Translate this page into:
Production of a compound against methicillin resistant Staphylococcus aureus (MRSA) from Streptomyces rubrolavendulae ICN3 & its evaluation in zebrafish embryos
Reprint requests: Dr S.G. Prakash Vincent, Coordinator, International Centre for Nanobiotechnology (ICN) Centre for Marine Science & Technology (CMST), Manonmaniam Sundaranar University Rajakkamangalam 629 502, Kanyakumari District, TN, India e-mail: vsgprakash.icn@gmail.com
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Antibiotic resistance in pathogens has become a serious problem worldwide. Therefore, the search for new antibiotics for drug resistanct pathogens is an important endeavor. The present study deals with the production of anti-methicillin resistant Staphylococcus aureus (MRSA) potential of Streptomyces rubrolavendulae ICN3 and evaluation of anti-MRSA compound in zebrafish embryos.
Methods:
The antibiotic production from S. rubrolavendulae ICN3 was optimized in solid state fermentation and extracted. The antagonistic activity was confirmed against MRSA and purified in silica gel column and reverse phase - HPLC with an absorption maximum at 215 nm. Minimal inhibitory concentration of the compound was determined by broth microdilution method. Zebrafish embryos were used to evaluate the extract/compound for its minimal inhibition studies, influences on heart beat rates, haematopoietic blood cell count and lethal dose values.
Results:
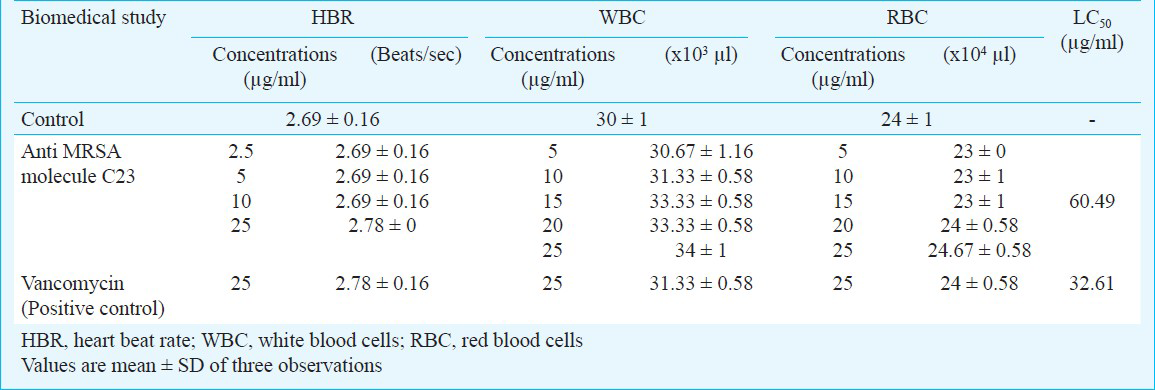
Streptomyces rubrolavendulae ICN3 showed potent antagonistic activity against MRSA with a zone of 42 mm. The minimum inhibitory concentration was calculated as 500 μg/ml of the crude extract and the purified C23 exhibited 2.5 μg/ml in in vitro assay. The LC50 value of the anti MRSA compound C23 was calculated as 60.49 μg/ml and the MRSA treated embryos survived in the presence of purified compound C23 at a dose of 10 μg/ml.
Interpretation & conclusions:
Our results suggested that the compound was potent with less toxic effects in zebrafish embryonic model system for MRSA infection. Further structural evaluation and analysis in higher mammalian model system may lead to a novel drug candidate for drug resistant Staphylococcus aureus.
Keywords
Anti-MRSA molecule
heart beat rate
RP-HPLC
solid state fermentation
Streptomyces
Streptomyces rubrolavendulae
zebrafish model
Methicillin resistant Staphylococcus aureus (MRSA) has become a common cause of infections especially in hospital environment, because of its systemic antibiotic resistance during infections1. Also, studies have shown a significant increase in methicillin resistance in clinical isolates of S. aureus in European countries up to 20 per cent2, and its colonization in community associated (CA)-MRSA infections has been reported to be 23 per cent in USA3. Thus, the increasing antibiotic resistance demands to discover new agents effective against MRSA to overcome the problems worldwide. Soil actinomycetes have been screened as source for antibiotics that are active against drug resistant pathogens4. Streptomycetes, the dominant members of the actinomycetes, which live in marine environment are poorly understood and only a few reports are available pertaining to actinomycetes from mangroves5. These have developed unique metabolic and physiological capabilities to produce diverse compounds with potential activities67. These are also shown to be potential antibiotic producers especially anti-MRSA compounds such as arenimycin, abyssomicin C, fijimycins A-C and etamycin A8910.
