Translate this page into:
Risk of type 2 diabetes mellitus after gestational diabetes mellitus: A systematic review & meta-analysis
For correspondence: Prof Anjiang Lei, No. 1416, Section 1, Chenglong Avenue, Jinjiang District, Chengdu 610 000, PR China e-mail: hxleianjiang@163.com
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background &objectives:
Women with gestational diabetes are at an increased risk of being diagnosed as type 2 diabetes, but the postpartum screening rate is low. To provide evidence-based data for health providers and promote postpartum screening, this systematic review and meta-analysis was conducted to access the risks of type 2 diabetes mellitus (T2DM) diagnosis after gestational diabetes mellitus (GDM) in different demographic and maternal subgroups.
Methods:
MEDLINE, Embase and Cochrane Library were searched systematically. Unadjusted relative risks (RRs) and 95 per cent confidence intervals (CIs) were calculated and pooled using a random-effects model. Heterogeneity was assessed with Cochrane’s Q text and by calculating I2 values. Subgroup analyses were conducted to address the disparities of type 2 diabetes conversion after gestational diabetes in different demographic and maternal subgroups.
Results:
1809 publications were screened and 39 cohort studies including 2,847,596 women were selected. In these studies, 78,893 women were diagnosed as T2DM at six weeks or later after delivery. The unadjusted RRs of women diagnosed T2DM at six weeks or later after delivery ranged from 1.32 (95% CI, 0.46-3.37) to 47.25 (95% CI, 2.95-758.01) with a pooled unadjusted RR of 8.92 (95% CI, 7.84-10.14). Older women, women with a family history of diabetes, Black and non-Hispanic White women and women living in Europe and South-East Asia had a higher risk of developing T2DM after GDM.
Interpretation & conclusionsxs:
It is suggested that healthcare providers may focus on older women with GDM and women with GDM and a family history of diabetes. Black and non-Hispanic White women with GDM may receive more attention, and healthcare providers, especially those in Europe and South-East Asia, may pay more attention to preventive measures for postpartum T2DM.
Keywords
Blood glucose
gestational diabetes mellitus
OGTT screening
postpartum
type 2 diabetes mellitus
Gestational diabetes mellitus (GDM) is defined as glucose intolerance first detected during pregnancy. The prevalence of GDM has increased more than 30 per cent over the past two decades1. As reported, the median prevalence of GDM globally ranges from 1.8 to 22.3 per cent2. GDM is associated with short and long-term adverse outcomes of both mothers and their respective offsprings, and is a well - known risk factor for developing type 2 diabetes mellitus (T2DM) after delivery. The rates of T2DM diagnosis after GDM range from two to 70 per cent, from six weeks to 28 yr postpartum3. Increasing prevalence of GDM and T2DM and their related complications lead to huge healthcare and economic costs45.
In light of these risks and the opportunity for preventive intervention, women with GDM are advised to have oral glucose tolerance test (OGTT) assessed at 6-12 wk postpartum6. However, studies reported that postpartum screening rates range from 13 to 82 per cent varying across geography, ethnicity and practice patterns. and is underused789. Furthermore, while there are various barriers of postpartum diabetes screening patient compliance with diabetes screening recommendations are inadequate10. Systematic review and meta-analysis previously showed that women with a history of GDM have a sevenfold risk of being diagnosed as T2DM than those without although the results of this study were synthesized despite heterogenous differences11. In the present study the relative risks (RRs) among all selected studies were included and sensitivity and subgroup analyses were conducted to identify the sources of the heterogeneity. Moreover, risks of being diagnosed as T2DM vary widely2, and therefore the disparities of T2DM diagnosis after GDM in different demographic subgroups to help health providers focus on the high-risk patient were assessed.
Material & Methods
Literature search and inclusion criteria: Twenty studies were hand-searched from the previous systematic review11 and did an electronic search of MEDLINE and Embase from January 1, 2009 to July 31, 2019 and did not apply any restrictions. The search of the Cochrane Library was from inception to July 31, 2019, without restrictions. Search terms were a combination of ‘gestational diabetes mellitus’, ‘pregnancy diabetes mellitus’, ‘diabetes, gestational’, ‘type 2 diabetes mellitus’, ‘diabetes mellitus, type 2’ and ‘non-insulin dependent diabetics mellitus’. In addition to the electronic search, reference lists and citations of relevant reviews and articles were hand-searched.
Prospective and retrospective cohort studies (PCS and RCS) in which women were diagnosed with GDM and normal blood glucose were searched for. The outcome was the diagnosis of T2DM at six weeks or later after delivery. The criteria of GDM and T2DM were not restricted. Studies of women with pre-existing diabetes mellitus were excluded.
Methodological quality assessment: The quality of included studies was assessed by a standardized checklist based on the Newcastle–Ottawa Scale (NOS)12. The NOS is a star rating system (0-9 stars) used for observational studies. For cohort studies, the criteria cover three domains: selection of participants, between-group comparability and ascertainment of outcome. Each item can get one star in selection and outcome domains and two stars in comparability domain if appropriate methods were reported1213.According to the final score, studies were classified as high (c7-9 stars), medium (5-6 stars) or low (0-4 stars) quality. Low quality (c7) study might reduce the credibility of results, so we excluded low quality studies in this meta-analysis.
Data abstraction: Participant and study characteristics and cumulative incidences of T2DM in the GDM and non-GDM groups were independently extracted by two authors using standardized tables. Disagreements were solved by discussion with the third author. If more than one report based on the same population was identified, the one with the most relevant and complete information was selected.
Statistical analysis: A Meta-analysis was carried out using Stata/MP (Version 14.0, StataCorp LLC, Texas, USA). Unadjusted, pooled relative risks (RRs) and 95 per cent confidence intervals (CIs) were calculated. Heterogeneity was assessed with Cochrane’s Q text and by calculating I2 values. High heterogeneity was defined by either P≤0.10 or I2 ≥60 per cent, median heterogeneity was defined by either P ≤0.10 or 30 per cent ≤ I2< 60 per cent and little or no heterogeneity was defined by either P>0.10 or I2< 30 per cent14. In cases of high heterogeneity, a random-effects model was used. Sensitivity analyses were conducted to identify the outliers by testing the outcome robustness after one study was removed. Subgroup analyses were performed to explore the sources of heterogeneity among studies by stratification according to mean maternal age, body mass index (BMI) at follow up, race/ethnicity, region, family history of diabetes mellitus, time interval of postpartum OGTT performed, GDM criteria, T2DM criteria and number of confounders matched. Begg’s test and Egger’s test were performed to investigate small sample bias and publication bias. A P<0.05 was considered statistically significant.
