Translate this page into:
Role of microbial dysbiosis in carcinogenesis & cancer therapies
For correspondence: Dr S. Kannan, Laboratory of Cell Cycle Regulation & Molecular Oncology, Division of Cancer Research, Regional Cancer Centre, Thiruvananthapuram 695 011, Kerala, India e-mail: kannans@rcctvm.gov.in
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The human body supports a heterogeneous population of microorganisms. Every microorganism has the ability to contribute to the unique microenvironment around it. The aim of this review is to discuss the changes in the microbial population and their relative abundance across different ecosystems of the human body, the interactions within the microbial communities, metabolites they secrete to their external environment, their immunomodulatory functions, their signal transduction pathways and how these respond to environmental stimuli such as various diets, alcohol and drug consumption, smoking and finally suggest new therapeutic approaches. The microbiota may leads to cancer through inflammation mediated mechanisms which modulate immune responses, or produce carcinogenic metabolites and genotoxins, or deregulate cell proliferative signalling pathways. The identification of these molecular mechanisms in carcinogenesis may lead to better treatment strategies. In this review we have tried to explore the changes in microbial composition between cancer and normal tissues and what molecular mechanisms provide a connecting link between microbial dysbiosis and cancer.
Keywords
Cancer therapies
carcinogenesis
dysbiosis
immunomodulation
microbiome
Microbiome plays an important role in maintaining the normal physiology of the human body. The changes occurring in the microbial composition induce production of toxins, chronic inflammations and carcinogenic metabolites through several mechanisms. Dysbiosis can be defined as an imbalance in number and types of microbial population, which can modulate microenvironment and homeostasis in host through over proliferation of a certain microbial species. Thus, dysbiosis may directly or indirectly contribute to carcinogenesis in human beings1. Studies on microbiome are mainly concentrated on regions such as hair2, mouth3, nostrils4, gut5, stomach6 and colon7 of the human body.
The conventional culture-based microbial studies are inadequate to understand species diversity and relative abundance. In the conventional microbial culture based technique, 16s rRNA gene sequencing is a PCR based technique, which provides a method for the identification of bacterial member in microbiome. The mechanism involves amplification of prokaryotic small ribosomal RNA (16s rRNA) gene by PCR89. The primer used in PCR binds to a conserved region of rRNA, while the extension step occurs on a highly variable region of rRNA, which is specific for each species. To overcome limitations, emerging molecular techniques for the microbiome research including next-generation sequencing, advanced culture technologies and its combination with metabolomics are also used in research10.
Role of dysbiosis of skin microbiome in carcinogenesis
Human skin microbiome contains a highly diverse community of hundreds of species inhabiting the skin11. Each area of skin provides a different environment with varying, temperature, moisture, pH, salinity, sebum content and intrinsic factors. These differences along with other lifestyle related factors determine the composition of microbiota on different skin habitats. Skin contains highly uneven surface having many invaginations with hairs and follicles, sebaceous glands; these protruding structures along with the presence of sweat glands make skin a diverse habitat and consequently, the microbes present on the skin are different in each part of the body12. The microbial diversity is more abundant in moist body sites, where the sweat gland produce unfavourable growth condition for the microorganisms and thereby allow the colonization of only certain microorganisms. Sebaceous glands secrete hydrophobic lipid rich sebum which acts as an antimicrobial agent in hairy areas of the skin1314 whereas, Staphylococcus epidermidis survive in commensal relationship with the skin surface and forms a part of commensal microbiota in skin. It secretes a serine protease enzyme that inhibits Staphylococcus aureus colonization15. These regulatory mechanisms on skin surface prevent changes in microbial population and resist over proliferation of certain microbial strains over the other. The microbiome inside the body is involved in the activation of immune cells, especially cells that produce inflammation16.
An in vivo study on skin cancer mice model demonstrated that the colonization of certain flagellated microorganisms promoted carcinogenesis through inherent signalling mechanism in the host. The microenvironments around the wound provide favourable environment for the growth of opportunistic microorganisms, especially flagellated bacteria like Escherichia coli and Pseuodomonas aeruginosa17. Flagellin and other microbial particles induce inflammation and activate toll-like receptor 5 (TLR-5) mediated signalling mechanisms in wound18. A study confirms that topical application of flagellin on the wound increases tumour growth, while antibiotic treatment and ablation of TLR-5 produce an anti-tumourogeic effect19. This indicates that through microbial dysbiosis human immune system can induce tumour formation. Multiple immunity-related mechanisms have been suggested to explain the link between skin microbiome and cancer20, but a detailed profiling of microbial population is necessary to unmask the molecular mechanisms which induce the carcinogenesis21.