Several preclinical strategies have been used to identify potential drug candidates by target-based screening, phenotypic screening, modification of natural substances and biologic-based approaches11. The preliminary data validation in any mammalian animal models (e.g. rat or rabbit) is a slow and costly procedure, resulting in a gap in drug development process. Zebrafish has been considered as an ideal in vivo vertebrate model for drug screening12. This model has been used for the generation of high-value knowledge on safety risks of novel drugs13. The present study was undertaken with the objective of using zebrafish as an embryonic model system to study and evaluate the novelty of anti-MRSA compound from Streptomyces rubrolavendulae ICN3 showing antagonistic property to the MRSA clinical isolate.
Material & Methods
The study was conducted at International Centre for Nanobiotechnology, Centre for Marine Science and Technology, Manonmaniam Sundaranar University, Rajakkamangalam and Sathyabama University, Chennai, India from January 2011 to March 2012. The study was carried out after approval of the protocol by the Institutional Animal Ethics and Biosafety Committee of Manonmaniam Sundaranar University (Approval number: MSU/Ethical /2009/5).
Isolation and identification of actinomycetes: The sample was collected from the rhizosphere soil region of the Mangrove Avicennia officinalis from Manakkudy estuary of Arabian coast, Kanyakumari, India (8◦ 05′ 50.43” N Latitude 77◦ 29′ 05.35” E Longitude) at 3 feet depth. Soil sample (1 g) was serially diluted in sterile water and spread plated over the medium containing soluble starch, 20g; KNO3, 1g; NaCl, 0.5g; K2HPO4, 0.5g; MgSO4, 0.5g; FeSO4, 20 μM; agar 15g; distilled water 1 l, and 15 μg nalidixic acid was added to inhibit the growth of other bacteria and incubated at 28◦C for 3-7 days. The antagonistic strain was identified by 16s rRNA gene sequencing and phylogeny analysis14.
Antimicrobial assay: The isolated actinomycetes were patched over the isolation medium and incubated for 3-7 days at 28◦C. The antagonistic activity of S. rubrolavendulae ICN3 was performed by double layer agar method5 against MRSA. The soft agar medium was adjusted to 5 × 105 cfu/ml using log phase culture of MRSA with 0.3 per cent of agar in the Mueller-Hinton (M-H) broth; (Hi Media Labs, India) 1ml of the soft agar with MRSA culture was overlaid and incubated at 37°C overnight to analyze zone of inhibition.
Media optimization and antibiotic production: The effects of temperature (25, 28, 37, 40 and 45°C), pH (5, 6, 7, 8 and 9), and salinity (0.25, 0.5, 1 and 1.5%) on antibiotic production were determined by solid state fermentations (SSF). The medium components with selective nutrient sources of carbon (glucose, maltose, sucrose, lactose and fructose) and nitrogen (yeast extract, peptone, beef extract, tryptone and sodium nitrate) were used to optimize the antibiotic production by substituting components present in the basal medium. Nutrient optimized culture condition (NOCC)15 was further used for the production of anti MRSA compound. Each experiment was performed thrice and the values were analyzed to show mean ± SD for triplicate data. Statistical significances was estimated by one way ANOVA.
