Translate this page into:
Streptococcus pyogenes pharyngitis & impetigo in a rural area of Panchkula district in Haryana, India
##For correspondence: Dr Rajesh Kumar, Professor & Head, School of Public Health, PGIMER, Chandigarh 160 012, India dr.rajeshkumar@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Sir,
To justify development of a vaccine against Streptococcus pyogenes [Group A Streptococcus (GAS)], the burden of streptococcal diseases needs to be estimated. There are several reports on the occurrence of GAS infection in India but information on the incidence of pharyngitis and impetigo is still limited1–4. Hence, incidence of GAS pharyngitis and impetigo was determined and GAS emm types were identified in rural children aged 7-11 yr in four primary schools of Raipur Rani Community Development Block of Panchkula district in Haryana, India. These schools were selected in villages where health care workers were available to assist project field staff. Permission from the respective schools authorities was taken to conduct the study.
After approval of study protocol by Institute Ethics Committee of Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh and the Institute Review Board of National Institute of Allergy and Infectious Diseases (NIAID), USA, a general physical examination of the registered children was done at each weekly visit by a physician except during holidays. The student volunteers were asked about symptoms of upper respiratory infections, i.e., sore throat, cough, nasal discharge, and the common cold. A diagnosis of pharyngitis was made if there were symptoms of sore throat, and on physical examination there was tonsillar and/or pharyngeal exudate, and/or pharyngeal erythema. Similarly, all children were examined for skin rash or eruption/lesion. If present, the nature of the lesion was noted, and cultured for a bacteriologic diagnosis. If the throat or skin cultures were positive for GAS, these patients were given a diagnosis of GAS pharyngitis and GAS impetigo, respectively. Pharyngitis patients were given a 10-days course of oral penicillin and impetigo patients were given betadine ointment for local application.
Standard methodology5 was employed for transportation of swabs, identification of ß-haemolysis of streptococci on sheep blood agar plates, and to determine Group A streptococcus using a Streptex Murex test kit (Remel Europe Ltd, UK). GAS was further processed for emm typing in the Molecular Genetics Laboratory at Department of Experimental Medicine and Biotechnology, PGIMER, Chandigarh using methods described earlier6. The emm gene sequence was searched for homology by BLAST (Basic Length Alignment Search Tool) search analysis (http://www.ncbi.nlm.nih.gov/BLAST/Blast.cgi). To be more precise the sequences were also submitted to CDC website (http://www.cdc.gov/ncidod/biotech/strep/strepblast.htm). Strains showing > 95 per cent sequence homology having maximum alignment with the reference strain in the CDC Gene Bank database were selected and designated particular parental emm type.
Weekly surveillance was conducted from July 2002 to April 2004 on 241 student volunteers aged 7 to 11 yr. Sample size calculations had assumed incidence of S. pyogenes pharyngitis to be at least 15/100 child-year with absolute precision of 5/100 child-year7. There were a total of 13,168 weekly observations done, out of a possible 18,222 due, with a participation rate of 72 per cent. The child-weeks at risk were derived from the number of weekly observations carried out among cohort children over the study period. The incidence (of GAS pharyngitis/impetigo) was estimated by dividing the number of (GAS pharyngitis/ impetigo) cases that occurred during the study period with the number of child-weeks at risk [Incidence per 1000 child-weeks (A) = GAS cases/13168 child-weeks X 1000 and incidence per 1000 child-years (B) = AX52 wk]. Isolation of GAS with the same emm type within a period of 14 days from the same child was considered a single infection (from one child same GAS emm type was identified within 14 days). Epi Info version 6 software (CDC, Altanta, USA & WHO, Geneva) was used to calculate 95 per cent confidence interval (CI) and odds ratio (OR).
There were 42 cases of GAS pharyngitis. The incidence of GAS pharyngitis was 3.2 cases/1000 child-weeks or 166 cases/1000 child-years (95% CI 122, 218). There were 97 GAS impetigo cases. The incidence of GAS impetigo was 7.4 cases/1000 child- weeks or 383 cases/1000 child-years (95% CI 323, 446) . GAS impetigo cases were more common in summer, whereas GAS pharyngitis was higher during winters (Fig.).