Dysbiosis of oral microbiota influences carcinogenesis
Oral microbiome occupies different ecological niches in an oral cavity, and each niche contains a different composition of microbiota. It is one of the most diverse microbial communities with more than 500-700 species22. Each part of the oral cavity including lips, gingivae, teeth, hard palate, buccal mucosa, tongue and floor of the mouth provides an optimum environment for the proliferation of different microorganisms in a symbiotic manner. This environmental diversification contributes to the existence of a large number of species in a small ecosystem. Epidemiological studies indicate differences in the species abundance from one site to another23. The oral cavity itself contains several physical and chemical factors secreted by the commensal microbiota, which render protection from various microbial pathogens through immune system activation and resource competition24. The commensal microbiota not only provides resistance against infectious agents but also plays a vital role in the digestion, maintenance of homeostasis, signal transduction and other cellular processes25.
In some oral cancers, an over proliferation of some commensal microbial species such as Streptococcus anginosus or Fusobacterium nucleatum has been observed. The infection of S. anginosus in oral mucosa induces overproduction of nitric oxide which leads to DNA damage and finally lead to carcinogenesis26. Similar to S. anginosus, F. nucleatum is a pro-inflammatory, anaerobic, adherent bacterial oral pathogen. Typically, F. nucleatum is seen in the oral cavity, but in chronic periodontitis, the balance between the host-microbiota interactions is broken27. Increase in the number of F. nucleatum represents an example of an opportunistic infection at an immunocompromised site28. The chronic periodontitis leads to chronic inflammation, so the infected cells have a high risk to develop into premalignant lesions and finally into tumour29. A study using chronic inflammation associated tumourigenesis animal model has demonstrated that F. nucleatum and Porphyromonas gingivalis modulate Interleukin 6 (IL-6) – signal transducer and activator of transcription 3 (STAT 3) axis of inflammatory signalling pathways27. These bacteria also help in tumour progression through atypical activation of immunocytes, which then produce DNA damage through generation of reactive chemical species27. In addition to IL-6 mediated immune responses, F. nucleatum also promotes lipopolysaccharide-induced production of inflammatory cytokines such as tumour necrosis factor α (TNF α), IL-1β and IL-12 and IL-1730. These immune responses result in the upregulation of inflammation induced transcription factor such as nuclear factor kappa beta (NF-κB), which promotes tumourigenesis at the site of infection31. Another bacterium P. gingivalis increases prostaglandin-endoperoxide synthase expression through cyclooxygenase-2 gene expression and thereby causes symptoms of inflammation by bringing pro-inflammatory mediators into the site of infection32. These microbial mechanisms can indirectly induce inflammation mediated carcinogenesis and tumour progression.
Role of dysbiosis of gut microbiota in carcinogenesis
F. nucleatum is rarely seen in healthy human gastrointestinal tract. F. nucleatum has the ability to induce damage to the epithelial lining of colon30. An in vivo studies with murine models has demonstrated that specific myeloid cell group penetrates into the tumour and produces pro-inflammatory signals3132. Cancer promoting property of F. nucleatum is mediated through FadA adhesion mechanism33. FadA bind to E-cadherin in the host cell and activates the β-catenin signalling pathways. FadA also promotes expression of oncogenes and regulates the inflammatory responses when the Fusobacterium adheres to it34. Unlike the chronic inflammation mediated mechanism of tumourigenesis, Fusobacterium can induce tumour formation by reconstruction of microenvironment around the host cells and in turn infiltrates into the tumour and promotes cell proliferation. The binding and activation of the F. nucleatum to the FadA motif promotes the signalling mechanism that alters epithelial barrier which leads to the loss of tight junction interactions, the mucus layer integrity, epithelial cell polarity and cell to cell adhesion35. These alterations in the epithelial barrier induce the invasion of the Fusobacterium species and accelerate cell-mediated immunity against the bacterium inside the tumour36. The inflammatory genotoxic condition produced by the immune cells triggers DNA damage and finally promotes cell proliferation37 (Figure). Similar to the β-catenin signal activation by F. nucleatum, the pathogenic bacteria species Bacteroides fragilis produces a toxin with an additional oncogenic function. This toxin cleaves β-catenin and activates signalling pathway for cell proliferation. In addition to the interactions between the immune cells and the microbial products, it will also induce DNA damage and contribute to tumourogenesis in the host. The epithelial barrier functions to separate the immune cells from this microbial microenvironment. When a tumour developes, the immune/inflammatory cells (CD4, TH1 cells, TH-17 cells, etc.) infiltrates the epithelial barrier and come in contact with the tumour as well as the microbial community38. Activated microbial products will induce the production of IL-17 and IL-23 from myeloid cells35. These ILs are mediators of inflammation and promote a chronic inflammation around the tumour to form a favourable microenvironment for the growth of the tumour39 (Figure).