Results
Selection of studies: In total, 1957 records were identified through electronic database searching, 30 additional publications were identified through reference lists and 20 publications were included from a previous systematic review. Altogether,1809 titles and abstracts were screened after 198 duplicates were removed. Of 343 publications that were selected for full-text review, 304 were excluded for various reasons. Finally, 39 cohort studies involving 2,847,596 women were included in this meta-analysis. In these studies, 78,893 women were diagnosed as T2DM at six weeks or later after delivery (Fig. 1).
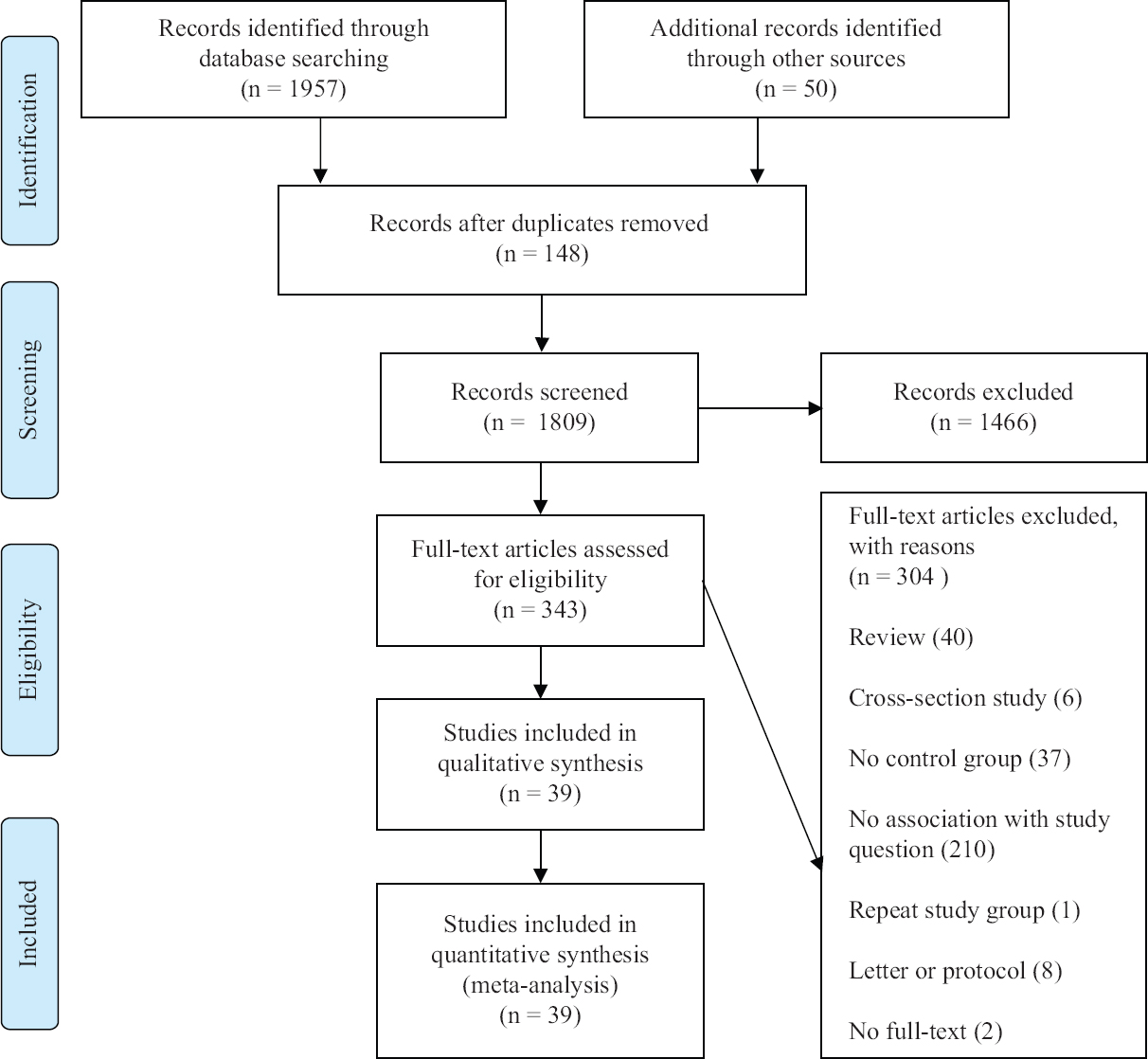
- PRISMA flow diagram showing literature search.
Characteristics of the studies: A total of 26 retrospective415161718192021222324252627282930313233343536373839 and 13 prospective cohort studies>5404142434445464748495051 conducted in different countries were considered for this meta-analysis. The participants varied widely in maternal age, BMI, family history of diabetes mellitus, ethnicity, length of follow up and time interval of postpartum OGTT performed. Moreover, diagnostic criteria of GDM and T2DM varied by country as well. In 15.4 per cent (6/39) of studies, the dropout rate was under 30 per cent. In 5.1 per cent (2/39) of studies, the dropout rate is between 30 and 60 per cent. In 38.5 per cent (15/39) of studies, none of the women dropped out. In 41.0 per cent (16/39) studies, the dropout rate was not recorded. In 76.9 per cent (30/39) of studies, women in two groups were matched by different confounders. In 23.1 per cent (9/39) of studies, confounders adjustment was not recorded (Table).
