Translate this page into:
Role of LRRK2 variant p.Gly2019Ser in patients with Parkinsonism
For correspondence: Dr Jharna Ray, S.N. Pradhan Centre for Neurosciences, University of Calcutta, 35, Ballygunge Circular Road, Kolkata 700 019, West Bengal, India e-mail: jharnaray@gmail.com
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Parkinsonian disorder, including Parkinson's disease (PD), is an aetiologically complex neurodegenerative disorder. Mutations in leucine-rich repeat kinase 2 (LRRK2) gene have been implicated in an autosomal dominant form of PD with variable penetrance. The identification of a common LRRK2 variant (p.Gly2019Ser) in dementia with Lewy bodies indicated its potential role in Parkinsonian disorder. The current study was aimed to identify the p.Gly2019Ser variant in Indian patients with Parkinsonian disorder.
Methods:
The patient group consisting of 412 classical PD patients, 107 PD patients with cognitive impairment, 107 patients with Parkinson plus syndrome and 200 unrelated controls were recruited from eastern part of India. The allele representing p.Gly2019Ser variant was screened by polymerase chain reaction followed by restriction fragment length polymorphism analysis.
Results:
The p.Gly2019Ser variant was identified in an East Indian young-onset female PD patient in a heterozygous state having several motor and autonomic problems without disturbed cognition. Her younger brother, sister and elder son harbouring the same mutation were asymptomatic carriers for the variant. However, the influence of DNM3 on decreased disease onset in this family was not clear.
Interpretation & conclusions:
Identification of the p.Gly2019Ser variant in only one patient among a large number of Indian patients (n=626) with Parkinsonian disorder in our study suggests a limited role of the LRRK2 variant towards disease pathogenesis.
Keywords
Gly2019Ser mutation
LRRK2
Parkinson plus
Parkinson's disease
parkinsonism
Parkinsonism is an umbrella term that includes a large number of neurological disorders, of which Parkinson's disease (PD) is the most common. Other Parkinsonian disorders show some clinical features overlapping with PD and others, including bilateral and symmetric onset, lack of long-term benefit of levodopa, absence of rest tremor and early dementia. PD is the second most common aetiologically complex neurodegenerative disorder and is characterized by tremor, rigidity, bradykinesia and postural instability1. About 10 per cent of patients with PD are familial and result from mutations in causal genes2. The LRRK2 (leucine-rich repeat kinase 2) gene has been implicated in an autosomal dominant form and often sporadic PD. LRRK2, containing 51 exons, encodes a 280 kDa multifunctional protein with multiple domains, which can phosphorylate itself as well as other PD-associated proteins such as parkin and alpha-synuclein (SNCA)1. More than 100 nucleotide variants have been reported in the LRRK2 gene in the Parkinson Disease Mutation Database (http://www.molgen.vib-ua.be/PDMutDB), among which p. Gly2019Ser (c.6055G>A) is the most common and well-characterized mutation1. The age of onset differs greatly between p. Gly2019Ser carriers across the world1. A single nucleotide polymorphism (SNP) in dynamin 3 (DNM3) (i.e., rs2421947) has been reported as a modifier of age of onset in LRRK2 Gly2019Ser Parkinsonism3. LRRK2 interacts with dynamin superfamily GTPases (Dnm1, Dnm2 and Dnm3) to regulate membrane dynamics for endocytosis and mitochondrial morphology4. However, no significant alteration in interaction between p. Gly2019Ser and Dnm1 compared to wild-type LRRK2 has been reported4.
The p. Gly2019Ser variant has also been identified in dementia patients with Lewy bodies (DLB) in Caucasians5. Experimental evidence suggests that abnormal substrate phosphorylation and cytotoxicity by p. Gly2019Ser are responsible for irreversible neuronal loss - a common pathology for Parkinsonism6.
