Translate this page into:
Promise(s) of using mesenchymal stem cells in reproductive disorders
Reprint requests: Dr Vijayalakshmi Venkatesan, Scientist ‘E’, Department of Biochemistry/Stem Cell Research, National Institute of Nutrition, (ICMR), Jamai-Osmania (P.O), Hyderabad 500 007, Telangana, India e-mail: v.venkateshan@gmail.com
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
In recent times, infertility among both man and woman has become a major concern affecting about 20 per cent of the population worldwide and has been attributed in part to several aetiological factors such as changes in lifestyle, which includes sedentary life, dietary habits, sleep anomalies, environmental pollution, etc. Assisted reproductive technologies (ART) have come to the rescue of many such couples, but presence of metabolic disorders such as obesity, diabetes with insulin resistance (IR) and its secondary complications (micro- and macro-vascular complications), become confounders to the outcome of ART. Cell therapies are arising as a new hope in the management of reproductive disorders and currently, the efficacy of mesenchymal stem cells (MSCs) harvested from the adult sources finds wide application in the management of diseases like stroke, neuropathy, nephropathy, myopathy, wounds in diabetes, etc. Given the capacity of MSCs to preferentially home to damaged tissue and modulate the cellular niche/microenvironment to augment tissue repairs and regeneration, the present review outlines the applications of MSCs in the management of infertility/reproductive disorders.
Keywords
Diabetes
infertility
mesenchymal stem cells
metabolic syndrome
obesity
WNIN obese mutant rats
Introduction
Stem cells (SCs) are known to have a capacity of self renewal and differentiation into various cell types on receiving the desired stimulus. Based on the proliferation rates and differentiation capabilities (potency/plasticity), SCs have been broadly classified into embryonic (ESCs) and adult stem cells (ASCs)/somatic stem cells1. ESCs, the pluripotent SCs derived from inner cell mass of the blastocyst not only replicate indefinitely, but also have a high differentiation capacity to form cell types/tissues from all the three germ layers (ecto-, endo- and mesoderm) and hence carry immense application in tissue regeneration/ replacement following injury/disease. However, several ethical issues, accessibility and availability limit their applications in the clinical world, opening horizons for application of ASCs. ASCs unlike ESCs are multipotent undifferentiated cells derived from juvenile/adult tissues, with limited capacities of self-renewal and plasticity/differentiation to form different cell types2. Based on the source/tissue of derivation, ASCs have also been classified as haematopoietic (HSCs), mammary (MaSCs), intestinal (ISCs), mesenchymal (MSCs), endothelial (EnSCs), neural (NSCs), testicular (TSCs) and olfactory (OSCs) SCs, etc., ASCs have profound therapeutic applications in the management of several complications such as spinal cord injury3, liver cirrhosis4 and peripheral vascular disease5 with some success. However, limitations on the yield of these SCs, successful homing and/or differentiation to the tissue/organ of interest in vivo from most of the adult sources have limited their clinical application. On the other hand, MSCs among the ASCs have gained importance as an obvious choice for cell therapy because of the potent beneficial effects of immunomodulation, targeted differentiation to tissue of interest, higher proliferation rates, etc.
MSCs are multipotent adult progenitor cells derived from the embryonic mesoderm. In 1970 Friendstein and co-workers isolated this population of cells from the bone marrow as colony-forming unit-fibroblasts (CFU-F) with abilities to differentiate into at least adipo-, chondro- and osteocyte lineages6. In the past four decades, these multipotent progenitors have been isolated from most of the adult tissues such as adipose tissue, synovium, cartilage, bone, foetal tissues and extra embryonic tissues such as placenta, amniotic fluid and umbilical cord blood and umbilical cord matrix7 and with extended plasticity to other mesodermal cell types such as myocytes and cardiomyocytes, and transdifferentiation to ectodermal (skin and neurons) and endodermal (insulin-secreting like cells/islet-like cellular aggregates, hepatocytes) lineages8. Studies have shown that MSCs could also be differentiated into endothelial cells and haematopoietic cells9. Several studies on MSCs from different sources have shown a similar phenotypic pattern of CD90+ CD29+ CD105+/CD34- CD45- CD11b- and are immunologically inert [HLA-DR- and do no elicit a mixed lymphocyte reaction (MLR) on co-culture with lymphocytes]10. Long viewed as the cell type in tissue regeneration, studies in the last few decades have demonstrated their immunomodulatory functions attributed to the secretion of various cytokines and inhibitors of oxidative stress, adding new horizons for clinical applications of MSCs10. High yield, relatively easier propagation techniques, immunological inertness and immunomodulation highlight the potential role of MSCs in cell replacement therapies for treating several human diseases1011 such as cardiac ischaemia12, Parkinson's disease13 and muscular dystrophies14, diabetes and its complications1516, more recently in the management of infertility (both male and female)17.