| Author | Study type | Region | Race/ethnicity | Mean maternal age (yr; overall or GDM/non-GDM) | BMI at followup (kg/m2; overall or GDM/non-GDM) | Family history (GDM/non-GDM, %) | Time interval of postpartum OGTT performed | GDM criteria | T2DM criteria | Dropout rate (%) | Confounders matched |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Daly et al15, 2018 | RCS | Europe | Other | 33/33 | Not recorded | Not recorded | Three years | Not recorded | Clinical codes | Not recorded | Age |
| Shen et al40, 2018 | PCS | Western Pacific | Asian | 29.7/30.1 | 22.9/24.2 | 27.1/35.7 | 4.4 yr | WHO, 1999 | ADA, 2018 | Not recorded | Smoking exposure |
| Herath et al16, 2017 | RCS | South-East Asia | Other | 31.7/27.7 | Not recorded | Not recorded | One year | WHO, 1999 | WHO, 1999 | Not recorded | Ethnicity, education, family income per month, sex of infant, exclusive breastfeeding duration |
| Ajala et al17, 2015 | RCS | North America | Other | 32.1/31.4 | 28.9/26.6 | 52.2/52.5 | One year | CDA, 2008 | Local | None | Age, ethnicity, BP, smoking exposure, amount of alcohol consumed and time of physical activity |
| Cormier et al4, 2015 | RCS | North America | Other | 36.4/35.6 | 27.7/25.6 | Not recorded | Three-four years | Not recorded | CDA, 2013 | None | Age, parity, time to follow up after delivery |
| Hakkarainen et al5, 2015 | PCS | Europe | Non-Hispanic White | 30.8 | 26.88/28.38 | Not recorded | Not recorded | Local | ADA, 2011 | Not recorded | Infant birth weight |
| Pintaudi et al41, 2015 | PCS | Europe | Hispanic | 35.7 | Not recorded | Not recorded | Not recorded | ADA, 2004 | Local | Not recorded | Propensity score |
| Mai et al18, 2014 | RCS | Western Pacific | Asian | 30.6/27.2 | 22.7/21.5 | 19.5/6.3 | Six months | ADA, 2004 | ADA, 2010 | None | Family history, parity, length of follow up, DBP and hip circumference |
| Barden et al42, 2013 | PCS | Western Pacific | Non-Hispanic White | 32.9/32.6 | Not recorded | 60.70/53.4 | Six months | Australasian diabetes, 1998 | Local | 27.90 | Not recorded |
| Feig et al19, 2013 | RCS | North America | Other | 28.8 | Not recorded | Not recorded | 5.4 yr | Canadian Institute for Health Information Discharge Abstract Database | Ontario Diabetes Database | Not recorded | Not recorded |
| Hummel et al20, 2013 | RCS | Europe | Non-Hispanic White | Not recorded | Not recorded | Not recorded | Not recorded | German Diabetes Association, 2001 | German Diabetes Association, 2011 | None | Not recorded |
| Anderberg et al21, 2012 | RCS | Europe | Non-Hispanic White | Median 32 | Not recorded | Not recorded | Not recorded | Local | Not recorded | None | Age, year of delivery and residence |
| Mukerji et al22, 2012 | RCS | North America | Other | 20-49 | Not recorded | Not recorded | Not recorded | Ontario Ministry of Health and Long-Term Care, Registered Persons Database | Ontario Diabetes Database | Not recorded | Not recorded |
| Tam et al43, 2012 | PCS | Western Pacific | Asian | 28.8/28.2 | 24.7/24.4 | Not recorded | Not recorded | WHO, 1999 | WHO, 1999 | 31.50 | Age, parity, smoking exposure, BMI, waist-hip ratio, fat (%), LDL, HDL, cholesterol and metabolic syndrome |
| Tehrani et al23, 2012 | RCS | Middle East | Other | 33.6/33.7 | 30.0/29.8 | 37.9/34.5 | Not recorded | WHO, 1998 | ADA, 2009 | Not recorded | Age, BMI, parity, family history, BP, blood glucose, cholesterol, LDL and HDL |
| Wang et al44, 2012 | PCS | North America | Other | 26.8/24.3 | Not recorded | Not recorded | Not recorded | ADA, 2004 or WHO, 1998 | ADA, 2004 or WHO, 1998 | Not recorded | Postpartum DBP, current smoker and annual family income |
| Akinci et al24, 2011 | RCS | Europe | Non-Hispanic White | 31.9/31.4 | 26.51/21.79 | 46.7/26.8 | One year | Carpenter and Coustan | ADA, 2009 | None | Age and length of followup |
| Ramezani et al45, 2011 | PCS | Middle East | Other | 36.3 | Not recorded | Not recorded | Not recorded | ADA, 2009 | ADA, 2009 | None | Not recorded |
| Xiang et al25, 2011 | RCS | North America | Other | 32.4/32.3 | Not recorded | Not recorded | Not recorded | Carpenter and Coustan | ADA, 2010 | 4.82 | Ethnicity |
| Feig et al26, 2008 | RCS | North America | Non-Hispanic White | 29.3 | Not recorded | Not recorded | Six months | Canadian Institute for Health Information Discharge Information | Ontario Diabetes Database | 3.90 | Not recorded |
| Lee et al27, 2008 | RCS | Western Pacific | Asian | 33.6 | 23.5/22.5 | 36.5/11.9 | Six weeks | NDDG, 1979 | Local | Not recorded | Age, smoking exposure, hip circumference and DBP |
| Madarász et al28, 2008 | RCS | Europe | Non-Hispanic White | 33.1/30.0 | Not recorded | Not recorded | Not recorded | WHO, 1999 | WHO, 1999 | Not recorded | Not recorded |