Extraction and column purification of bioactive compound: A loopful of the strain S. rubrolavendulae ICN3 (three well-developed colonies on the isolation medium) was streaked in the nutrient-optimized culture conditions and incubated for 7 days. The secondary metabolites were extracted using HPLC grade methanol by cold percolation method for 3 days at room temperature and concentrated in vacuum concentrator (Eppendorf 5301, Germany) at 30°C after filtration in 0.22 μm syringe filter (Hi-media, Mumbai). Silica gel (60-120 mesh) (Hi Media Labs, India) was packed in a dry glass column (2.5 × 50 cm width and length) using hexane. The concentrated sample (500 mg) was mixed with 1 g of silica gel and loaded in the column. The silica gel powder was added over the loaded sample to avoid disturbance while pouring the solvents. The fractions were eluted using benzene: methanol in the ratio of 10: 100 per cent to 100:10 per cent16 and HPLC grode methanol was used to elute the remaining compounds to get 50 fractions and concentrated at 30°C. The absorbance maxima of the active fractions were determined in a UV-Vis spectrophotometer (Techcomp, UV Vis 8500, Hong Kong). The active fraction (25 mg) was dissolved in 1 ml of HPLC mobile phase (acetonitrile) and centrifuged at 6500 g for 5 min (Spinwin, Tarsons). The supernatant (250 μl) was made up to 2 ml with the HPLC mobile phase and the absorbance values were read between 200 nm to 800 using UV-VIS spectrophotometer.
Reverse phase HPLC purification of anti-MRSA molecule: Isolation and analysis of the molecule was performed in a HPLC system (Cyberlab, USA) with C-18 column using the solvents acetonitrile: water (HPLC grades) in the ratio of 65:35 (v/v) as mobile phase. The mobile phase was sonicated for 15 min before using in HPLC; 25 μl of the sample was injected in HPLC column with an isocratic elution for a flow rate of 1 ml/min at 215 nm. The elution and retention rime (RT) analysis was carried out for a total run time of 8 min in the chromatogram.
Susceptibility testing: Minimal inhibitory concentration (MIC) assay was performed by broth microdilution method17. A loopful of freshly grown MRSA colonies was resuspended in M-H broth and incubated at 37 °C for 18 h to give a turbidity of 0.5 McFarland standard (1 × 108 Cfu/ml). Final inoculate was adjusted to 5 × 105 Cfu/ml to dispense 100 μl in the 96 well plates. Further, the MIC was determined for anti-MRSA growth for 24 h incubation at 37°C using vancomycin as positive control. Anti-MRSA disk susceptibility test of the column purified fractions was performed according to the Clinical and Laboratory Standard Institute (CLSI) guidelines17 along with vancomycin (30 μg/disk) to confirm the antagonistic activity in the column elution.
Breeding and maintenance of zebrafish embryos: Zebrafish were maintained in Fish Culture facility of the International Centre for Nanobiotechnology, Centre for Marine Science and Technology, M S University, Kanyakumari, Tamil Nadu, India. Following successful breeding, the eggs were subsequently collected from the bottom of tanks and the embryos were raised in embryo rearing solution (5 mM NaCl, 0.17 mM KCl, 0.4mM CaCl2 and 0.16 mM MgSO4).
Toxicity assessment and compound evaluation in zebrafish embryos: The embryos was treated with 103, 104, 105, 106 and 107 cfu/ml of MRSA in the 48 well plates and observed after 24 h of incubation at 37°C to monitor infection and lethality in a sterile laminar flow chamber. The column purified small molecule C23 was tested at 1, 2 and 4x of the MIC along with MRSA for its inhibitory effect in parallel to the treatment of 4, 8 and 12 μg/ml of vancomycin in 1 per cent dimethyl sulphoxide (DMSO) as vehicle. Cardiac assay or heart beat rate (HBR)18 was studied in 3 days post fertilization (dpf) developing embryo at 1, 2, 4 and 10x concentrations of the MIC. The embryos were anaesthetized by 1 per cent tricaine (Sigma, USA) to calculate the HBR and blood cell count enumeration19 for 5-25 μg/ml of C23 compound. Toxicity evaluations were done between 10-100 μg/ml to observe the phenotypic deformities. LC50 values were calculated by probit analysis using SPSS12 (SPSS Inc, USA).
Results
Identification and antimicrobial activity of actinomycetes: Anti MRSA activity was found in the four strains of the 23 actinomycete isolates. Among the four the strain ICN3 strongly inhibited the growth of the clinical isolate MRSA with an inhibitory zone of 21 mm radius to the culture patch on double layer agar method. The antagonistic strain was identified and confirmed as S. rubrolavendulae ICN3 (NCBI GenBank: JN187862) using 16S rRNA gene sequence analysis by showing 99 per cent similarity to its nearest neighbor S. rubrolavendulae strain D43 in NCBI database. GC content was calculated as 59.5 per cent.