Metabolic syndrome (MS) and infertility
Metabolic disorders such as insulin resistance (IR) resulting from the increasing incidence of obesity, have serious ramifications on the progression of lifetime health problems such as type II diabetes (T2D), cardiovascular disease (CVD), dyslipidaemia and hypertension. Obesity a component of metabolic syndrome characterized by adipocyte hypertrophy, hypertriglyceridaemia, hyperleptinaemia, hypertriglyceridaemia, etc. is a risk factor for development of diabetes and/or IR, while diabetes another component characterized by hyperglycaemia and/or hyperinsulinaemia is in turn a risk factor for onset and progression of obesity. Diabetes and obesity share several similar causes, are inter-related and have reached the state of an epidemic with the incidence expected to reach 330 million worldwide by 203018. Obesity and/or diabetes demonstrate hyperglycaemia, hyperinsulinaemia, hyperleptinaemia, hypercholesterolaemia with or without IR attributing to a wide spectrum of secondary complications with cardiovascular disease, stroke, glomerular nephropathy, diabetic retinopathy, sciatica at one end of the spectrum and infertility (due to an associated disturbance in the hormonal milieu thus affecting the reproductive system) at the other end19. Development of obesity and IR commonly go hand-in-hand with the development of fertility problems, but the link between this metabolic state and infertility remains to be defined.
With no preferences, infertility affects both the sexes equally with its own set of complications. The common infertility related complications in obese women include altered ovarian physiology and associated ovulatory disturbances (oligomenorrhoea), increased risk of miscarriages, polycystic ovary syndrome (PCOS), impaired outcomes of assisted reproductive technologies (ART) and pregnancy, and some hormone-dependent forms of cancer, while among obese males it could lead to hypogonadism, decreased sperm concentrations, sperm motility and fertility rates20.
In recent years increased body mass index (BMI) has been shown to be associated with reproductive parameters such as poor semen quality, decreased sperm concentration, decreased normal-motile sperm cells and increased DNA fragmentation index21 among men. However, contradicting studies also showed little or no relation between obesity, sperm concentration and motility or morphology21 in males, even when serum reproductive hormone levels were altered22. On the contrary, studies performed on diet-induced obese mice demonstrated decrease in sperm motility23, fertilization rate24, number of plugs and pregnancy rate23, increase in sperm DNA damage and sperm intracellular reactive oxygen species (ROS)24.
Mechanisms underlying infertility in MS
Reproductive function declines at both extremes of human energy balance. A significant proportion of the infertile or sub-fertile populations is obese or overweight, with a plethora of reproductive complications including menstrual dysfunction, anovulation and miscarriage25. Both male and female reproductive (hormones) systems are under stringent regulation by the hypothalamus and pituitary glands. Interestingly, reproductive hormones and their analogues, which act through estrogen and testosterone receptors, are targets for obesogenic agents such as endocrine disruptors, thiazolidinediones etc. The common derivation of hormones from cholesterol biosynthesis, which is in turn regulated by signaling molecules such as insulin26 and leptin, the key molecules in diabetes/obesity-induced obesity, undoubtedly depicts cross-talk between cholesterol, insulin and leptin signaling pathways with the hormonal signaling pathways27. Interestingly, adipokines such as leptin, adiponectin, resistin, etc., and enterokines such as ghrelin have also been implicated in the reproductive functions (normal sexual maturation and reproduction) since these modulate glucose homeostasis, fat homeostasis, influence insulin action and thus may mechanistically link obesity, IR and fertility among both the sexes28. A dysregulation of all these adipokines/enterokines is seen with onset and progression of obesity and the condition worsens with the setting in of associated secondary complications such as IR and diabetes mellitus (DM).