| Vambergue et al29, 2008 | RCS | Europe | Other | 27.0/28.8 | Not recorded | Not recorded | Six years | Carpenter and Coustan | ADA, 1997 | 29 | Pregnancy-induced hypertension and caesarean section |
| Ferraz et al46, 2007 | PCS | South America | Hispanic | 26.9/25.1 | 26.34/25.33 | Not recorded | 6.2 yr | WHO, 1999 | WHO, 1999 | None | BMI, BP and blood glucose at follow up |
| Gunderson et al47, 2007 | PCS | North America | Other | 18-30 | 24.45 | Not recorded | 5-20 yr | Obstetric Laboratory Reports | ADA, 1997 | 28 | Age, smoking exposure and marital status |
| Krishnaveni et al48, 2007 | PCS | South-East Asia | Other | 19.6/33.1 | 25.5/23.5 | 57.1/27.2 | Six months | Carpenter and Coustan | WHO, 1999 | None | Parity, BMI at follow up, height, family history and waist-hip ratio |
| Lee et al30, 2007 | RCS | Western Pacific | Other | 30.7/30.5 | Not recorded | 16.7/24.0 | Six weeks | Australian Diabetes in Pregnancy Society Guidelines | WHO, 1998 | 56.20 | Height, parity and Infant birth weight |
| Morimitsu et al49, 2007 | PCS | South America | Other | 32/27 | 29.6/24.4 | Not recorded | Four-six months | ADA, 1997 | ADA, 1997 | Not recorded | Age, LDL and HDL |
| Järvelä et al31, 2006 | RCS | Europe | Non-Hispanic white | 31.6/31.3 | Not recorded | Not recorded | Not recorded | Finnish Diabetes Association | Medication for T2DM linked to database 13 | Not recorded | Age, parity and date of delivery |
| Albareda et al50, 2003 | PCS | Europe | Hispanic | 30.7/30.4 | 24.5/24.8 | 53.7/43.9 | Six weeks | Second and Third Workshop-Conferences on Gestational Diabetes | WHO, 1998 | Not recorded | Family history, subsequent pregnancies and BMI at followup |
| Aberg et al32, 2002 | RCS | Europe | Hispanic | 35.7 | Not recorded | Not recorded | One year | ADA, 2004 | Local | Not recorded | Age, parity, pregnancy weight, pregnancy duration and infant birth weight |
| Linné et al33, 2002 | RCS | Europe | Non-Hispanic white | 32.6/30.6 | 25.7/24.7 | Not recorded | Not recorded | Local | Local | None | Age, weight before first pregnancy, fat (%), BP, cholesterol, LDL, HDL and number of children |
| Bian et al34, 2000 | RCS | Western Pacific | Asian | 29 | Not recorded | Not recorded | 5-10 yr | NDDG, 1979 | WHO, 1985 | None | Age, social background and Ga of birth |
| Ko et al35, 1999 | RCS | Western Pacific | Asian | 34.0/34.4 | 22.7/24.8 | Not recorded | Six weeks | Local | WHO, 1985 | None | Age |
| Osei et al36, 1998 | RCS | North America | Black | 31.3/36.0 | 34/27.0 | Not recorded | Not recorded | NDDG, 1979 | NDDG, 1979 | None | Age, BMI, fat (%) and lean body mass |
| Damm et al37, 1994 | RCS | Europe | Non-Hispanic white | 30.1/26.7 | 21.0/23.1 | Not recorded | Two months | Local | WHO, 1985 | 19.90 | Not recorded |
| Benjamin et al38, 1993 | RCS | North America | Other | 27.2/26.5 | Not recorded | Not recorded | 1-10 yr | Local | NDDG, 1979 | None | Age, BMI, parity and length of follow up |
| O’Sullivan et al51, 1984 | PCS | North America | Other | Not recorded | Not recorded | Not recorded | Not recorded | Not recorded | WHO, 1985 | Not recorded | Not recorded |
| Persson et al39, 1991 | RCS | Europe | Non-hispanic white | 31/30 | 24.18/21 | Not recorded | Not recorded | WHO, 1985 | WHO, 1985 | None | Pre-pregnancy weight, Ga at OGTT and Infant birth weight |
Ga, gestational age; RCS, retrospective cohort studies; PCS, prospective cohort studies; GDM, gestational diabetes mellitus; OGTT, oral glucose tolerance test; BMI, body mass index; T2DM, type 2 diabetes mellitus; NDDG, national diabetes data group; BP, blood pressure; LDL, low-density lipoprotein; HDL, high-density lipoprotein; DBP, diastolic blood pressure; ADA, age discrimination Act; CDA, Canadian diabetes association
As per the NOS scores as shown in Fig. 2, 87 per cent (34/39) of studies included in this meta-analysis were of high quality, and 13 per cent (5/39) studies were of medium quality. The unadjusted RRs of women diagnosed as T2DM at six weeks or later after delivery ranged from 1.32 (95% CI, 0.46-3.37) to 47.25 (95% CI, 2.95-758.01), with a pooled unadjusted RR of 8.92 (95% CI, 7.84-10.14).The heterogeneity was defined as high with P<0.01, and I2=94.1 per cent (Fig. 3). Sensitivity analyses were conducted by recalculating the pooled RRs with included studies removed one by one. The results indicated that the pooled RRs were not affected by the exclusion of any individual study (Fig. 4).
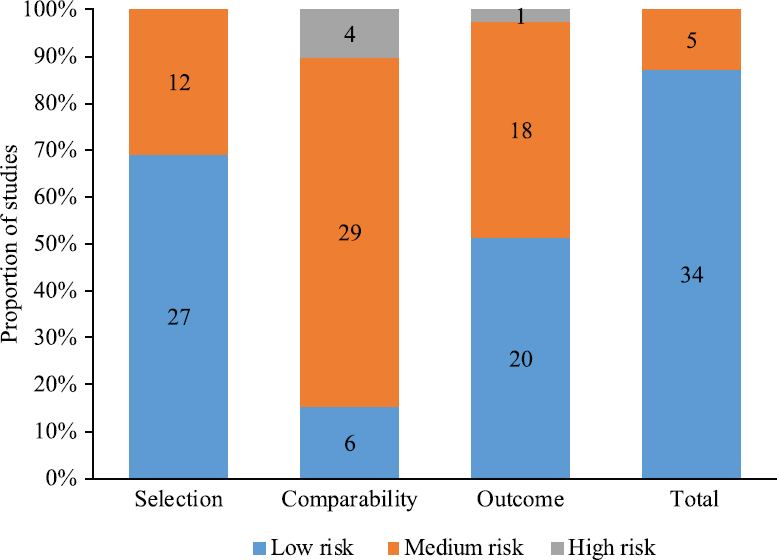
- Newcastle–Ottawa Scale scores of 39 included studies in meta-analysis.
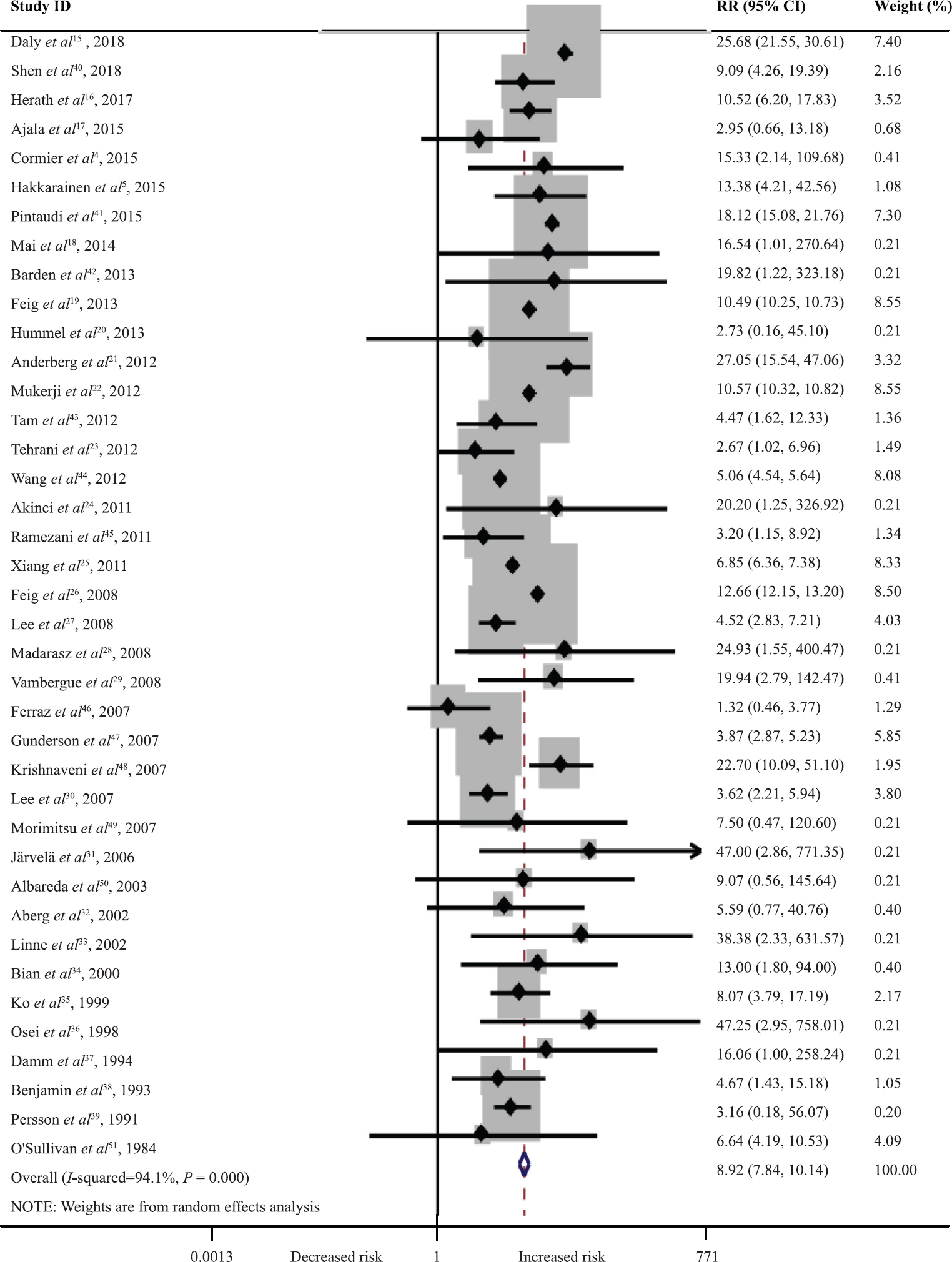
- Forest plot of the risk of women diagnosed as type 2 diabetes mellitus (DM) after gestational DM. X-axis is plotted in log scale. Solid squares and horizontal lines indicate relative ratios and 95 per cent confidence intervals. The diamond represents the pooled relative risk (RR).