Genetic screening of LRRK2 was independently performed from three geographical regions of India (north, south and east)789. The screening of common pathogenic mutations (viz., p. Arg1441Gly, p. Arg1441Cys, p. Arg1441His, p. Tyr1699Cys and p. Gly2019Ser) and a risk variant common among Asians (p. Gly2019Ser) was not observed in the eastern Indian cohort7. However, the presence of the Gly2019Ser variant in LRRK2 has been reported in a single PD patient in a heterozygous state in only one study9. The present study was conducted to specifically look for this mutation which is common in other world populations, in eastern Indian patients with PD and Parkinson plus, to assess the role of LRRK2 in patients with a wide spectrum of Parkinsonian phenotypes.
Material & Methods
A total of 626 Indian patients were recruited for this study. The patients were examined at Bangur Institute of Neurosciences (BIN), Kolkata (36 Parkinson plus patients; 412 classical PD patients; 107 PD patients with cognitive impairment); Burdwan Medical College and Hospital, Burdwan (15 Parkinson plus patients); National Neurosciences Centre Calcutta, Kolkata (38 Parkinson plus patients); Alzheimer's Related Disorder Society of India, Kolkata Chapter, Kolkata (18 Parkinson plus patients). In addition, 200 unrelated controls (mean age, 48.3±8.2 yr) with no personal or family history of Parkinsonism or any other neurological symptoms were selected from the eastern part of India for the present study. These healthy controls were unrelated family members and spouses of the patients. Their enrollment was done on the basis of disclosure about negative personal/familial history of PD along with clinical examination by a neurologist.
The study patients comprised 412 classical PD patients, 107 PD patients with cognitive impairment, 107 Parkinson plus patients from eastern India. Patients with PD having at least two of the cardinal features (tremor, rigidity, bradykinesia and postural instability) were selected. The Parkinson plus patients included DLB, progressive supranuclear palsy and multiple system atrophy. The demographic details of the patients are described in Table I.
| Category (patient studied) | Male:female | Familial:sporadic | Early onset:late onset | Age on onset (AO) (yr) mean±SD |
|---|---|---|---|---|
| Classical PD (412) | 282:130 | 127:285 | 151:261 | 44.33±13.62 |
| PD with CI (107) | 75:32 | 22:85 | 20:87 | 54.43±14.84 |
| Parkinson plus | ||||
| DLB (40) | 34:6 | 7:33 | 3:37 | 60.23±10.3 |
| PSP (55) | 36:19 | 2:53 | 0:55 | 60.17±6.62 |
| MSA (12) | 6:6 | 0:12 | 5:7 | 49.08±9.41 |
PD with CI, Parkinson’s disease with cognitive impairment and dementia; DLB, dementia with Lewy body; PSP, progressive supranuclear palsy; MSA, multiple system atrophy; early onset, AO ≤45 yr; late onset, AO >45 yr
The study protocol was approved by the Institutional Ethics Committees of the respective institutes and written informed consent was obtained from all participants.
Collection of blood samples and genomic DNA preparation: Peripheral blood samples (10 ml) were collected in ethylenediaminetetraacetic acid (EDTA) from patients and controls. Genomic DNA was prepared from fresh whole blood by a conventional salting-out method using sodium perchlorate followed by isopropanol precipitation10. Genomic DNA was dissolved in Tris-EDTA (TE) buffer (10 mM Tris-HCl, pH 8.0 and 0.1 mM EDTA).
Multiplex ligation-dependent probe amplification (MLPA) analysis: MLPA was done on 250 patients with PD with early age of onset and/or a positive family history using the SALSA MLPA P051 Parkinson mix 1 probemix (MRC-Holland, the Netherlands), which assays for LRRK2 variant c.6055 G>A (p. Gly2019Ser) along with gene dosage mutations in PRKN, PINK1, DJ-1 and SNCA.