The mechanisms leading to/affecting male infertility are mostly ambiguous and undefined29 and may contribute to a dysregulation of the hypothalamic-pituitary-gonadal (HPG) axis. An increase in adipocyte hypertrophy/hyperplasia as a result of obesity can result in both physical changes (increased scrotal temperatures, sleep apnoea and erectile dysfunction) and hormonal changes [altered levels of leptin, oestrogen, insulin (increased) and testosterone (decreased)], which in turn contribute to oligozoospermia, azoospermia, increased DNA fragmentation index (DFI), decreased semen volume, etc. In addition, excess adipose tissue increases peripheral aromatization of androgen to oestrogen due to increased activity of aromatase cytochrome P450 enzyme20. Animal studies demonstrate deleterious impact of high oestrogen levels on spermatogenesis30. In addition, diminished sex hormone-binding globulin (SHBG) seen in obesity also enhances bioavailable testosterone and estradiol, thus affecting the central negative feedback of excess oestrogen, thereby contributing to decreased hypothalamic pituitary signalling. Oestrogen acts on the hypothalamus to negatively regulate the release of pulses of gonadotropin releasing hormone (GnRH) as well as release of luteinizing hormone (LH) and follicle stimulating hormone (FSH) from the anterior pituitary gland, and estrogen agonists have been shown to have an inhibitory effect on androgen biosynthesis30.
Obesity also involves a higher secretion of several adipokines such as leptin, resistin, tumour necrosis factor-α (TNF-α), etc. Excess levels of leptin due to increased secretion from adipose tissue are known to have a deleterious effect on sperm production and the production of androgens by Leydig cells31 thus directly affect sperm via the endocrine system independent of changes in the HPG axis32. Hyperinsulinaemia, which often occurs in obese men, has an inhibitory effect on normal spermatogenesis and can be linked to decreased male fertility by affecting the amount of nuclear and mitochondrial DNA damage in the sperm33. In addition, insulin levels also influence the levels of sex hormone binding globulin (sHBG), a glycoprotein that binds to sex hormones. High circulating insulin levels inhibit sHBG synthesis in the liver34, leading to a decrease in sHBG thus binding less oestrogen leaving more biologically active, free oestrogen. Increased oxidative stress/ROS as seen in obesity is an independent marker of male infertility leading to DNA damage, deformity and damaged plasma membrane integrity of sperm, acting by impairing the feedback regulation of the HPG axis, in turn affecting spermatogenesis and male reproductive function35. A study by Fernandez et al36 on diet-induced obesity in a Wistar rat model demonstrated decreased sperm motility without affecting other parameters such as reproductive organ weights and sperm counts in the testis and epididymis, sexual behaviour, testosterone levels, etc., causing a slight reduction in fertility potential36.
Obesity is associated with many ovulatory disorders (anovulation) and is known to worsen the metabolic and reproductive abnormalities in women with PCOS37. Interestingly, many of women with PCOS demonstrate hyperinsulinaemia and/or IR38. However, other anovulation disorders associated with obesity are characterized by hypogonadotrophic hypogonadism (in contrast to the relative hypergonadotrophic hypergonadism of PCOS) such as in Prader-Willi syndrome39.
Obesity also potentially adversely affects the endometrium, implantation and early foetal development40, thus increasing the risk of miscarriage41. Decreased in vitro fertilization (IVF) pregnancy rates in obese women could be partially attributed to the relative gonadotrophin resistance and fewer oocytes retrieved. Similar studies on obese hyperleptinaemic leptin resistant mice demonstrated that fewer embryos reach the blastocyst stage than normal controls42 possibly implicating for the effects of adipokine/enterokine on ovulatory function. Excess bioavailable androgen may also have detrimental effects on the oocyte, follicle and endometrium.
These mechanisms have been summarized in the Figure.