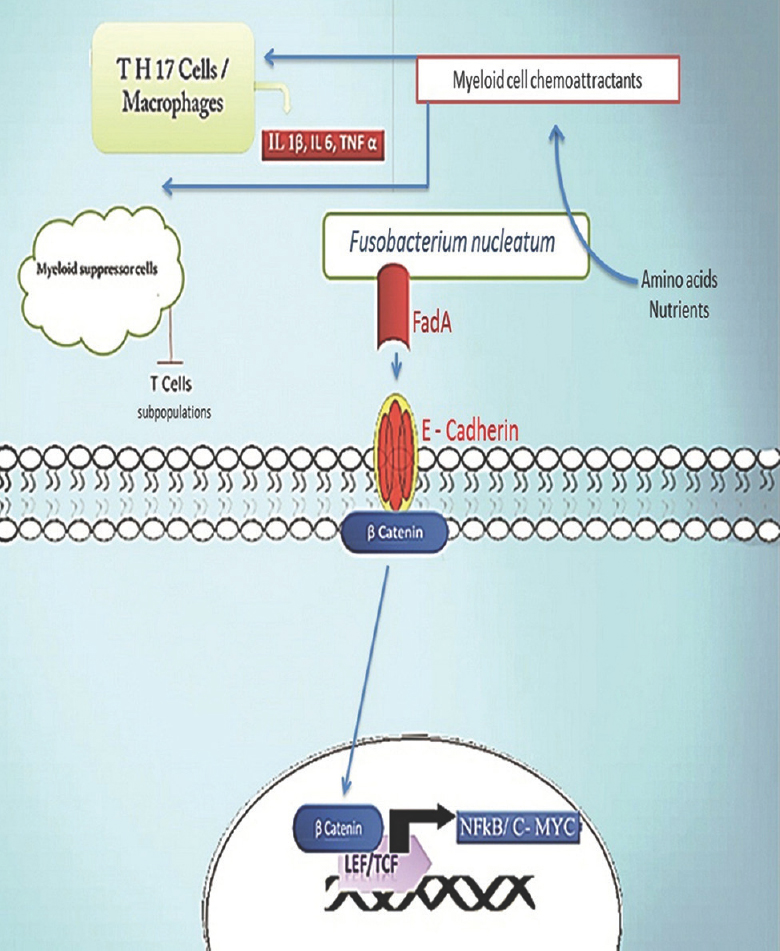
-
Fusobacterium nucleatum through FadA activates NF- kB and C-MYC transcription factors mediated cell proliferation pathway. Fusobacterium species produces short peptides or short chain fatty acids which act as chemo-attractants, and attracts TH 17 cells and myeloid derived suppressor cells (MDSCs) to the tumour site. TH 17 cells produce interleukin 1 beta (IL-1β), interleukin 6 (IL-6) and tumour necrosis factor α (TNF α), while myeloid suppressor cells suppress some T cell subpopulations such as CD4+ cells in tumour microenvironment. Fusobacterium also leads to the increased presence of tumour associated macrophages near tumour. Fusobacterium express FadA ligand molecules on its surface which binds to E cadherin which is an adhesion molecule in epithelial cells and activates E cadherin mediated β catenin cell proliferation pathway. Activation of β catenin signalling leads to its translocation inside the nucleus where it leads to increase in expression of oncogenes such as C-MYC and NF-kB by binding to coactivator transcription factors lymphoid enhancer factor (LEF) and T cell factor (TCF).
Enterococcus faecalis forms a part of the intestinal microbiome. The abnormal proliferation of this bacterial strain induces DNA damage and generates colorectal carcinomas40. E. faecalis can produce extracellular superoxide (O*2), hydroxyl radical and H2O2 through demethylmenaquinone-mediated autoxidation. The ability of E. faecalis to induce DNA damage was proved in a mice model and it was found that these free radicals were the cause of chromosomal instability and these were associated with colorectal cancer41. The gastrointestinal tract develops adaptive immunity with the help of intestinal microbiota in the peripheral region. The interaction between intestinal mucosa and microbiota is mediated through Toll – like receptors, (TLRs) these interactions play a vital role in maintaining homeostasis. When the mucosal barrier ruptures, immune cells such as T cells and macrophages activate TLR-dependent pathway which increases epithelial cell proliferation and recruits inflammatory cells to the site. The TLRs may also be activated by the infectious microorganism like; Helicobacter pylori and Listeria monocytogenes through which this natural mechanism turns into a carcinogenic mechanism4243.