PCR and restriction fragment length polymorphism (RFLP) analysis: The targeted region harbouring the nucleotide change c.6055 G>A (p. Gly2019Ser) in exon 41 of the LRRK2 was amplified using a mismatched forward primer to create a PstI restriction enzyme site: 5′-CATTGCAAAGATTGCTGACTGC-3′, the reverse primer: 5′-GAGGTCAGTGGTTATCCATCCT-3′7. The amplicon (134 bp) was digested with PstI (New England Biolabs, USA) using the conditions specified by the manufacturer and electrophoresed on a seven per cent polyacrylamide gel. The wild-type amplicon (134 bp) remained undigested, whereas the mutant allele containing a PstI site generated two DNA fragments (111 and 23 bp).
The SNP rs2421947 of DNM3 was genotyped exclusively for a patient harbouring p.G2019S and for her family members by the PCR-RFLP method described above7. The primer set 5′-CATGTTCCCCTCTACCTGGA-3′ (forward) and 5′-TAAAGTCCTTGGCGTTTTGC-3′ (reverse) (Sigma-Aldrich, USA) was used for PCR, and the AlwNI (New England Biolabs, USA) restriction enzyme was used for RFLP. After digestion, the C allele produced two fragments (209 and 33 bp), whereas the G allele remained undigested (242 bp). RFLP products were separated by electrophoresis on polyacrylamide gel (7%).
DNA sequencing: Because mutation of the PRKN gene is the most common cause of PD, all exons of the PRKN gene were analyzed for the individual harbouring an LRRK2 mutation by Sanger sequencing11.
Results & Discussion
The p. Gly2019Ser variant of LRRK2was identified in only one young-onset female PD patient in a heterozygous state by MLPA analysis from eastern part of India. No additional variants were observed in other causal genes analyzed in this patient. Screening of other patients and 200 controls for the Gly2019Ser variant by RFLP did not reveal any additional mutant alleles. As shown in Figure A and B, genetic analysis by RFLP and Sanger sequencing of family members of the proband revealed the presence of the same variant in her asymptomatic brother (29 yr), younger sister (25 yr) and elder son (18 yr) but not in her mother (60 yr), elder sister (41 yr) and two paternal aunts (60 and 55 yr). This suggested that the variant allele might have been inherited from her paternal side. However, DNA from her asymptomatic father (I-1), who died at the age of 45 yr, was not available. Because there was no report on the clinical examination of the proband's father available in his lifetime, he might have had subclinical phenotypes not noticeable to a naïve person. Genotyping for the variant was not done in her two younger sons (14 and 7 yr), Figure D.