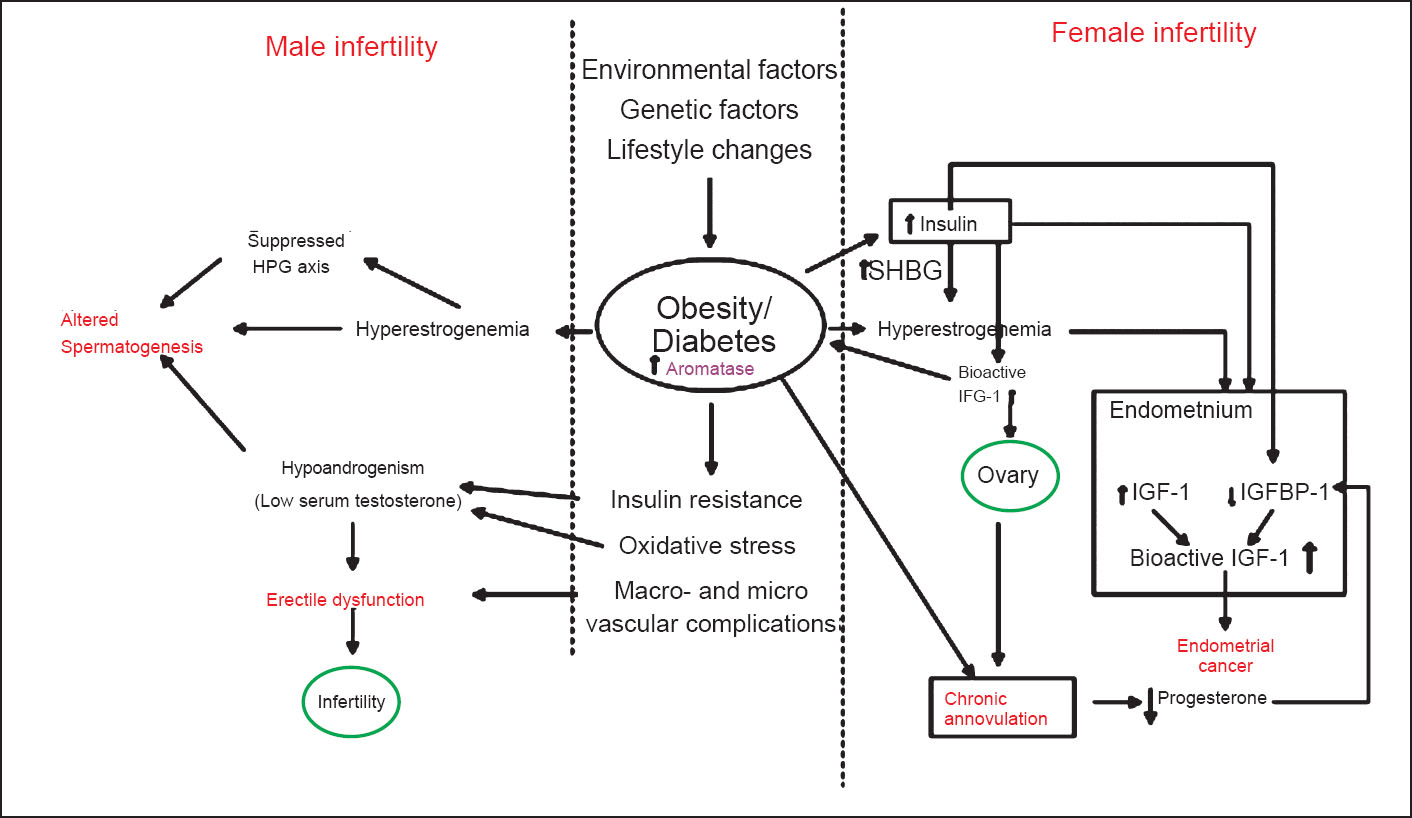
- Schematic diagram showing different mechanisms/pathways resulting in male and female infertility with the primary contributing factors being obesity and diabetes with insulin resistance. HPG, hypothalamus-pitutary-gonadal axis; SHBG, sex hormone-binding globulin; IGF-1, insulin growth factor-1; IGFBP-1, insulin growth factor binding protein-1.
Animal models of infertility
Similar to many other disease models such as obesity, diabetes, cancers, pancreatitis, etc. animal models of infertility are also available. While the stubby mice43, A-myb knockout mouse44 and many others45 demonstrate experimental (genetic) male infertility, ALADIN deficient mice (meiosis and maturation of oocytes affected)4647 demonstrate genetic models of female infertility. Overall, more models of male infertility exist compared to those on female infertility. Recently, chemically induced infertility rodent models are also being explored4849.