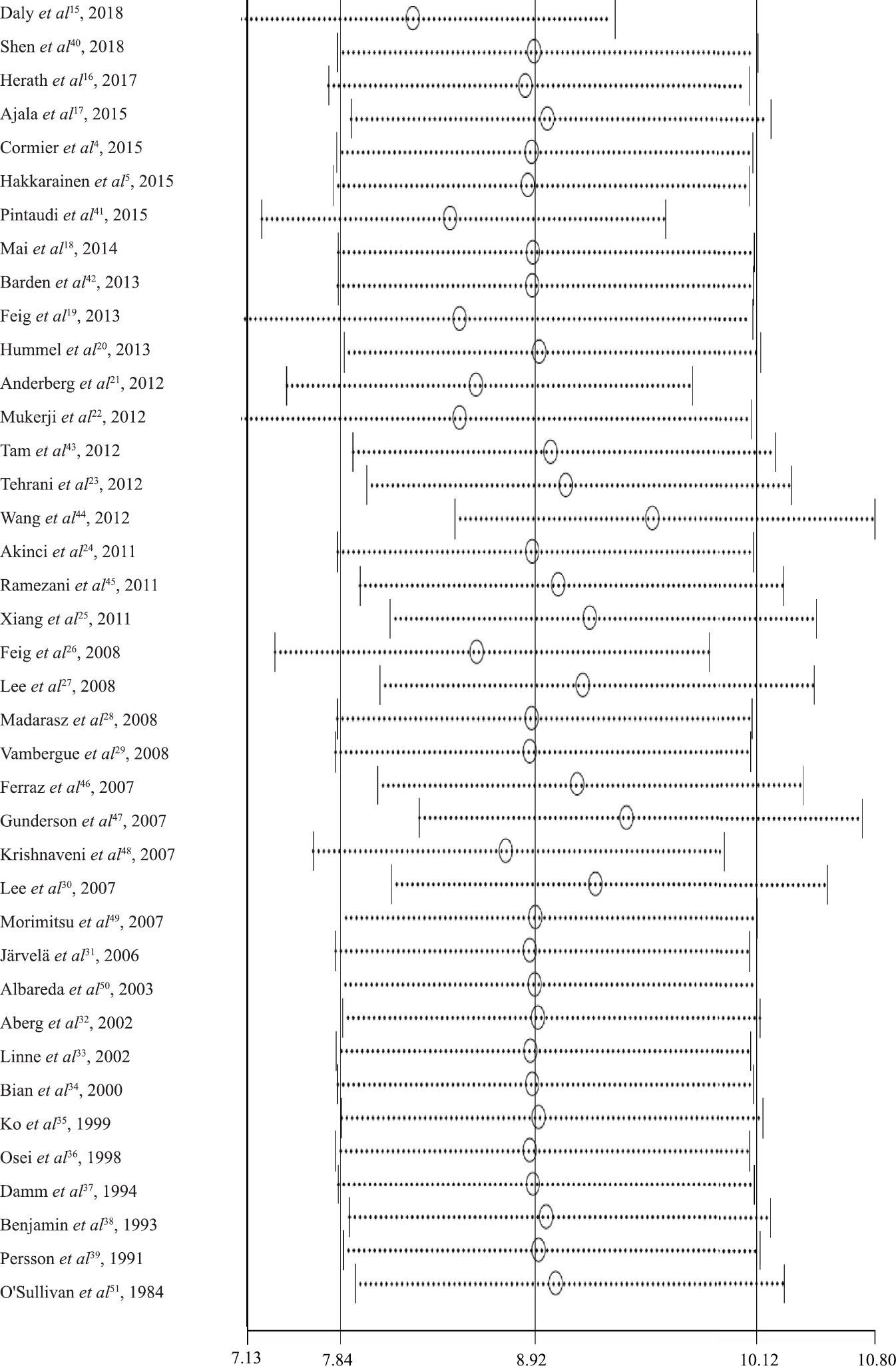
- Sensitivity analysis of women diagnosed as type 2 DM after gestational DM. Three vertical lines indicate the pooled RR and 95 per cent CI of all studies. Circles and horizontal dashed lines indicate recalculated RRs and 95 per cent CIs.
Subgroup analyses indicated that maternal characteristics and the time interval of postpartum OGTT performed was associated with the RR of T2DM onset after GDM. Older maternal age and family history of diabetes mellitus increased the risk of T2DM after GDM. The incidence of T2DM after GDM is the highest within the first year after delivery. The RR of diagnosing T2DM after GDM is variable when studies were grouped according to race/ethnicity and geographic region. The RR of diagnosing T2DM after GDM was lower when more confounders were matched (Fig. 5).

- Risk of women diagnosed as type 2 DM after gestational diabetes mellitus grouped by maternal characteristics, study characteristics and diagnostic criteria. The diamond represents the subtotal relative risk.
These results suggest that race/ethnicity, region, family history and time interval of postpartum OGTT performed could explain the reason behind the heterogeneity among studies. However, mean maternal age, BMI at follow up, GDM criteria, T2DM criteria and number of confounders matched could not explain the same.