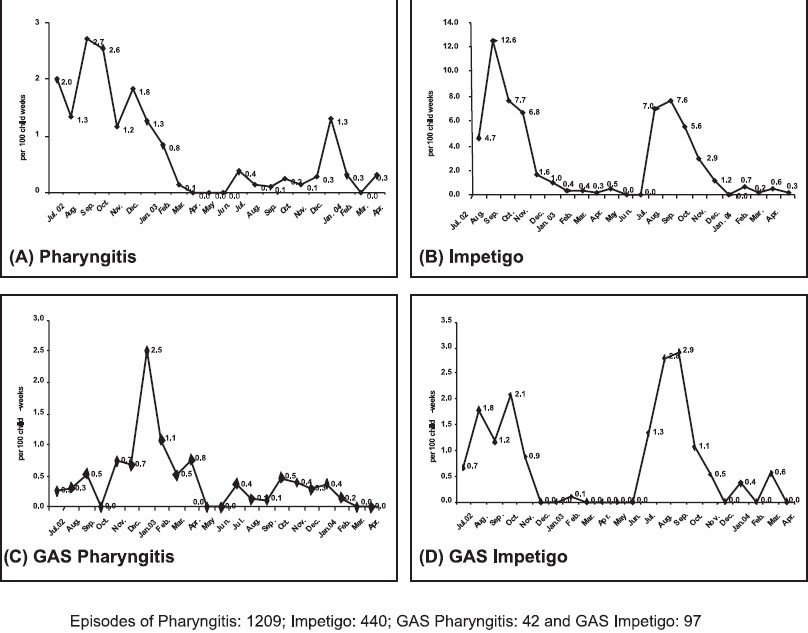
- Monthly incidence of pharyngitis, impetigo and Group A streptococcal infections during 2002-2004.
Approximately 15 per cent of school age children (150/1000 child-years) are estimated to have GAS pharyngitis each year7. For example, in an affluent population of Melbourne, the incidence of pharyngitis was 0.14 cases/child/ year (140 cases/1000 child-years), a rate similar to that seen in this study8. The incidence is estimated to be appreciably greater in developing countries7. Although India is a less developed country, the school children of our study lived in villages in a relatively prosperous rural region about 40 km from Chandigarh. In contrast, in an earlier study on school age children in a crowded slum area of Chandigarh, the incidence of GAS pharyngitis was reported to be 950 cases/1000 child-years3.
There is a consensus that it is difficult to make a diagnosis of GAS pharyngitis on the basis of signs and symptoms and physical examination alone, because virus infections are a frequent cause of pharyngitis. Also, there is an appreciable pharyngeal carriage of GAS in the general population. Recovery of GAS at the time of viral pharyngitis may lead to an erroneous diagnosis and give a false estimate of the incidence of GAS pharyngitis. For these reasons an alternative approach can be employed to estimate the incidence that relies on an estimate of the GAS pharyngeal carrier rate. To illustrate this strategy, the number of GAS positive pharyngitis patients (n=42) in 1209 pharyngitis cases that occurred during the study, were compared to the GAS pharyngeal carriage rate (n=26) in a monthly survey of children without symptoms (n=2016). This carrier study was conducted a year earlier in the same region as the four study villages6. Comparison revealed the odds ratio of GAS pharyngitis to be 2.62 (95% CI 1.55 - 4.04). While this suggests that patients with GAS positive pharyngitis were likely to have had a GAS infection, the conclusion is only tentative because the carrier survey was not done during the same year (although in the same locale) and not on the same children.
Twenty two of the student volunteers in our study had only one attack of pharyngitis. Seven children had GAS pharyngitis more than once; four children had twice, two had thrice, and one had GAS pharyngitis four times. In most instances, different emm types of GAS were isolated from children who had more than one episode of pharyngitis. GAS impetigo occurred a single time in 62 students. Seventeen children had GAS impetigo on two occasions. The same emm type was isolated from two of these children, but the second occurrence of impetigo was greater than 100 days. It is likely these were re-infections. There were 13 children who showed signs and symptoms of both GAS pharyngitis and impetigo during the two year follow up. In eight children, the impetigo and the pharyngitis were due to the same emm type, but the interval between the two events was between 68 and 271 days. Pharyngitis and impetigo due to the same emm type was uncommon. This was not surprising, because almost all impetigo occurred in the summer, when the attack rate of GAS pharyngitis was low, and one-third of the impetigo cases were due to emm types of GAS that did not cause pharyngitis.
Wannamaker had first recognized that impetigo was due to multiple M types that were often different from those that cause pharyngitis, although the same M type can cause both infections in children9. GAS M protein serotypes like M 1, 3, 4, 5, 12, 14, 18, 19, and 24 were found to be associated with throat infection, while M serotypes 2, 49, 57, 59, 60 and 61 are considered to be associated with impetigo10. In our study GAS emm44 and emm112 were predominantly associated with skin infection. The GAS emm1-2.2, first time identified in India was also from skin infected patients. Certain emm types have the potential for infecting both throat and skin sites11. There are numerous cases in which the same M type can be isolated simultaneously from both the pharynx and the impetigo lesion.
The emm types are not always adequate strain markers, as these can be shared by unrelated clonal types also. More so the strain variations have been noted within particular M types, and virulence has been linked with a particular isolate rather than being broadly related to a given serotype. GAS emm type 81.1 (23.8%, 10/42) was the most prevalent type recovered from pharyngitis. GAS emm type 81.1 and emm type 112.2 were recovered from 33% (32/97) of the patients with impetigo. Fourteen emm types/subtypes that caused impetigo did not cause pharyngitis, and six emm types/subtypes of GAS caused pharyngitis that did not cause impetigo (Table). The distribution of emm types of GAS isolated from impetigo was similar in both years. There was no evidence of clustering of pharyngitis or impetigo cases in any of the four schools under observation. The total number of pharyngitis patients for each school was similar.