WNIN obese mutant rats developed at the animal facility of National Institute of Nutrition, Hyderabad, India, similar to other models of obesity such as Zucker rats, ob/ob mice, etc. demonstrate infertility. Homozygous animals (both sexes) among these mutants did not breed, though reversal of infertility could be achieved partially50. Similar to human and other model studies, males among both these mutant models were normal in terms of sperm count, sperm motility and testis histology, however, the decreased libido observed was possibily due to a low circulating testosterone levels, which could also account for observed low testis and accessory gland weights in line with other obese models such as Zucker rats and human subjects. Female mutant rats on the other hand, exhibit delayed puberty, irregular oestrus cycles with small ovaries and short and stumpy uterine horns. Absence of FSH peak during oestrus stage of the sexual cycle and E2 peak of normal oestrus cycle, elevated progesterone levels throughout the sexual cycle possibly attribute the observed female infertility to abnormal gonadosteroid levels51.
Similarly, studies in obese male Zucker rats demonstrate an inadequate sexual behaviour52, low pituitary weight and low concentration of LH and FSH53, with low testis weight, low levels of circulating testosterone, and low weights of levator ani (LA) muscle54.
Management of infertility
Though both the sexes are at risk/ are contributors for infertility, 30-40 per cent of the reproductive failure is contributed by the male counterpart. Grossly, causes of infertility among females could be grouped into ovulatory problems (ammhenorrhoea, abnormalities of thyroid gland, increased stress levels) (35%), pelvic and tubal factors (50%) (endometriosis, scarred tissue/adhesions and distorted fallopian tubes) and other such as cervical (stenosis, sperm allergy) and uterine (uterine polyps or fibroids) factors (10%), while on the other hand, male infertility could be because of a number of genetic and environmental factors in addition to low sperm counts, abnormal sperm morphology (shape), and low sperm motility and varicocele55.
Management of infertility in animal models has been successful to some extent. Most commonly employed strategies include food restriction (50-70%), adrenelectomy, in both sexes, administration of hormones such as testosterone or estrogen, etc., However these results have not been to satisfaction since neither techniques restore infertility completely50. ART such as in vitro fertilization (IVF), intrauterine insemination (IUI) etc. and administration of hormones such as oestrogen, testosterone, glucocorticoids etc. are among the choices for management of infertility in both males and females. However, being invasive, expensive and hence non-affordable and with several side-effects, all of these strategies have their own disadvantages, though some success has been demonstrated. Studies in experimental models have shown promises and stem cell therapy for management of infertility is gaining importance.
Management of female infertility: Ovulation is a complex event in which hormonal signals and physical events are linked in a delicate balance. Women ovulate most effectively in their late teens and early twenties. By age 35-38, most women may experience a decline in the ability to ovulate effectively. Ovulation can be seriously affected by (i) abnormalities of the thyroid gland, (ii) overproduction of prolactin (a hormone leading to breast milk production), (iii) excessive male hormone (androgens) and (iv) physical stress, psychological stress and extreme lifestyle changes.
Premature ovarian failure is another cause of female infertility. Studies by Azmy et al56, have shown the reversal of experimentally induced (using cyclophosphamide) premature ovarian failure in rat model. Their studies revealed that intravenous injection of bone marrow (BM)-MSCs derived from male rats reversed hypoestrogenic and hypergonadotrophic state of experimental rats in the treated group as evidenced by serum levels of FSH and E2, resurrection of ovarian folliculogenesis and corpus luteum formation confirmed from the cytological and histopathological studies. Dectection of sry gene expressing Y chromosome in the ovarian tissues in the treated group corroborates the concept that injected/infused MSCs augment tissue regeneration by homing/incorporating into the tissue structure and/or by paracrine effects. Johnson et al54, showed that bone marrow grafts to female mice, and possibly humans, could produce new follicles and oocytes in the recipient ovary.
Another important cause of female infertility is endometroisis. Endometrium is a dynamic tissue that undergoes cycles of growth and regression with each menstrual cycle. This preparation of the endometrium is associated with a period of hyperproliferation and angiogenesis58, which points towards the dynamics of resident stem cell population. Endometrial polyps arise from endometrial overgrowth and may cause intermenstrual bleeding, irregular bleeding, and menorrhagia59. Several studies have shown that transplanted BM-MSCs are recruited to uterine endometrium in endometriosis. Zhou et al60, demonstrated the inhibitory effects of smoking on both recruitment and differentiation of BM-MSCs to the uterus. Other studies6162 have demonstrated presence of ‘endometrial stem cells’, the resident stem cells from endometrium as mesenchymal-like stem cells in structure and function demonstrating clonality; long-term culturing properties; expression of CD146, CD105, CD90, CD73, MSI1, NOTCH1, SOX2; absence of CD34 and CD14 expression and multilineage differentiation potential. These endometrial stem cell transplantation have potential applications in restoration of endometriosis as an autologous source and restoration of dopamine production in Parkinson's disease model63.