Dysbiosis of microbiome and gynaecologic cancers
As mentioned earlier, inflammatory mediated mechanisms and cytotoxic mechanisms induce tumour progression mediated by the human microbiota. The gynaecologic cancers also form a part of such cancers which develop into tumours through dysbiosis of microbiota. Studies on dysbiosis have revealed the association between change in microbial community and human papillomavirus infection44. A woman whose vaginal microbiota is low in Lactobacillus gasseri species and high in Atopobium possesses a healthy composition of the microbiota, while HPV-positive women show higher number of L. gasseri and Gardnerella vaginalis species45. The pH of the vaginal region supports the invasion and sustainability of the HPV virus46. Similarly, ovarian cancer is also influenced by the upper reproductive tract microbiota. The colonization of the pathogenic bacteria activates the inflammatory pathways in uterus and fallopian tubes which promote immunomodulation and tumourigenesis47.
The vaginal microbiota and uterine cancer are linked by two inflammatory diseases viz. pelvic inflammatory disease (PID) and bacterial vaginosis. Bacterial vaginosis promotes the dysbiosis of vaginal microbiota, especially the population of G. vaginalis, Mycoplasma hominis and Ureaplasma urealyticum4849. Changes in the relative population size lead to PID, as a consequence of which epithelial dysfunction and chronic inflammation occur simultaneously in the uterine region. These conditions together promote tumour growth and invasion49.
Factors influencing dysbiosis of microbiome
Studies indicate that development of cancers is also linked to lifestyle-related risk factors. These factors form a major cause of dysbiosis, and these include smoking, diet imbalance, alcohol consumption, obesity and lack of physical exercise505152. These changes can be studied through metagenomic studies, which help to identify dysbiosis and their relationship with genetic susceptibility in a comprehensive way. Diet imbalance or a specific food pattern may contribute towards cancer development via dysbiosis53. The risk factors such as alcohol consumption and tobacco use form a common cause of cancer when compared to other lifestyle-related factors. Although the diet related disorders account for a negligible portion of cancer cases, but some dietary habits such as high meat or pork intake, low vegetable or fruit consumption increase susceptibility to cancer54. Cancer preventive diet can overcome some effects caused due to changes in the microbial community. The diet with green vegetables and fruits has high level of antioxidants and carotenoids that protect cells from triplet sensitizers, singlet oxygen, and radical intermediates and maintains a normal commensal microflora inside the body55.
Acetaldehyde is considered as teratogenic, genotoxic and mutagenic agent because it reacts with DNA and produces a N2-ethylidenedeoxyguanosine adduct565758. It also interacts with deoxyguanosine in the DNA to form 1 N2-propano-2-deoxyguanosine adduct59. Therefore, the accumulation of acetaldehyde may lead to permanent alterations in the genetic material. Many investigations on microorganisms in the oral cavity have shown that one of the reasons for an increase in the alcohol dehydrogenase level when compared to aldehyde dehydrogenase is due to dysbiosis in the oral microbiome60. Neisseria is the part of commensal microflora of oral cavity, some of the non-pathogenic Neisseria species produce extremely high amount of alcohol dehydrogenase enzyme in the oral cavity and may lead to carcinogenesis6162. On the other hand, an elevated level of Streptococcus salivarius, Candida albicans and some Gram-positive bacteria and yeast show ability to produce acetaldehyde in the presence of alcohol in the oral cavity63.
Another reason for the dysbiosis in the oral cavity is tobacco consumption. The immunomodulatory functions of tobacco allow invasion of pathogenic microorganisms to the oral cavity, nasal mucosa, throat, trachea and lungs64. In a comparative study on smokers and non-smokers, Treponema denticola, Prevotella intermedia, F. nucleatum, P. gingivalis, Tannerella forsythensis, Campylobacter rectus, Eikenella corrodens, Peptostreptococcus micros, Aggregatibacter actinomycetemcomitans and tar-resistant S. aureus infections were present in most smokers656667. These infectious agents can activate inflammatory molecules; which mainly involves an increase in the production of TNF α, IL-1β and IL-661. Therefore, the increase in free radical exposure, reduced nutrient metabolism, tumour-promoting enzyme activation, blockage of detoxifying enzyme, change in the hormone status and carcinogenic components from alcohol or smoke may promote cancer through dysbiosis in the oral cavity.