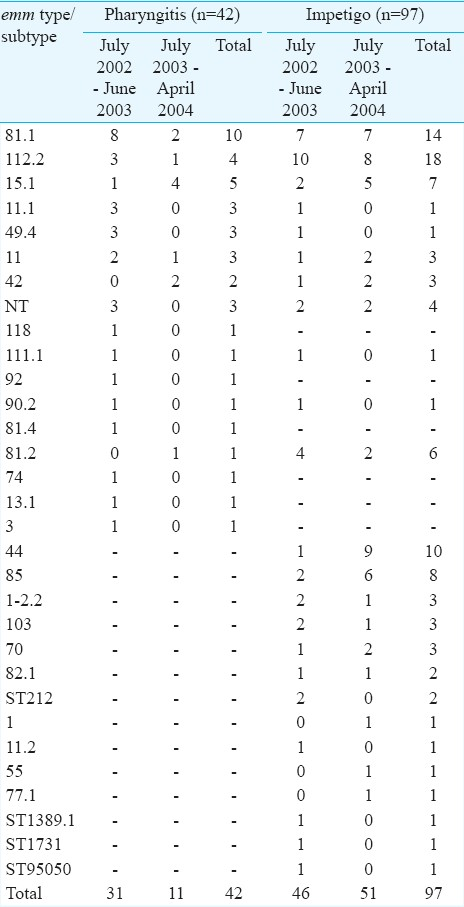
In our study, 23.8 per cent of the pharyngitis cases were caused by GAS emm type 81.1, with 76.2 per cent due to 16 different emm types/subtypes. Our findings support the earlier observations about the genetic heterogeneity among Indian skin and throat isolates1213. Such diversity was also seen in other settings14. It appears that serotypes present within a population vary between distinct geographical locations and may also change with passage of time. Kaplan et al15 reported displacement of M1 GAS with M6 in USA. Thus, surveillance is needed to identify the diversity of M types of GAS, and the emergence of M types not previously present in the population.
There are a few instances today where only several predominant GAS M types cause the majority of infections. Notable exceptions are: M 18 causing the majority of endemic ARF in the Salt Lake City area16, and M 3 as the primary cause of necrotizing fascitis in Canada and elsewhere17.
This study was financially sponsored and supported by Department of Biotechnology, Government of India, New Delhi, and also in part by the intramural program of the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH), USA.
Conflict of interest: none.
References
- Epidemiology of streptococcal pyoderma in an orphanage community of a tropical country. J Trop Med Hyg. 1988;91:306-14.
- [Google Scholar]
- A study on sore throat and β hemolytic streptococcal pharyngitis among rural school children in Varanasi, with reference to age and season. Indian J Pub Health. 1988;32:191-8.
- [Google Scholar]
- Group A streptococcal sore throat in a periurban population of northern India: a one year prospective study. Bull World Health Organ. 2001;79:528-33.
- [Google Scholar]
- Bacteriological and molecular studies of group A streptococcal pharyngitis in a south Indian hospital. Indian J Med Microbiol. 2008;26:197-8.
- [Google Scholar]
- Laboratory diagnosis of group A streptococcal infections: a laboratory manual. Geneva: World Health Organization; 1996. p. :16-9.
- Epidemiology of Group A Streptococcal pharyngitis and impetigo: A cross sectional and follow-up study in a rural community of Northern India. Indian J Med Res. 2009;130:765-71.
- [Google Scholar]
- The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005;5:685-94.
- [Google Scholar]
- The burden of group A streptococcal pharyngitis in Melbourne families. Indian J Med Res. 2004;119:144-7.
- [Google Scholar]
- Differences between streptococcal infections of the throat and of the skin. N Engl J Med. 1970;282:78-85.
- [Google Scholar]
- Can we eradicate rheumatic fever in the 21st century? Indian Heart J. 2001;53:25-34.
- [Google Scholar]
- The dynamics of streptococcal infections in a defined population of children: serotypes associated with skin and respiratory infections. Am J Epidemiol. 1976;104:652-66.
- [Google Scholar]
- emm types of Streptococcus pyogenes in Chennai. Indian J Med Microbiol. 2001;19:161-2.
- [Google Scholar]
- Molecular heterogeneity among north Indian isolates of group A streptococcus. Lett App Microbiol. 2004;39:84-8.
- [Google Scholar]
- Global emm type distribution of group A streptococci: systematic review and implications for vaccine development. Lancet Infect Dis. 2009;9:611-6.
- [Google Scholar]
- Dynamic epidemiology of group A streptococcal serotypes associated with pharyngitis. Lancet. 2001;358:1334-7.
- [Google Scholar]
- Genome sequence and comparative microarray analysis of serotype M18 group A streptococcus strains associated with acute rheumatic fever outbreaks. Proc Natl Acad Sci USA. 2002;99:4668-73.
- [Google Scholar]
- Genome-wide molecular dissection of serotype M3 group A Streptococcus strains causing two epidemics of invasive infections. Proc Natl Acad Sci USA. 2004;101:11833-8.
- [Google Scholar]