Management of male infertility: Male infertility is commonly due to deficiencies in the semen, and semen quality is used as a surrogate measure of male fecundity. Male germ cells are derived from a founder population of primordial germ cells (PGCs) that are set aside early in embryogenesis. After birth, PGCs differentiate to spermatogonial stem cells in the male which are responsible for maintaining spermatogenesis throughout life by continuous production of daughter cells that differentiate into spermatozoa6465. Causes of male infertility are more attributed to sperm dysfunctions in terms of their number, morphology and motility. Erectile dysfunction is another emerging cause for male infertility. MSCs have again come to rescue in the management of male infertility.
Drusenheimer and co-workers66, showed that hBM-MSCs could transdifferentiate into putative male germ cells that could sustain sperm production. Their studies revealed that these transdifferentiated male germ cells expressed early germ cell markers Oct4, Fragilis, Stella and Vasa and male germ cell specific markers Dazl, TSPY, Piwil2 and Stra8. This was also substantiated by other studies6768 which demonstrated that adult ESCs/ BM-MSCs from mouse/human could differentiate into Leydig cells or adrenocortical cells (i.e., steroidogenic cells), thus paving way for treatment of infertility related complications. In addition, MSCs derived from adipose tissue69 and umbilical cord blood70 have also shown similar potential. Further studies in this direction have also come from the Japanese group working at Kyoto University towards differentiation of embryonic stem cell from mouse to form functional spermatogonial cells in vitro and in vivo, with a capacity to fertilize mouse oocyte when implanted into female mice71.
Limitations for therapeutic use
There are several problems that limit the therapeutic use of MSCs at present7273. Poor engraftment and limited differentiation under in vivo conditions are major obstacles for efficient therapeutic use of MSCs73. Since the frequency of spontaneous differentiation of MSCs in the host tissue is extremely rare, therapeutic use of MSCs depends on the ability to control their in vivo differentiation into functional cells with high efficiency and purity73. An additional limitation is the potential of MSCs to differentiate into unwanted mesenchymal lineages74, which could impair their therapeutic use.
Conclusion
Because of their immunomodulatory ability, self-renewal and differentiation capacity, MSCs are promising therapeutic agents for improvement of cardiac function and treatment of cardiomyopathy, nephropathy, and wounds in diabetic patients. Among stem cells, MSCs have several advantages for therapeutic use such as ability to migrate to the sites of tissue injury, strong immunosuppressive effects, better safety after infusion of allogenic MSCs, and lack of ethical issues, such as those related to the application of human embryonic stem cells. However, there are several outstanding problems including potential risk of malignant transformation of MSCs, unwanted mesenchymal lineages differentiation, and suboptimal targeted differentiation, which need to be addressed before MSCs are defined as a novel and efficient therapeutic agent in the management of infertility.
References
- Stem Cell Basics. 2009. Stem Cell Information. Bethesda, MD: National Institutes of Health, U.S. Department of Health and Human Services; Available from: http://stemcells.nih.gov/info/basics/defaultpage
- [Google Scholar]
- Autologous multiple injections of in vitro expanded autologous bone marrow stem cells for cervical level spinal cord injury - A case report. J Stem Cells Regen Med. 2010;6:175-6.
- [Google Scholar]
- Improved liver function in patients with liver cirrhosis after autologous bone marrow cell infusion therapy. Stem Cells. 2006;24:2292-8.
- [Google Scholar]
- Application of autologous bone marrow mononuclear cells in six patients with advanced chronic critical limb ischemia as a result of diabetes: our experience. Cytotherapy. 2011;13:993-9.
- [Google Scholar]
- The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393-403.
- [Google Scholar]
- Mesenchymal stem cells derived from bone marrow of diabetic patients portrait unique markers influenced by the diabetic microenvironment. Rev Diabet Stud. 2009;6:260-70.
- [Google Scholar]
- Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-7.
- [Google Scholar]
- Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells. 2004;22:377-84.
- [Google Scholar]
- Immunomodulatory effect of mesenchymal stem cells. Braz J Med Biol Res. 2010;43:425-30.