Dietary changes may induce carcinogenesis through microbial activity in intestines. For example, protein-rich diet will increase protein fermentation and production of amino acid derivatives in the intestine. Some of the amino acid derivatives such as branched-chain fatty acids and phenylacetic acids are produced by bacteria belonging to the phylum Bacteriodetes and Firmicutes68. The overproduction of these amino acid derivatives leads to the nitrosation of these products leading to Liposuction of N-nitroso compounds. These compounds are potential carcinogens; and may damage DNA through alkylation and cause mutations69. Red meat consumption also modulates the microenvironment in the intestinal region. The tumour-associated microorganism F. nucleatum produces hydrogen sulphide in the presence of red meat which cause DNA damage in the colonic epithelium and the cells surrounding the tumour aid in the tumour formation and progression70. The polyamines are organic molecules derived from the arginine produced in the host tissues; these play vital role in maintaining the integrity of cell membrane and synthesis of nucleic acids71. Protein-rich diet will lead to the activation of protein fermenting microbiota and an increase in their number. This alteration causes overproduction of polyamines not only from the host tissues but also from gut microbial population72 such as B. fragilis, Salmonella enterica, H. pylori, Streptococcus pneumoniae, Shigella flexneri, etc. The catabolism of polyamines produces oxidative stress and DNA damage in the intestinal mucosa73.
Future research and therapeutic applications of the microbiome in cancer
Changes in microbial composition tend to disturb the cell microenvironment leading to an increase in proliferation of a particular species of microorganism. These alterations may cause DNA damage or tumour development, but in some exceptional cases, it might inhibit tumour as well74. The microbiome has the ability to enhance the efficacy of therapeutic treatments by modulation of host immune system. Therefore, microbiome does not always act to promote tumour formation but also play a vital and unique role to suppress tumour development with the aid of certain composition of microbes7576. The tumour cells adapt certain immune checkpoint pathways to desensitize the host immune system against T cell and the antibody attack77. The immune checkpoint pathways are a self-defensive mechanism of the host to prevent autoimmune disorders. If these pathways are inhibited in tumour, the immune system can attack the tumour cells without any resistance78. The bacterial species of the commensal microbiome, Bifidobacterium strengthen the immunity against the tumour and intensify the activity of host dendritic cells77. It also suggests the manipulation of gut microbiome to enhance the efficiency of cancer immune therapy77 (Table I).
| Tumour site | Microbial dysbiosis | Carcinogenic mechanism | References |
|---|---|---|---|
| Skin | An increase in Staphylococcus aureus, while a decrease in abundance of S. epidermidis | More likely to develop atopic dermatitis and non-melanoma skin cancer or basal cell carcinomas | 1516 |
| Population of flagellated bacteria like Escherichia coli, Psuedomonas aeruginosa, Shigella are increases | Flagellin-mediated TLR 5 activation and inflammatory responses | 171819 | |
| Oral cavity including lips, gingivae, teeth, hard palate, cheek mucosa, mobile tongue and floor of the mouth | Over proliferation of Streptococcus anginosus | DNA damage through overproduction of nitric oxide | 26 |
| Increased population of Porphyromonas gingivalis, Fusobacterium nucleatum | Produces reactive oxygen species and nitrogen species which interact with bacterial or viral particles to form peroxynitrite leads to DNA damage | 27 | |
| Overproliferation of S. anginosus, F. nucleatum | Infection causes chronic periodontitis which leads to chronic inflammation, high risk to become pre-malignant lesions | 27282930 | |
| Neisseria, Streptococci and some other gram-negative microorganisms and yeast in presence of alcohol | Produces an extremely high amount of alcohol dehydrogenase enzyme and acetaldehyde which is, genotoxic and mutagenic | 616263 | |
| Gastrointestinal tract | Elevated level of F. nucleatum | FadA adhesion mechanism, also promotes cell proliferation with oncogene activation | 343536 |
| Abnormal proliferation of Enterococcus faecalis | Produces extracellular superoxide, which induces chromosomal instability, leads to colorectal cancer | 4041 | |
| Ovary, fallopian tube, uterus, cervix, vagina and vulva | Elevated level of Gardnerella vaginalis, Mycoplasma hominis, Ureaplasma urealyticum | Through chronic inflammation and immunomodulation | 454849 |
TLR-5, toll like receptor 5
Colorectal cancer shows high permeability towards inflammatory cytokine-producing cells. These tumour-infiltrating cells are able to activate intracellular pathways which help the colorectal tumour cells to grow79. The spent medium from the culture of tumour-infiltrating leukocytes has been shown to enhance the growth of colorectal carcinoma cell lines by activation of intracellular pathways and transcription factors such as STAT 3 and NF-kB79. T helper 17 cells from the tumour infiltrating leukocytes produce a large number of pro-inflammatory cytokines (IL-17A, IL-17F, IL-21 and IL-22), TNF-α and IL-6. These cytokines increase proliferation of colorectal cancer cells80. The gut microbiome can shift through intake of an oral probiotic, which contains a unique probiotic mixture enriched with certain beneficial bacterial genera like, Alistipes, Butyricimonas, Mucispirillum, Oscillibacter, Parabacteroides, Paraprevotella and Prevotella81. Presence of these microorganisms downregulates the Th 17 cell infiltration and presence of inflammatory cytokines in the tumour. The metabolites released from the probiotic bacterial community inhibit the tumour-promoting activity of immune cells by activate the polarization of anti-inflammatory Type 1 regulatory T cells (Treg/Tr1) and promote their differentiation in the gut region82. Experiments conducted on hepatocellular carcinoma cell lines showed that these probiotic infused therapies might reduce tumour volume up to 40 per cent when compared to control81.