Publications bias: No apparent asymmetry was observed in the Begg’s funnel plot (Fig. 6) and Egger’s publication bias plot (Fig. 7). Results of the Begg’s test (P=0.200) and Egger’s test (P=0.380) were not significant.
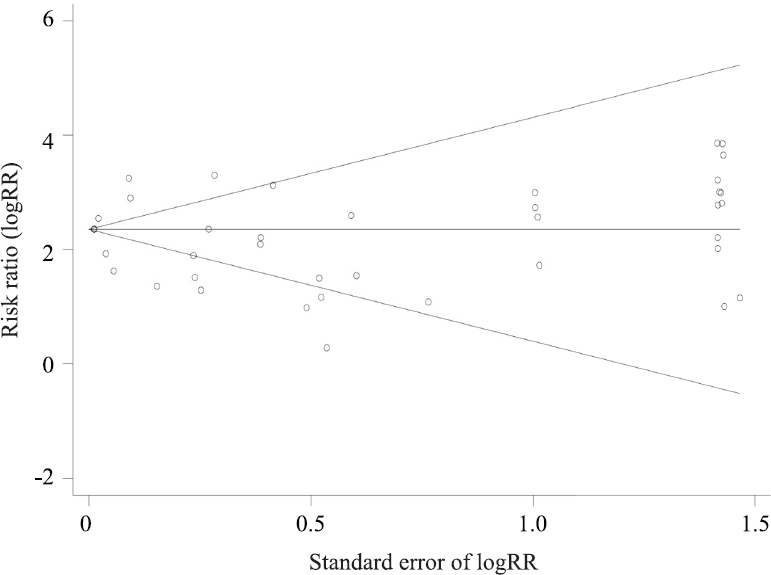
- Begg’s funnel plot of 39 publications.
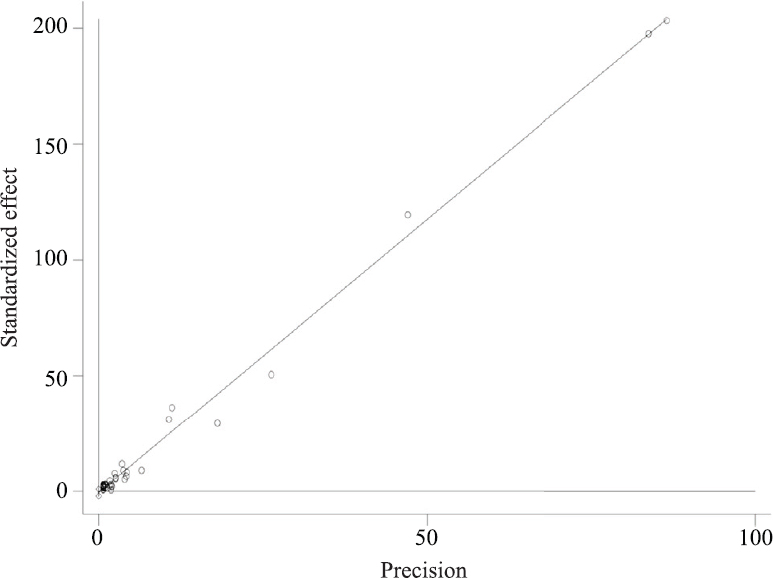
- Egger’s publication bias plot of 39 publications.
Discussion
This meta-analysis indicates that women with a history of GDM have near nine fold increased risk of being diagnosed as T2DM in the future compared with those without GDM. The magnitude of the association between GDM and T2DM suggests that more frequent assessment and effective interventions targeting eligible women are needed. American Diabetes Association and other professional organizations recommend diabetes screening at 6-12 wk postpartum for women with GDM5253. Despite the emphasis of multiple guidelines, the postpartum screening compliance rates are still typically low5455. In addition, from the present study it was evident that within the first year after delivery, the progression of T2DM increased steeply. So, healthcare providers should emphasize the importance of continuity in treatment and healthcare and women with GDM should attend the follow up programmes earlier and conduct OGTT at 6-12 wk postpartum. Furthermore, later long-time screening strategies and optimal screening frequency may be needed further studies to explore.
Maternal age, BMI, race/ethnicity and family history are associated with the prevalence of GDM and T2DM11. In this meta-analysis, the results of subgroup analyses corroborated that maternal age and family history of diabetes might be the risk factors for T2DM after GDM. Thus, older women or those with a family history should value antepartum counselling and postpartum diabetes screening more than other women with GDM.
It has been suggested previously that the prevalence of GDM varies with race/ethnicity225, with Asians and Hispanics reported to have a higher GDM prevalence than non-Hispanic Whites and Blacks5657. In the present study it was observed that Blacks and non-Hispanic Whites had a higher RR of developing T2DM after GDM than Hispanics and Asians, which was consistent with a large multi-ethnic cohort study25. Another study reported that Hispanics and Asians had the highest RR of T2DM after GDM44 however, the sample size was small and CIs were wide44. This inconsistency could be attributed to the sample size. Large multi-ethnic cohort studies are needed to verify that conjecture.
Besides race/ethnicity, regional disparity (geographic level) is an important influence factor of GDM prevalence. The Middle East and North Africa had the highest prevalence of GDM, followed by South-East Asia, Western Pacific, South America, Africa and North America, whereas Europe had the lowest prevalence2. Despite the relatively high prevalence, no eligible studies from North Africa or Africa were identified in our search, and only two studies from South-East Asia were included. The subgroup analysis indicated that the RR of T2DM after GDM in Europe and South-East Asia was higher than other geographic regions. Although the GDM prevalence in Europe was the lowest, the RR of T2DM after GDM in Europe was the highest. Moreover, RRs in South America and Middle East were relatively low. Taken together, the RR of T2DM after GDM was not associated with GDM prevalence.