- [Google Scholar]
- Derivation, growth and applications of human embryonic stem cells. Reproduction. 2004;128:259-67.
- [Google Scholar]
- Bone marrow-derived mesenchymal stem cell therapy as a candidate disease-modifying strategy in Parkinson's disease and multiple system atrophy. J Clin Neurol. 2009;5:1-10.
- [Google Scholar]
- Mesenchymal stem cells: emerging therapy for Duchenne muscular dystrophy. PMR. 2009;1:547-59.
- [Google Scholar]
- Islet-like cell aggregates generated from human adipose tissue derived stem cells ameliorate experimental diabetes in mice. PLoS One. 2011;6:e20615.
- [Google Scholar]
- Mesenchymal stem cell treatment of the complications of diabetes mellitus. Stem Cells. 2011;29:5-10.
- [Google Scholar]
- Stem cells as new agents for the treatment of infertility: Current and future perspectives and challenges. Biomed Res Int 2014 2014 507234
- [Google Scholar]
- The global burden. Diabetes and impaired glucose tolerance. In: Sicree R, Shaw J, Zimmet J, eds. IDF diabetes atlas (4th ed). Brussels, Belgium: International Diabetes Federation; 2009.
- [Google Scholar]
- Direct effects of sex steroid hormones on adipose tissues and obesity. Obes Rev. 2004;5:197-216.
- [Google Scholar]
- Treatment of male infertility secondary to morbid obesity. Nat Clin Pract Endocrinol Metab. 2008;4:415-9.
- [Google Scholar]
- Body mass index in relation to semen quality, sperm DNA integrity, and serum reproductive hormone levels among men attending an infertility clinic. Fertil Steril. 2010;93:2222-31.
- [Google Scholar]
- Is overweight a risk factor for reduced semen quality and altered serum sex hormone profile? Fertil Steril. 2008;90:619-26.
- [Google Scholar]
- Diet-induced obesity in male mice is associated with reduced fertility and potentiation of acrylamide-induced reproductive toxicity. Biol Reprod. 2010;82:94-104.
- [Google Scholar]
- The effect of paternal dietinduced obesity on sperm function and fertilization in a mouse model. Int J Androl. 2011;346:402-10.
- [Google Scholar]
- Adipokines. Implications for female fertility and obesity. Reproduction. 2005;130:583-97.
- [Google Scholar]
- Endocrine and paracrine control of follicular development and ovulation rate in farm species. Anim Reprod Sci. 2004;82-83:461-77.
- [Google Scholar]
- Insulin and leptin acutely regulate cholesterol ester metabolism in macrophages by novel signaling pathways. Diabetes. 2001;50:955-61.
- [Google Scholar]
- Obesity and the role of gut and adipose hormones in female reproduction. Hum Reprod Update. 2006;12:585-601.
- [Google Scholar]
- The effect of obesity on sperm disorders and male infertility. Nat Rev Urol. 2010;7:153-61.
- [Google Scholar]
- Leptin and androgens in male obesity: evidence for leptin contribution to reduced androgen levels. J Clin Endocrinol Metab. 1999;84:3673-80.
- [Google Scholar]
- Serum immunoreactive leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292-5.
- [Google Scholar]
- Insulin dependant diabetes mellitus: implications for male reproductive function. Hum Reprod. 2007;22:1871-7.
- [Google Scholar]
- Decreased androgen levels in massively obese men may be associated with impaired function of the gonadostat. Int J Obes Relat Metab Disord. 2000;24:1433-7.
- [Google Scholar]
- Effect of obesity and fertility status on sex steroid levels in men. Urology. 1993;42:171-4.
- [Google Scholar]
- Diet-induced obesity in rats leads to a decrease in sperm motility. Reprod Biol Endocrinol. 2011;9:32.
- [Google Scholar]
- Insulin resistance in the sisters of women with polycystic ovary syndrome: association with hyperandrogenemia rather than menstrual irregularity. J Clin Endocrinol Metab. 2002;87:2128-33.
- [Google Scholar]
- Insulin-lowering agents in the management of polycystic ovary syndrome. Endocr Rev. 2003;24:633-67.
- [Google Scholar]
- Endocrine dysfunction in Prader–Willi syndrome: a review with special reference to GH. Endocr Rev. 2001;22:787-99.