As mentioned earlier the interaction between the tumour-associated immune cells and the microbiota induce inflammation, which contributes to the development of cancer. Therefore, the removal of the microorganism from the tumour surface may greatly reduce the production of a pro-inflammatory cytokine by the tumour-associated immune cells16. CpG-oligonucleotide immunotherapy and platinum chemotherapy are common treatment methodologies followed for gastrointestinal cancers83.
Conclusion
The unique environment present in the human body promotes a specific composition of microbes to grow and contribute distinct functions. Through mutual interactions, each and every microorganism contributes toward the proper functioning of the human body. The diversity and relative abundance of the microbiome vary from site to site. Therefore, alterations in the microbiome can be uncovered only through comparative studies between normal and disease conditions. The microbiota may possibly give rise to cancer through the inflammation mediated mechanisms such as modulation of immune responses, or production of carcinogenic metabolites and genotoxins, or through activation of cell proliferative signalling pathway. The identification of these molecular mechanisms of carcinogenesis will aid to improve treatment strategies. Along with new improved cancer therapies favourable microbiota inducing diet, anti-inflammatory drugs administration, prebiotic or probiotic treatment, transplantation of microbiome, administration of genotoxin neutralizing agents may aid in improving treatment outcomes.
Acknowledgment:
In first author (JV) acknowledges the University Grants Commission, Government of India, New Delhi for providing Junior Research Fellowship.
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- Microbiome in healthy skin, update for dermatologists. J Eur Acad Dermatol Venereol. 2016;30:2038-47.
- [Google Scholar]
- Characterization of the upper and lower respiratory tract microbiota in Piedmontese calves. Microbiome. 2017;5:152.
- [Google Scholar]
- The human gut microbiome – A potential controller of wellness and disease. Front Microbiol. 2018;9:1835.
- [Google Scholar]
- Molecular characterization of the human stomach microbiota in gastric cancer patients. Front Cell Infect Microbiol. 2017;7:302.
- [Google Scholar]
- Effect of water flow and chemical environment on microbiota growth and composition in the human colon. Proc Natl Acad Sci U S A. 2017;114:6438-43.
- [Google Scholar]
- An ecological and evolutionary perspective on human-microbe mutualism and disease. Nature. 2007;449:811-8.
- [Google Scholar]
- Emerging technologies for gut microbiome research. Trends Microbiol. 2016;24:887-901.
- [Google Scholar]
- The normal flora of the skin in different age groups. Br J Dermatol. 1969;81:248-58.
- [Google Scholar]
- Epithelial antimicrobial defence of the skin and intestine. Nat Rev Immunol. 2012;12:503-16.
- [Google Scholar]
- Staphylococcus epidermidis Esp degrades specific proteins associated with Staphylococcus aureus biofilm formation and host-pathogen interaction. J Bacteriol. 2013;195:1645-55.
- [Google Scholar]
- Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342:967-70.
- [Google Scholar]
- Interaction of the microbiome with the innate immune response in chronic wounds. Adv Exp Med Biol. 2012;946:55-68.
- [Google Scholar]
- Innate sensing of microbial products promotes wound-induced skin cancer. Nat Commun. 2015;6:5932.
- [Google Scholar]
- AllergoOncology: Opposite outcomes of immune tolerance in allergy and cancer. Allergy. 2018;73:328-40.
- [Google Scholar]
- Oral cancer in southern India: The influence of smoking, drinking, paan-chewing and oral hygiene. Int J Cancer. 2002;98:440-5.
- [Google Scholar]
- The role of the microbiota in inflammation, carcinogenesis, and cancer therapy. Eur J Immunol. 2015;45:17-31.