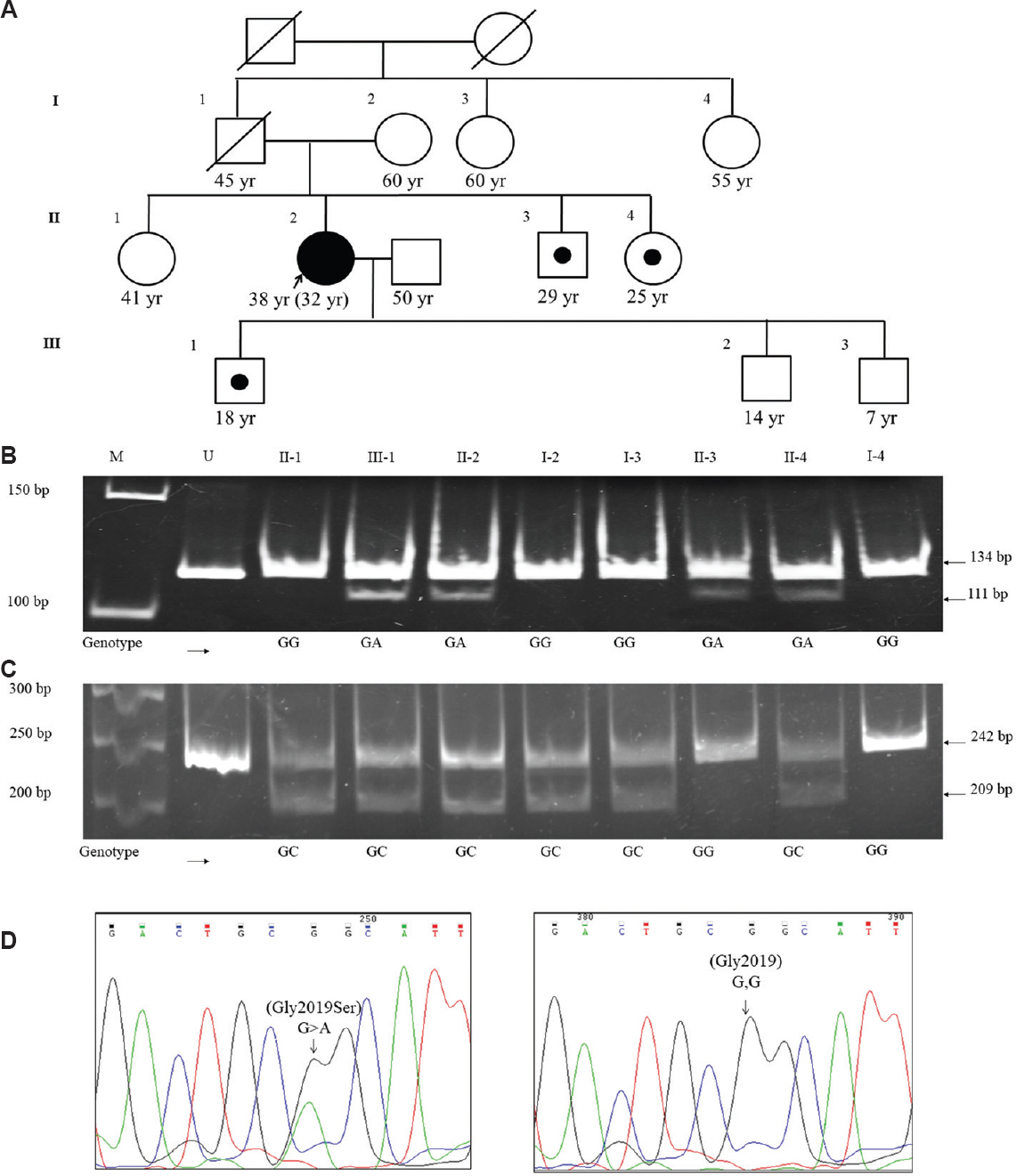
- Screening the p.Gly2019Ser variant (c.6055G>A) in the leucine-rich repeat kinase 2 (LRRK2) gene and rs2421947 (G>C) of DNM3 in a family affected with Parkinson's disease. (A) The upper panel represents a three-generation pedigree showing age and sex of each individual. The filled symbols indicate symptomatic LRRK2 mutants; the arrow indicates the proband and a black circle within a square indicates an asymptomatic male harbouring the mutant allele. Both the current age and age at onset (in parenthesis) are given for the proband. (B) Segregation pattern of the LRRK2 variant allele in family members, as represented by PstI digested polymerase chain reaction products, separated by polyacrylamide gel electrophoresis. Genotypes of the index case and family members are shown. (C) Genotyping details of rs2421947 of DNM3 in family members as represented by AlwNI digested polymerase chain reaction (PCR) products, separated as above. Lane U, undigested PCR product; lane M, 50 bp DNA ladder molecular weight marker. The sizes of the digested DNA fragments and their molecular weights are shown on the right and left side of the gels, respectively. (D) Chromatograms of the DNA sequence from the patient (II-2) showing the heterozygous condition of c.6055G>A of LRRK2 and the homozygous ‘wild’ genotype of the mother (I-2).
It has been reported that disease onset is accelerated by about 12.5 yr for p. Gly2019Ser carriers harbouring the GG genotype at rs2421947 of DNM33. However, genotyping of adult family members including non-carriers, mutant individuals in the pedigree (except the brother of the patient) was found to be heterozygous for rs2421947 of DNM3 (Figure C). Therefore, using data from a single family, it is difficult to comment on the role of DNM3 as a genetic modifier of p. Gly2019Ser-mediated PD pathogenesis in Indians. However, our study was consistent with a study on a large number of PD patients demonstrating that disease onset was about five years earlier in women with LRRK2 mutations12. Therefore, it is possible that the brother of the index case in our study may develop the disease in the future.