Optimization of anti-MRSA production media: The antibiotic production was calculated to be higher at pH 7 and 37°C with inhibitory zones of 33.33 ± 1.15 and 22.67 ± 1.53 mm, respectively. Also 0.01 per cent of NaCl showed inhibition of 30.67±1.53 mm and considered as control for the optimization of NaCl. Among the carbon and nitrogen sources maximum inhibition was seen with glucose (Fig. 1) and sodium nitrate (Fig. 2) supplemented medium. Sodium nitrate exhibited the maximum inhibition 58.67 ± 3.05 mm while the yeast extract showed the limited inhibition at 28°C. The nutrient-optimized culture conditions showed the antagonistic activity of 43 ± 1.53 mm in double layer agar method at optimized temperature of 37°C and at pH 7, and considered as NOCC for further studies.
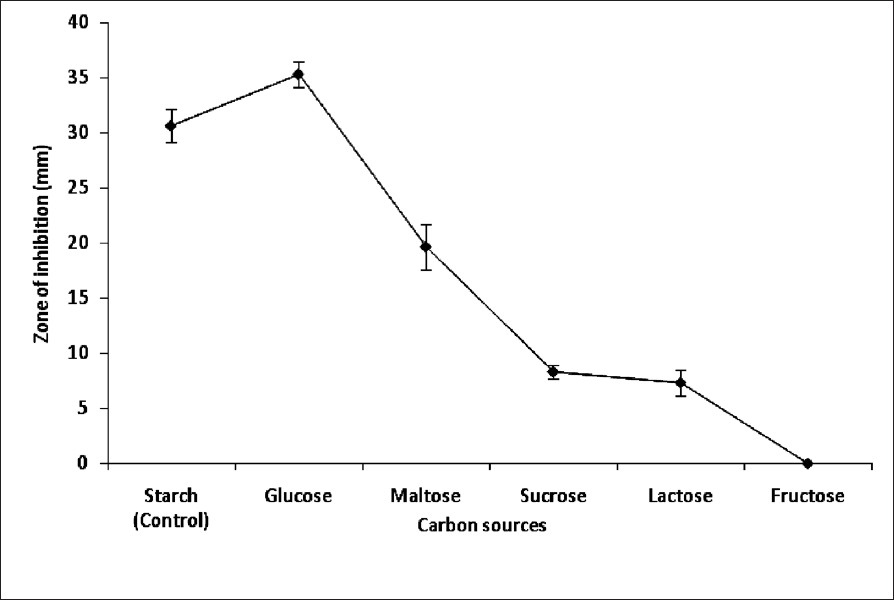
- Anti-MRSA activity of S. rubrolavendulae ICN3 supplemented with different carbon sources. Values are mean ± SD of triplicate experiments.

- Anti-MRSA activity of S. rubrolavendulae ICN3 supplemented with different nitrogen sources. Values area mean ± SD of triplicate experiments.
Extraction and purification of bioactive compounds: Crude compound (687 mg) was obtained from 200 ml of methanolic extract from NOCC in 1 liter medum preparation by SSF. Further, 500 mg of crude sample was purified using silica gel column chromatography. The fractions were scanned between 200 to 800 nm using UV-VIS spectrophotometer, and 36 mg of C23 was obtained from 500 mg crude compound and quantified as 5.24 per cent of the total yield. The UV-VIS spectrophotometric analysis was carried out to find the wavelength for HPLC analysis and purification. The retention time of the RP-HPLC purified molecule C23 is 2.062 min.
Anti-MRSA assay: The minimal inhibitory concentration of the crude metabolites from S. rubrolavendulae ICN3 grown in NOCC was 500 μg/ml and the purified molecule C23 showed 2.5 μg/ml against MRSA by micro-dilution method. MIC of the positive control vancomycin was 2 μg/ml in the broth micro-dilution method.
Biomedical studies in zebrafish embryos: Treatment of 1 × 105 cfu/ml (1μl volume) of the MRSA inoculum to the dechorionated 2 dpf (days post fertilization) embryos in 48 well microtitre plates incubated for 24 h showed infection. The infected embryos were survived in the presence of 5 μg/ml of C23 with infections and muscle deformities in eye, yolk sac, pericardial cavity and trunk region (Fig. 3a–f). MRSA infected embryos were survived in the presence of purified molecule C23 at a dose of 10 μg/ml. Vancomycin treated (12 μg/ml) embryos did not show any deformities (Fig. 3). There was an increase of HBR and WBC level but no changes in RBCs (Table). The tail flexure and spinal truncation, cardiac malformation and yolk sac oedema were observed in the embryos treated with C23 (Fig. 4). LC50 was determined by probit analysis as 60.49 μg/ml (Table).