- [Google Scholar]
- Obesity is associated with increased risk of first trimester and recurrent miscarriage: matched case-control study. Hum Reprod. 2004;19:1644-6.
- [Google Scholar]
- Declining fertility in the lethal yellow mouse is related to progressive hyperleptinemia and leptin resistance. Reprod Nutr Dev. 2005;45:143-50.
- [Google Scholar]
- Animal models of physiologic markers of male reproduction: genetically defined infertile mice. Environ Health Perspect. 1987;74:15-29.
- [Google Scholar]
- Arrest of spermatogenesis and defective breast development in mice lacking A-myb. Nature. 1997;386:713-7.
- [Google Scholar]
- Mice lacking the nuclear pore complex protein ALADIN show female infertility but fail to develop a phenotype resembling human triple A syndrome. Mol Cell Biol. 2006;26:1879-87.
- [Google Scholar]
- Contemporary genetic technologies and female reproduction. Hum Reprod Update. 2011;17:829-47.
- [Google Scholar]
- Chemotherapy-induced female infertility and protective action of gonadotropin-releasing hormone analogues. J Obstet Gynaecol. 2007;27:20-4.
- [Google Scholar]
- Ethanol-induced male infertility: impairment of spermatozoa. J Pharmacol Exp Ther. 1983;225:479-86.
- [Google Scholar]
- Animal models of obesity & their usefulness in molecular approach to obesity. Indian J Med Res. 1998;108:225-42.
- [Google Scholar]
- Effect of estradiol on uterine weight, thyroid function, food intake, and pituitary weight of genetically obese (fatty Zucker) and lean rats. Proc Soc Exp Biol Med. 1976;153:88-91.
- [Google Scholar]
- A long-acting anabolic steroid: 4-hydroxy-19-nortestosterone-17-cyclopentylpropionate. Endocrinology. 1963;72:494-5.
- [Google Scholar]
- The biology of infertility: research advances and clinical challenges. Nat Med. 2008;14:1197-213.
- [Google Scholar]
- Stem cells restored ovarian function and folliculogenesis in rats following induced Ovarian failure. IFFS 2010 World Congress on Fertility and Sterility, Munich; 12-16 September; 2010.
- [Google Scholar]
- Oocyte generation in adult mammalian ovaries by putative germ cells in bone marrow and peripheral blood. Cell. 2005;122:303-15.
- [Google Scholar]
- Enhanced differentiation and clonogenicity of human endometrial polyp stem cells. Differentiation. 2011;81:172-80.
- [Google Scholar]
- Cigarette smoke inhibits recruitment of bone-marrow-derived stem cells to the uterus. Reprod Toxicol. 2011;31:123-7.
- [Google Scholar]
- Endometrial regenerative cells: a novel stem cell population. J Transl Med. 2007;5:57.
- [Google Scholar]
- Characterization of endometrial mesenchymal stem-like cells obtained by endometrial biopsy during routine diagnostics. Fertil Steril. 2011;95:423-6.
- [Google Scholar]
- Endometrial stem cell transplantation restores dopamine production in a Parkinson's disease model. J Cell Mol Med. 2011;15:747-55.
- [Google Scholar]
- Germ and somatic cell lineages in the developing gonad. Mol Cell Endocrinol. 2000;163:3-9.
- [Google Scholar]
- Putative human male germ cells from bone marrow stem cells. Soc Reprod Fertil Suppl. 2007;63:69-76.
- [Google Scholar]
- Bone morphogenetic proteins induce germ cell differentiation from human embryonic stem cells. Stem Cells Dev. 2006;15:831-7.
- [Google Scholar]
- Differentiation of adult stem cells derived from bone marrow stroma into Leydig or adrenocortical cells. Endocrinol. 2006;147:4104-11.
- [Google Scholar]
- Recent advances in andrology-related stem cell research. Asian J Androl. 2008;10:171-5.
- [Google Scholar]
- Differentiation of umbilical cord mesenchymal stem cells into steroidogenic cells in comparison to bone marrow mesenchymal stem cells. Cell Prolif. 2012;45:101-10.
- [Google Scholar]
- In vitro production of fertile sperm from murine spermatogonial stem cell lines. Nat Commun. 2011;2:472.
- [Google Scholar]
- Opportunities and challenges for mesenchymal stem cell-mediated heart repair. Curr Opin Lipidol. 2007;18:645-9.
- [Google Scholar]