Our previous study on 308 PD patients and another study on 150 PD patients from eastern part of India did not identify the Gly2019Ser variant713. However, this mutation was reported previously in another female patient from north India9. Therefore, taking into consideration various Indian studies, p. Gly2019Ser-mediated pathogenesis may be considered a rare event (2/1996) in Indian patients with PD (Table II). The reported frequency of alleles for p. Gly2019Ser varies widely across the world15. Our genetic data corroborated with other Asian studies (<1%)16.
| Mutations screened | Demographic distribution | Patient studied | Gly2019Ser mutation/frequency of mutation (%) | Reference |
|---|---|---|---|---|
| Gly2019Ser, Arg1441Cys, Arg1441Gly, Arg1441His, Ile2012Thr, Ile2020Thr | North and South | 800 | 1 (0.125) | 9 |
| Gly2019Ser | South | 140 | 0 | 8 |
| Gly2019Ser | South | 186 | 0 | 14 |
| Arg1441Gly, Arg1441Cys, Arg1441His, Gly2019Ser, Tyr1699Cys, Ile2020Thr and Ile2012Thr | East | 150 | 0 | 13 |
| Arg1441Cys, Arg1441Gly, Arg1441His, Tyr1699Cys, Gly2019Ser and Gly2385Arg | East | 308 | 0 | 7 |
| Gly2019Ser | East | 412 | 1 (0.243) | Present study |
| Total | 1996 | 2 (0.1002) | ||
The only female patient, in the present study harbouring the p. Gly2019Ser mutation in a heterozygous state, developed tremulousness in her left limbs (both upper and lower), slowness of activity and dragging of her left lower limb while walking at the age of 32 years. Within the next year, she also developed tremor in her right limbs, both during rest and in action. Three years later, when she visited BIN, Kolkata, for the follow up, she had whole-body rigidity, postural instability, gait disturbances and complete dependence on family members. On examination, she was found to have other clinical features including stooped posture, abnormal positioning of neck (laterocolis and retrocolis), loss of facial expression, hypophonia with slurring of speech, gait disturbances, early-morning dystonia of her left foot, difficulty in turning in bed, as well as diurnal variation, insomnia and knee pain. However, she did not experience any subjective or objective memory impairment. Taking together all the clinical features, her total Unified Parkinson's Disease Rating Scale (UPDRS)17 score was 108 in off phase [Mentation, Behaviour and Mood subscore: 2; Motor subscore: 72 and Activities of Daily Living (ADL) subscore: 34].
The patient did not take any medications since disease onset due to economic constraints. For only the last six months, she has started levodopa-carbidopa therapy with good response, as revealed by her improved UPDRS score (57 in on phase) (Mentation, Behaviour and Mood subscore: 2; Motor subscore: 34 and ADL subscore: 21).
Both features such as tremor and postural instability have been reported to be quite common disease phenotypes in carriers of the Gly2019Ser variant118. However, lower tremor scores have been reported using the UPDRS in p. Gly2019Ser carriers compared to PD patient non-carriers of this variant18. Although no signs of cognitive decline were observed, the onset of PD in her lower extremities and the better quality of life on levodopa treatment were more consistent with LRRK2 mutation-related Parkinsonism than idiopathic PD1. The other patient reported from India9 harbouring the same pathogenic variant presented with similar clinical features including age of onset (35 vs. 32 yr) except for tremor and sleep disorder.
In conclusion, p. Gly2019Ser mutation in a patient with classical PD was observed from eastern parts of India. However, this variant was not detected in other forms of Parkinsonian patients. The female patient with this mutation exhibited both tremor and postural instability phenotypes with no disturbances in cognition. The overall low frequency of the p. Gly2019Ser variant restricts the identification of population-specific genetic modifiers in Indian patients with PD and suggests its limited role in PD manifestation in Indians.
