Biol. 2000;20:4210-23.
- [Google Scholar]
- Survivin, a cancer target with an emerging role in normal adults tissues. Mol Cancer Ther. 2006;5:1087-98.
- [Google Scholar]
- A novel anti-apoptosis gene: re-expression of survivin messenger RNA as a prognosis marker in non-small-cell lung cancers. J Clin Oncol. 1999;17:2100-4.
- [Google Scholar]
- Prognostic value of nuclear survivin expression in oesophageal squamous cell carcinoma. Br J Cancer. 2003;88:115-9.
- [Google Scholar]
- Expression of survivin and its relationship to loss of apoptosis in breast carcinomas. Clin Cancer Res. 2000;6:127-34.
- [Google Scholar]
- Survivin mRNA expression in patients with breast cancer. Anticancer Res. 2002;22:1839-43.
- [Google Scholar]
- Expression of survivin is correlated with cancer cell apoptosis and is involved in the development of human pancreatic duct cell tumors. Cancer. 2001;92:271-8.
- [Google Scholar]
- Expression of survivin, a novel inhibitor of apoptosis and cell cycle regulatory protein, in pancreatic adenocarcinoma. Br J Cancer. 2002;86:886-92.
- [Google Scholar]
- Survivin expression in ovarian carcinoma: correlation with apoptotic markers and prognosis. Mod Pathol. 2003;16:574-83.
- [Google Scholar]
- Comparison of pHH3, Ki-67, and survivin immunoreactivity in benign and malignant melanocytic lesions. Am J Dermatopathol. 2008;30:117-22.
- [Google Scholar]
- Expression of the antiapoptosis gene, survivin, predicts death from recurrent colorectal carcinoma. Gut. 2000;46:645-50.
- [Google Scholar]
- Expression of survivin messenger RNA correlates with poor prognosis in patients with hepatocellular carcinoma. Diagn Mol Pathol. 2002;11:33-40.
- [Google Scholar]
- Survivin promotes cell proliferation in human hepatocellular carcinoma. Hepatology. 2000;31:1080-5.
- [Google Scholar]
- Increased expression of survivin in gastric cancer patients and in first degree relatives. Br J Cancer. 2002;87:91-7.
- [Google Scholar]
- Immunohistochemical localization of the IAP protein survivin in bladder mucosa and transitional cell carcinoma. Appl Immunohistochem Mol Morphol. 2002;10:134-8.
- [Google Scholar]
- Expression of the antiapoptosis gene survivin in human leukemia. Int J Hematol. 2002;75:161-5.
- [Google Scholar]
- Detection of survivin and p53 in human oral cancer: correlation with clinicopathologic findings. Head Neck. 2009;31:1039-48.
- [Google Scholar]
- Immunoprofiling of oral squamous cell carcinomas reveals high p63 and survivin expression. Oral Dis. 2014;20:e76-80.
- [Google Scholar]
- A survivin gene signature predicts aggressive tumor behavior. Cancer Res. 2005;65:3531-4.
- [Google Scholar]
- Down-regulation of survivin by oxaliplatin diminishes radioresistance of head and neck squamous carcinoma cells. Radiother Oncol. 2010;96:267-73.
- [Google Scholar]
- Oncoapoptotic signaling and deregulated target genes in cancers: special reference to oral cancer. Biochim Biophys Acta. 2013;1836:123-45.
- [Google Scholar]
- dbSNP-database for single nucleotide polymorphisms and other classes of minor genetic variation. Genome Res. 1999;9:677-9.
- [Google Scholar]
- Evaluation of p53 codon 72 polymorphism in adenocarcinomas of the colon and rectum in La Plata, Argentina. World J Gastroenterol. 2006;12:1426-9.
- [Google Scholar]
- The common germline Arg72Pro polymorphism of p53 and increased longevity in humans. Cell Cycle. 2008;7:158-63.
- [Google Scholar]
- Prognostic significance of p53 codon 72 polymorphism differs with race in colorectal adenocarcinoma. Clin Cancer Res. 2009;15:2406-16.
- [Google Scholar]
- Importance of TP53 codon 72 and intron 3 duplication 16bp polymorphisms in prediction of susceptibility on breast cancer. BMC Cancer. 2008;8:32.
- [Google Scholar]
- Functional polymorphisms in promoter survivin gene and its association with susceptibility to bladder cancer in North Indian cohort. Mol Biol Rep. 2012;39:5615-21.
- [Google Scholar]
- Association of survivin gene polymorphism with endometrial cancer. Int J Gynecol Cancer. 2012;22:35-7.
- [Google Scholar]
- Association of polymorphisms in survivin gene with the risk of hepatocellular carcinoma in Chinese Han population: a case control study. BMC Med Genet. 2012;13:1.