In this meta-analysis (P<0.01, I2=94.1%) high heterogeneity was noted similar to a previous study10 (P<0.01, I2=85%). In this meta-analysis, sensitivity analysis indicated that no individual study contributed to the heterogeneity and the subgroup analyses, indicated that maternal age, BMI at follow up, GDM and T2DM criteria, and number of confounders matched could not explain the heterogeneity. Nevertheless, race/ethnicity, region, family history and time interval of postpartum OGTT performed might have contributed to the same. In subgroup analysis based on race/ethnicity, no significant evidence of heterogeneity was found in group ‘non-Hispanic White’ and ‘Asian’, but significant evidence of heterogeneity was found in group ‘Other’ and ‘Hispanic’. In group ‘Other’, most studies included mixed population and their racial/ethnic composition was different, which was considered the cause of the subgroup heterogeneity. In group ‘Hispanic’, two studies were carried out in Europe and one in South America; it was thus inferred that regional disparity might cause subgroup heterogeneity. In the results of subgroup analyses based on geographic regions, we only observed significant evidence of heterogeneity in the group ‘North America’. Such heterogeneity might be attributed to diversity in race/ethnicity, because the degree of diversification among population in North America was higher than that among the population of other geographic regions and most studies on this group included mixed population. In subgroup analysis based on family history, no heterogeneity was found in the group ‘<25 per cent’ and ‘>25 per cent’. In addition, in subgroup analysis based on time interval of postpartum OGTT performed, no heterogeneity was found in the groups ‘at six weeks’ and ‘<one year’ and high heterogeneity was seen in group ‘>one year’. Therefore, it was inferred that the family history of diabetes and time interval of postpartum OGTT performed might be the source of heterogeneity. Meanwhile, 76.9 per cent (30/39) studies did not record the family history information and 41.0 per cent (16/39) studies did not record the time interval of postpartum OGTT performed. Such absence of information might have caused a bias.
There were, however, two limitations in the present study. The RR was synthesized regardless of the huge variance in diagnostic criteria and screening protocol for GDM and T2DM. However, the diagnose criteria have been constantly changing over the last four decades. In 1997, the T2DM diagnosis threshold was reduced58. Moreover, recent studies using the new International Association of Diabetes and Pregnancy Study Group criteria show a higher prevalence of GDM58. Therefore, the inclusion of old studies might have caused the underestimation of the risk of having T2DM after GDM. Secondly, the main source of heterogeneity in this study could not be identified. Such heterogeneity in the present study might have been caused by the number of included studies and the differences in the participant characteristics.
In summary, the high risk of diagnosing T2DM after GDM suggests that healthcare providers need postpartum screening and follow up programmes, both of which are convenient and economic methods for early treatment of T2DM, thereby reducing the prematurity of cardiovascular, renal and retinal diseases59606162. Continuous assessment and effective interventions targeting eligible women are needed, in particular, older women with GDM or women with GDM and a family history of diabetes should value antepartum consulting and postpartum followup programmers more than other women with GDM only. Blacks and non-Hispanic Whites could receive more attention, and healthcare providers, especially those in Europe and South-East Asia, could pay more attention to preventive measures. Overall, it is concluded that the RR of diagnosing T2DM after GDM is not directly proportional to GDM prevalence among racial/ethnic groups or geographic regions. Whether the difference is due to lifestyle, genetics or environment needs to be investigated further.
Acknowledgment:
We thank Li Li for statistical support.
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- IDF Diabetes Atlas:Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271-81.
- [Google Scholar]
- The association between gestational diabetes and ASD and ADHD:a systematic review and meta-analysis. Sci Rep. 2021;11:5136.
- [Google Scholar]
- Gestational diabetes and the incidence of type 2 diabetes:a systematic review. Diabetes Care. 2002;25:1862-8.
- [Google Scholar]
- An explained variance-based genetic risk score associated with gestational diabetes antecedent and with progression to pre-diabetes and type 2 diabetes:A cohort study. BJOG. 2015;122:411-9.
- [Google Scholar]
- Post-challenge glycemia during pregnancy as a marker of future risk of type 2 diabetes:A prospective cohort study. Gynecol Endocrinol. 2015;31:573-7.
- [Google Scholar]
- American Diabetes Association Standards of Medical Care in Diabetes 2017. J Diabetes. 2017;9:320-4.
- [Google Scholar]
- Variation in postpartum glycemic screening in women with a history of gestational diabetes mellitus. Obstet Gynecol. 2016;128:159-67.
- [Google Scholar]
- Postpartum screening practices, progression to abnormal glucose tolerance and its related risk factors in Asian women with a known history of gestational diabetes:A systematic review and meta-analysis. Diabetes Metab Syndr. 2017;11(Suppl 2):S703-12.
- [Google Scholar]
- Postpartum diabetes screening among low income women with gestational diabetes in Missouri 2010-2015. BMC Public Health. 2019;19:148.
- [Google Scholar]
- Compliance with postpartum diabetes screening recommendations for patients with gestational diabetes. J Womens Health (Larchmt). 2018;27:498-502.
- [Google Scholar]
- Type 2 diabetes mellitus after gestational diabetes:A systematic review and meta-analysis. Lancet. 2009;373:1773-9.
- [Google Scholar]
- Meta-analysis of open surgical repair versus hybrid arch repair for aortic arch aneurysm. Interact Cardiovasc Thorac Surg. 2017;24:34-40.
- [Google Scholar]
- Association between SLCO1B1 ?521T>C and ?388A>G polymorphisms and risk of statin-induced adverse drug reactions:A meta-analysis. Springerplus. 2016;5:1368.
- [Google Scholar]
- Breastfeeding and maternal health outcomes:A systematic review and meta-analysis. Acta Paediatr. 2015;104:96-113.
- [Google Scholar]
- Increased risk of ischemic heart disease, hypertension, and type 2 diabetes in women with previous gestational diabetes mellitus, a target group in general practice for preventive interventions:A population-based cohort study. PLoS Med. 2018;15:e1002488.
- [Google Scholar]
- Gestational diabetes mellitus and risk of type 2 diabetes 10 years after the index pregnancy in Sri Lankan women - A community based retrospective cohort study. PLoS One. 2017;12:e0179647.
- [Google Scholar]
- Women with a history of gestational diabetes on long-term follow up have normal vascular function despite more dysglycemia, dyslipidemia and adiposity. Diabetes Res Clin Pract. 2015;110:309-14.
- [Google Scholar]
- Lipoprotein-associated phospholipase A2 and AGEs are associated with cardiovascular risk factors in women with history of gestational diabetes mellitus. Gynecol Endocrinol. 2014;30:241-4.
- [Google Scholar]
- Preeclampsia as a risk factor for diabetes:A population-based cohort study. PLoS Med. 2013;10:e1001425.
- [Google Scholar]
- Postpartum outcomes in women with gestational diabetes and their offspring:POGO study design and first-year results. Rev Diabet Stud. 2013;10:49-57.
- [Google Scholar]
- Use of healthcare resources after gestational diabetes mellitus:A longitudinal case-control analysis. Scand J Public Health. 2012;40:385-90.
- [Google Scholar]
- Impact of gestational diabetes on the risk of diabetes following pregnancy among Chinese and South Asian women. Diabetologia. 2012;55:2148-53.
- [Google Scholar]
- Follow-up of women with gestational diabetes in the Tehran Lipid and Glucose Study (TLGS):A population-based cohort study. J Obstet Gynaecol Res. 2012;38:698-704.
- [Google Scholar]
- Evaluation of postpartum carbohydrate intolerance and cardiovascular risk factors in women with gestational diabetes. Gynecol Endocrinol. 2011;27:361-7.