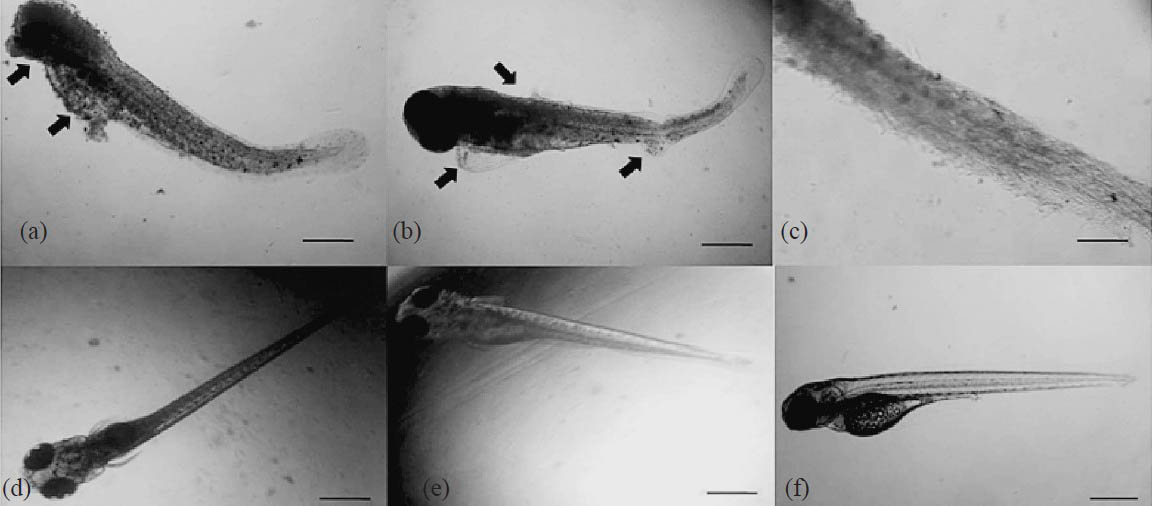
- MRSA infected zebrafish embryos treated with C23 elution at 3 dpf. (a) Arrows indicating muscle degradation in the infected eye and yolk sac of 1 μg/ml C23 treated embryo. Scale bar 200 μm. (b) Infected pericardial cavity and trunk region at 5 μg/ml. Scale bar 200 μm. (c) Trunk region showing muscle or somite damages in MRSA treated embryos at 5 μg/ml. Scale bar 50 μm. (d) Uninfected live embryo at 10 μg/ml of C23. Scale bar 200 μm. (e) Uninfected live embryo at 12 μg/ml of vancomycin treatment. Scale bar 200 μm. (f) Untreated embryo. Scale bar 200 μm.
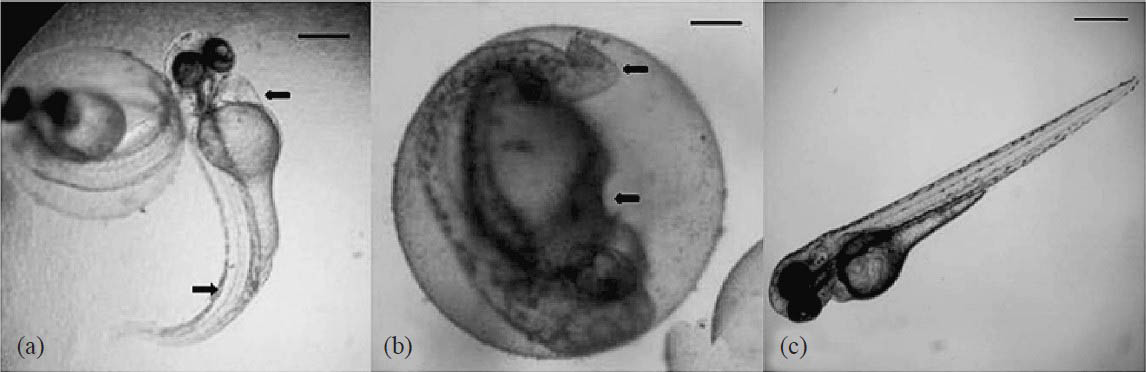
- Toxicity assessment of anti-MRSA molecule C23 from S. rubrolavendulae ICN3. (a) Arrows indicating the bradycardia and trunk flexure at 50 μg/ml. Scale bar 150 μm (2 dpf). (b) Trunk flexure and yolk sac oedema at 50 μg/ml. Scale bar 100 μm (2 dpf). (c) Untreated embryo. Scale bar 100 μm (2 dpf). LC50 was determined by probit analysis as 60.49 μg/ml (with 95% Confident limit). The lower and upper limits were calculated as 54.51μg/ml - 66.08 μg/ml.