- [Google Scholar]
- Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012;22:299-306.
- [Google Scholar]
- Periodontal pathogens Porphyromonas gingivalis and Fusobacterium nucleatum promote tumor progression in an oral-specific chemical carcinogenesis model. Oncotarget. 2015;6:22613-23.
- [Google Scholar]
- Chronic periodontitis and the incidence of head and neck squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2009;18:2406-12.
- [Google Scholar]
- Granulocyte-macrophage colony-stimulating factor amplification of interleukin-1beta and tumor necrosis factor alpha production in THP-1 human monocytic cells stimulated with lipopolysaccharide of oral microorganisms. Clin Diagn Lab Immunol. 1998;5:341-7.
- [Google Scholar]
- Fusobacterium nucleatum, inflammation, and immunity: The fire within human gut. Tumour Biol. 2016;37:2805-10.
- [Google Scholar]
- Fusobacterium nucleatum infection of colonic cells stimulates MUC2 mucin and tumor necrosis factor alpha. Infect Immun. 2011;79:2597-607.
- [Google Scholar]
- Inhibitory effect of low-level laser irradiation on LPS-stimulated prostaglandin E2 production and cyclooxygenase-2 in human gingival fibroblasts. Eur J Oral Sci. 2000;108:29-34.
- [Google Scholar]
- Crystal structure of FadA adhesin from Fusobacterium nucleatum reveals a novel oligomerization motif, the leucine chain. J Biol Chem. 2009;284:3865-72.
- [Google Scholar]
- Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe. 2013;14:195-206.
- [Google Scholar]
- Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491:254-8.
- [Google Scholar]
- Fusobacterium spp. and colorectal cancer: cause or consequence? Trends Microbiol. 2013;21:506-8.
- [Google Scholar]
- Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe. 2013;14:207-15.
- [Google Scholar]
- Fusobacterium nucleatum and the Immune System in Colorectal Cancer. Curr Colorectal Can Rep. 2019;15:149-56.
- [Google Scholar]
- Fusobacterium nucleatum Contributes to the Carcinogenesis of Colorectal Cancer by Inducing Inflammation and Suppressing Host Immunity. Translational Oncology. 2019;12:846-51.
- [Google Scholar]
- Enterococcus faecalis produces extracellular superoxide and hydrogen peroxide that damages colonic epithelial cell DNA. Carcinogenesis. 2002;23:529-36.
- [Google Scholar]
- Extracellular superoxide production by Enterococcus faecalis promotes chromosomal instability in mammalian cells. Gastroenterology. 2007;132:551-61.
- [Google Scholar]
- Listeria monocytogenes promotes tumor growth via tumor cell toll-like receptor 2 signaling. Cancer Res. 2007;67:4346-52.
- [Google Scholar]
- Role of Toll-like receptors in gastrointestinal malignancies. Oncogene. 2008;27:234-43.
- [Google Scholar]
- Microbiome factors in HPV-driven carcinogenesis and cancers. PLoS Pathog. 2020;16:e1008524.
- [Google Scholar]
- Comparison of the vaginal microbiota diversity of women with and without human papillomavirus infection: A cross-sectional study. BMC Infect Dis. 2013;13:271.
- [Google Scholar]
- A large, population-based study of age-related associations between vaginal pH and human papillomavirus infection. BMC Infect Dis. 2012;12:33.
- [Google Scholar]
- A prospective study of circulating C-reactive protein, interleukin-6, and tumor necrosis factor α receptor 2 levels and risk of ovarian cancer. Am J Epidemiol. 2013;178:1256-64.
- [Google Scholar]
- A cluster analysis of bacterial vaginosis-associated microflora and pelvic inflammatory disease. Am J Epidemiol. 2005;162:585-90.
- [Google Scholar]
- Lack of Vitamin D receptor causes dysbiosis and changes the functions of the murine intestinal microbiome. Clin Ther. 2015;37:996-1009.e7.
- [Google Scholar]
- Western lifestyle: A ‘master’ manipulator of the intestinal microbiota? Gut. 2014;63:5-6.
- [Google Scholar]
- Obesity, motility, diet, and intestinal microbiota-connecting the dots. Curr Gastroenterol Rep. 2019;21:15.
- [Google Scholar]
- Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015;16:1599-600.
- [Google Scholar]
- The Benefits and Risks of Certain Dietary Carotenoids that Exhibit both Anti- and Pro-Oxidative Mechanisms-A Comprehensive Review. Antioxidants (Basel). 2020;9:264.