- [Google Scholar]
- Racial and ethnic disparities in diabetes risk after gestational diabetes mellitus. Diabetologia. 2011;54:3016-21.
- [Google Scholar]
- Risk of development of diabetes mellitus after diagnosis of gestational diabetes. CMAJ. 2008;179:229-34.
- [Google Scholar]
- Prevalence of type 2 diabetes among women with a previous history of gestational diabetes mellitus. Diabetes Res Clin Pract. 2008;81:124-9.
- [Google Scholar]
- Metabolic syndrome after pregnancy complicated with gestational diabetes:Four-year follow-up. Orv Hetil. 2008;149:831-8.
- [Google Scholar]
- Increasing incidence of abnormal glucose tolerance in women with prior abnormal glucose tolerance during pregnancy:DIAGEST 2 study. Diabet Med. 2008;25:58-64.
- [Google Scholar]
- Gestational diabetes mellitus:Clinical predictors and long-term risk of developing type 2 diabetes:A retrospective cohort study using survival analysis. Diabetes Care. 2007;30:878-83.
- [Google Scholar]
- Gestational diabetes identifies women at risk for permanent type 1 and type 2 diabetes in fertile age:Predictive role of autoantibodies. Diabetes Care. 2006;29:607-12.
- [Google Scholar]
- Predictive factors of developing diabetes mellitus in women with gestational diabetes. Acta Obstet Gynecol Scand. 2002;81:11-6.
- [Google Scholar]
- Natural course of gestational diabetes mellitus:Long term follow up of women in the SPAWN study. BJOG. 2002;109:1227-31.
- [Google Scholar]
- Risk factors for development of diabetes mellitus in women with a history of gestational diabetes mellitus. Chin Med J (Engl). 2000;113:759-62.
- [Google Scholar]
- Glucose intolerance and other cardiovascular risk factors in Chinese women with a history of gestational diabetes mellitus. Aust N Z J Obstet Gynaecol. 1999;39:478-83.
- [Google Scholar]
- History of gestational diabetes leads to distinct metabolic alterations in nondiabetic African-American women with a parental history of type 2 diabetes. Diabetes Care. 1998;21:1250-7.
- [Google Scholar]
- Prevalence and predictive value of islet cell antibodies and insulin autoantibodies in women with gestational diabetes. Diabet Med. 1994;11:558-63.
- [Google Scholar]
- Diabetes in pregnancy in Zuni Indian women. Prevalence and subsequent development of clinical diabetes after gestational diabetes. Diabetes Care. 1993;16:1231-5.
- [Google Scholar]
- Follow-up of women with previous GDM. Insulin, C-peptide, and proinsulin responses to oral glucose load. Diabetes. 1991;40(Suppl 2):136-41.
- [Google Scholar]
- Gestational diabetes with diabetes and prediabetes risks:A large observational study. Eur J Endocrinol. 2018;179:51-8.
- [Google Scholar]
- The long-term effects of stillbirth on women with and without gestational diabetes:A population-based cohort study. Diabetologia. 2015;58:67-74.
- [Google Scholar]
- A simple scoring method using cardiometabolic risk measurements in pregnancy to determine 10-year risk of type 2 diabetes in women with gestational diabetes. Nutr Diabetes. 2013;3:e72.
- [Google Scholar]
- Cardiometabolic risk in Chinese women with prior gestational diabetes:A 15-year follow-up study. Gynecol Obstet Invest. 2012;73:168-76.
- [Google Scholar]
- Racial differences in the association between gestational diabetes mellitus and risk of type 2 diabetes. J Womens Health (Larchmt). 2012;21:628-33.
- [Google Scholar]
- Metabolic disorders in women with previous gestational diabetes mellitus, Tehran lipid and glucose study. Iranian J Endocrinol Metabol. 2011;13:339-45.
- [Google Scholar]
- C-reactive protein and features of metabolic syndrome in Brazilian women with previous gestational diabetes. Diabetes Res Clin Pract. 2007;78:23-9.
- [Google Scholar]
- A 20-year prospective study of childbearing and incidence of diabetes in young women, controlling for glycemia before conception:The Coronary Artery Risk Development in Young Adults (CARDIA) Study. Diabetes. 2007;56:2990-6.
- [Google Scholar]
- Gestational diabetes and the incidence of diabetes in the 5 years following the index pregnancy in South Indian women. Diabetes Res Clin Pract. 2007;78:398-404.
- [Google Scholar]
- Fibrinolytic dysfunction after gestation is associated to components of insulin resistance and early type 2 diabetes in Latino women with previous gestational diabetes. Diabetes Res Clin Pract. 2007;78:340-8.
- [Google Scholar]
- Diabetes and abnormal glucose tolerance in women with previous gestational diabetes. Diabetes Care. 2003;26:1199-205.
- [Google Scholar]
- The Boston Gestational Diabetes Studies:Review and Perspectives. In: Carbohydrate metabolism in pregnancy and the newborn IV. London: Springer-Verlag Berlin Heidelberg; 1984. p. :287-94.
- [Google Scholar]
- Diabetes Care. 2011;34(Suppl 1):S11-61.
- Practice bulletin no. 137: Gestational diabetes mellitus. Obstet Gynecol. 2013;122:406-16.
- [Google Scholar]
- Reasons for women's non-participation in follow-up screening after gestational diabetes. Women Birth. 2015;28:e157-63.
- [Google Scholar]
- Health systems approaches to diabetes screening and prevention in women with a history of gestational diabetes. Curr Diab Rep. 2015;15:114.
- [Google Scholar]
- Racial/ethnic disparities in the prevalence of gestational diabetes mellitus by BMI. Diabetes Care. 2012;35:1492-8.
- [Google Scholar]
- Racial/ethnic differences in the prevalence of gestational diabetes mellitus and maternal overweight and obesity, by nativity, Florida, 2004-2007. Obesity (Silver Spring). 2013;21:E33-40.
- [Google Scholar]
- Type 2 diabetes after gestational diabetes:The influence of changing diagnostic criteria. World J Diabetes. 2015;6:234-44.
- [Google Scholar]
- Economic impact of integrated care models for patients with chronic diseases:A systematic review. Value Health. 2016;19:892-902.
- [Google Scholar]
- Gestational diabetes:Implications for cardiovascular health. Curr Diab Rep. 2012;12:43-52.
- [Google Scholar]
- Blood pressure and proteinuria control remains a challenge in patients with type 2 diabetes mellitus and chronic kidney disease:Experience from the prospective observational ALICE-PROTECT study. BMC Nephrol. 2016;17:135.
- [Google Scholar]
- Evidence-based treatment of diabetic retinopathy. Seminars Ophthalmol. 2016;32:67-74.
- [Google Scholar]