- [Google Scholar]
- Effects of caspase-9 and survivin gene polymorphisms in pancreatic cancer risk and tumor characteristics. Pancreas. 2010;39:976-80.
- [Google Scholar]
- Environmental and heritable factors in the causation of cancer-analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000;343:78-85.
- [Google Scholar]
- Distribution of coding apoptotic gene polymorphisms in women with extreme phenotypes of breast cancer predisposition and tolerance. Tumori. 2011;97:248-51.
- [Google Scholar]
- The expression of BIRC5 is correlated with loss of specific chromosomal regions in breast carcinomas. Genes Chromosomes Cancer. 2008;47:299-308.
- [Google Scholar]
- Two survivin polymorphisms are cooperatively associated with bladder cancer susceptibility. Int J Cancer. 2011;129:1872-80.
- [Google Scholar]
- Association between survivin gene promoter -31C/G polymorphism and urothelial carcinoma risk in Taiwanese population. Urology. 2009;73:670-4.
- [Google Scholar]
- The survivin -31 snp in human colorectal cancer correlates with survivin splice variant expression and improved overall survival. Cell Oncol. 2011;34:381-91.
- [Google Scholar]
- Survivin -31G/C promoter polymorphism and sporadic colorectal cancer. Int J Colorectal Dis. 2009;24:145-50.
- [Google Scholar]
- Role of survivin gene promoter polymorphism (-31G>C) in susceptibility and survival of esophageal cancer in northern India. Ann Surg Oncol. 2011;18:880-7.
- [Google Scholar]
- The association between the survivin C-31G polymorphism and gastric cancer risk in a Chinese population. Dig Dis Sci. 2009;54:1021-8.
- [Google Scholar]
- Survivin -31C/G polymorphism and gastric cancer risk in a Brazilian population. Clin Exp Med. 2011;11:189-93.
- [Google Scholar]
- Analysis of polymorphism in the survivin gene promoter as a potential risk factor for head and neck cancers development. Srp Arh Celok Lek. 2013;141:304-7.
- [Google Scholar]
- Combining factors on protein and gene level to predict radioresponse in head and neck cancer cell lines. J Oral Pathol Med. 2011;40:739-46.
- [Google Scholar]
- Survivin T9809C, an SNP located in 3’-UTR, displays a correlation with the risk and clinicopathological development of hepatocellular carcinoma. Ann Surg Oncol. 2012;19(Suppl 3):S625-33.
- [Google Scholar]
- The association between the survivin -31G/C promoter polymorphism and hepatocellular carcinoma risk in a Turkish population. Cancer Epidemiol. 2011;35:555-9.
- [Google Scholar]
- Lack of Association of BIRC5 polymorphisms with clearance of HBV infection and HCC occurrence in a Korean population. Genomics Inform. 2009;7:195-202.
- [Google Scholar]
- Polymorphisms in the survivin gene and the risk of lung cancer. Lung Cancer. 2008;60:31-9.
- [Google Scholar]
- Prognostic significance of survivin polymorphisms on non-small cell lung cancer survival. J Thorac Oncol. 2010;5:1748-54.
- [Google Scholar]
- Increased expression of inhibitor of apoptosis proteins, survivin and XIAP, in non-small cell lung carcinoma. Int J Oncol. 2009;35:1449-62.
- [Google Scholar]
- Functional promoter -31G>C variant in survivin gene is associated with risk and progression of renal cell cancer in a Chinese population. PLoS One. 2012;7:e28829.
- [Google Scholar]
- Initial apoptosis is followed by increased proliferation of apoptosis-resistant endothelial cells. FASEB J. 2005;19:1178-80.
- [Google Scholar]
- Autologous dendritic cell vaccines for non-small-cell lung cancer. J Clin Oncol. 2004;22:2808-15.
- [Google Scholar]
- Phase I clinical study of antiapoptosis protein, survivin-derived peptide vaccine therapy for patients with advanced or recurrent colorectal cancer. J Transl Med. 2004;2:19.
- [Google Scholar]
- Lack of toxicity of therapy-induced T cell responses against the universal tumour antigen survivin. Vaccine. 2005;23:884-9.
- [Google Scholar]
- Targeting survivin in cancer: novel drug development approaches. BioDrugs. 2014;28:27-39.
- [Google Scholar]
- Survivin signaling in clinical oncology: a multifaceted dragon. Med Res Rev. 2013;33:765-89.
- [Google Scholar]
- Induction of apoptosis and sensitization of head and neck squamous carcinoma cells to cisplatin by targeting survivin gene expression. Curr Gene Ther. 2012;12:444-53.
- [Google Scholar]
- Biology of oral cancer key apoptotic regulators. Boca Ratan, FL, USA: CRC Press, Taylor and Francis Group; 2014. p. :272.
- [Google Scholar]