- [Google Scholar]
- Genotoxicity of acetaldehyde- and crotonaldehyde-induced 1,N2-propanodeoxyguanosine DNA adducts in human cells. Mutat Res. 2006;608:1-7.
- [Google Scholar]
- Alcohol intake interacts with functional genetic polymorphisms of aldehyde dehydrogenase (ALDH2) and alcohol dehydrogenase (ADH) to increase esophageal squamous cell cancer risk. J Thorac Oncol. 2019;14:712-25.
- [Google Scholar]
- DNA damage induced by endogenous aldehydes: Current state of knowledge. Mutat Res. 2011;711:13-27.
- [Google Scholar]
- Polyamines stimulate the formation of mutagenic 1, N2-propanodeoxyguanosine adducts from acetaldehyde. Nucleic Acids Res. 2005;33:3513-20.
- [Google Scholar]
- Non-pathogenic Neisseria: Members of an abundant, multi-habitat, diverse genus. Microbiology. 2015;161:1297-312.
- [Google Scholar]
- Acetaldehyde production by non-pathogenic Neisseria in human oral microflora: Implications for carcinogenesis in upper aerodigestive tract. Int J Cancer. 2000;88:342-50.
- [Google Scholar]
- Multiple alcohol dehydrogenases but no functional acetaldehyde dehydrogenase causing excessive acetaldehyde production from ethanol by oral streptococci. Microbiology. 2013;159:1437-46.
- [Google Scholar]
- Cigarette smoke effects on innate immune mechanisms in the nasal mucosa. Potential effects on the microbiome. Ann Am Thorac Soc. 2014;11(Suppl 1):S38-42.
- [Google Scholar]
- The interaction between smoking, alcohol and the gut microbiome. Best Pract Res Clin Gastroenterol. 2017;31:579-88.
- [Google Scholar]
- Subgingival microbiome in smokers and non-smokers in periodontitis: An exploratory study using traditional targeted techniques and a next-generation sequencing. J Clin Periodontol. 2013;40:483-92.
- [Google Scholar]
- Carcinogenic potential of tobacco tar-resistant Staphylococcus aureus in buccal cavity. J Cancer Res Clin Oncol. 2004;130:301-5.
- [Google Scholar]
- Relevance of protein fermentation to gut health. Mol Nutr Food Res. 2012;56:184-96.
- [Google Scholar]
- Role of N-nitroso compounds (NOC) and N-nitrosation in etiology of gastric, esophageal, nasopharyngeal and bladder cancer and contribution to cancer of known exposures to NOC. Cancer Lett. 1995;93:17-48.
- [Google Scholar]
- The microbiome and its potential as a cancer preventive intervention. Semin Oncol. 2016;43:97-106.
- [Google Scholar]
- Polyamines: Emerging players in bacteria-host interactions. Int J Med Microbiol. 2013;303:484-91.
- [Google Scholar]
- Alternative spermidine biosynthetic route is critical for growth of Campylobacter jejuni and is the dominant polyamine pathway in human gut microbiota. J Biol Chem. 2011;286:43301-12.
- [Google Scholar]
- Toxicity of polyamines and their metabolic products. Chem Res Toxicol. 2013;26:1782-800.
- [Google Scholar]
- Dual Role of Bacteria in Carcinoma: Stimulation and Inhibition. Internat J Microbiol 2020:4639761.
- [Google Scholar]
- Gut microbiota modulation: a novel strategy for prevention and treatment of colorectal cancer. Oncogene. 2020;39:4925-43.
- [Google Scholar]
- Dysbiosis of gut microbiota in promoting the development of colorectal cancer. Gastroenterol Rep (Oxf). 2018;6:1-12.
- [Google Scholar]
- Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350:1084-9.
- [Google Scholar]
- The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252-64.
- [Google Scholar]
- Th17-type cytokines, IL-6 and TNF-α synergistically activate STAT3 and NF-kB to promote colorectal cancer cell growth. Oncogene. 2015;34:3493-503.
- [Google Scholar]
- Role of T17 cytokines in the control of colorectal cancer. Oncoimmunology. 2013;2:E26617.
- [Google Scholar]
- Probiotics modulated gut microbiota suppresses hepatocellular carcinoma growth in mice. Proc Natl Acad Sci USA. 2016;113:E1306-15.
- [Google Scholar]
- Inflammation-induced cancer: Crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13:759-71.
- [Google Scholar]
- Immunotherapy with dendritic cells and CpG oligonucleotides can be combined with chemotherapy without loss of efficacy in a mouse model of colon cancer. Int J Cancer. 2006;118:2790-5.
- [Google Scholar]