Acknowledgment
Authors acknowledge Dr Ananya Ray-Soni, Department of Neurology, Massachusetts General Hospital and Harvard Medical School, Boston, USA, for correcting language and grammar.
Financial support & sponsorship: The study was supported by the Department of Science & Technology (DST)-PURSE, a grant from the DST-CSI Programme (SR/CSI/89/2010) to the last author (JR). The second author (AB) was supported by a postdoctoral fellowship from the same Research Initiative Programme (SR/CSI/PDF-32/2014 and DST/CSRI-P/2017/22), and the first author (DS) was supported by a predoctoral fellowship from the University Grants Commission, New Delhi.
Conflicts of Interest: None.
References
- Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson's disease: A case-control study. Lancet Neurol. 2008;7:583-90.
- [Google Scholar]
- Role of mendelian genes in ‘sporadic’ Parkinson's disease. Parkinsonism Relat Disord. 2012;18(Suppl 1):S66-70.
- [Google Scholar]
- DNM3 and genetic modifiers of age of onset in LRRK2 Gly2019Ser Parkinsonism: A genome-wide linkage and association study. Lancet Neurol. 2016;15:1248-56.
- [Google Scholar]
- Functional interaction of Parkinson's disease-associated LRRK2 with members of the dynamin GTPase superfamily. Hum Mol Genet. 2014;23:2055-77.
- [Google Scholar]
- LRRK2 variation and dementia with Lewy bodies. Parkinsonism Relat Disord. 2016;31:98-103.
- [Google Scholar]
- Parkinson's disease-associated mutations in LRRK2 link enhanced GTP-binding and kinase activities to neuronal toxicity. Hum Mol Genet. 2007;16:223-32.
- [Google Scholar]
- Evaluation of the role of LRRK2 gene in Parkinson's disease in an East Indian cohort. Dis Markers. 2012;32:355-62.
- [Google Scholar]
- The SNCA (A53T, A30P, E46K) and LRRK2 (G2019S) mutations are rare cause of Parkinson's disease in South Indian patients. Parkinsonism Relat Disord. 2012;18:801-2.
- [Google Scholar]
- Absence/rarity of commonly reported LRRK2 mutations in Indian Parkinson's disease patients. Neurosci Lett. 2006;409:83-8.
- [Google Scholar]
- Purification of human genomic DNA from whole blood using sodium perchlorate in place of phenol. Anal Biochem. 1989;180:276-8.
- [Google Scholar]
- Molecular pathogenesis of Parkinson's disease: Identification of mutations in the Parkin gene in Indian patients. Parkinsonism Relat Disord. 2006;12:420-6.
- [Google Scholar]
- Comparative study of Parkinson's disease and leucine-rich repeat kinase 2 p.G2019S parkinsonism. Neurobiol Aging. 2014;35:1125-31.
- [Google Scholar]
- Absence of commonly reported leucine-rich repeat kinase 2 mutations in Eastern Indian Parkinson's disease patients. Genet Test Mol Biomarkers. 2010;14:691-4.
- [Google Scholar]
- LRRK2 G2019S mutation does not contribute to Parkinson's disease in South India. Neurol India. 2011;59:157-60.
- [Google Scholar]
- Worldwide frequency of G2019S LRRK2 mutation in Parkinson's disease: A systematic review. Parkinsonism Relat Disord. 2010;16:237-42.
- [Google Scholar]
- LRRK2 G2019S mutation: Prevalence and clinical features in Moroccans with Parkinson's disease. Parkinsons Dis. 2017;2017:2412486.
- [Google Scholar]
- Phenotype in parkinsonian and nonparkinsonian LRRK2 G2019S mutation carriers. Neurology. 2011;77:325-33.
- [Google Scholar]
- Motor phenotype of LRRK2 G2019S carriers in early-onset Parkinson disease. Arch Neurol. 2009;66:1517-22.
- [Google Scholar]