Discussion
Identification of novel antagonistic molecule(s) is needed for MRSA due to emerging resistance20. Previous guidelines have recommended the use of vancomycin in the management of severe MRSA infection21. The data of intensive care units from 75 countries showed 29.1 per cent mortality rates due to MRSA infection22. Actinomycetes have been known to be the rich source of novel secondary metabolites producers623 including the commonly used antibiotics gentamicin, rifampin and vancomycin2425. Components of the medium could influence the antimicrobial activities of actinomycetes26. Medium optimization studies showed maximum production of anti-MRSA compound in the presence of sodium nitrate source with glucose at 28°C. The influence of different carbon sources on the production of anti MRSA compound was supported in Streptomyces sp. PVRK15. S. rubrolavendulae ICN3 showed maximum antibiotic production in 0.01 per cent NaCl and at pH7.
It is a common practice to use different separation techniques such as thin layer chromatography (TLC), column chromatography, flash chromatography and HPLC, to obtain pure compounds27. Similar approaches for anti-MRSA molecule purifications were carried out by column fractionation and RP-HPLC to obtain the antagonistic fraction 23 (C23) with a HPLC retention of 2.062 min consisting the absorption maxima at 215 nm. Arenimycin, an anti-MRSA compound was absorbed at 216 nm8 as shown for C23 in the present study. However, the λmax of the other anti-MRSA molecules fijimycins A-C reported as 203, 308, 360 nm10 showed significant variations to C23. Fijimycins A-C and etamycin A from the fermentation broth of Streptomyces sp. CNS-575 were reported with retention times of 27.7, 40.5, 17.5, 31 min, respectively10. The retention time of C23 (anti-MRSA compound of column purified elution from S. rubrolavendulae ICN3) was 2.062 min. Hence, the present antibiotic C23 may be considered to be unique while comparable to the analytical values of RP-HPLC and UV-Vis spectrum.
Abyssomicin C from Verrucosispora sp. showed the MIC value against MRSA as 4 μg/ml9. Fijimycins A–C, and etamycin A are shown to possess anti-MRSA activity with MIC values between 4 and 16 μg/ml10. TPU-0037-A to D from a marine derived Streptomyces platensis exhibited MIC in the range 3-13 μg/ml28. The minimal inhibitory concentration of the C23 was 2.5μg/ml against MRSA in the present study. Marinopyrrole A produced by a marine Streptomyces sp showed the MIC value as 0.61 μM with an IC50 value of 8.8 μM against HCT-116 cells in an in vitro study29. Though the MIC and IC50 values are comparably lower only in vitro cell lines, the LC50 values of compound from S. rubrolavendulae ICN3 is potentially higher in vivo zebrafish embryo study. Hence purified compound C23 can be considered for its lower level of toxicity in the live animal system. Biomedical evaluation of C23 did not show any toxicity in its effective drug assessments in zebrafish embryos by observing HBR, blood cells count and LC50 values. Zebrafish based assays have been developed for testing toxicity of drug candidates, including acute toxicity (LC50), organ-specific toxicity and developmental toxicity30. Also, in the infection challenge experiment using MRSA zebrafish embryos survived in the presence of purified molecule C23 at 10 μg/ml, and vancomycin showed the survival at 12 μg/ml. These findings support the novelty of the compound with less toxicity and for the further use of zebrafish as an emerging infection model for MRSA3132.
In conclusion, the novel compound C23 isolated from S. rubrolavendulae ICN3 showed potent anti-MRSA activity in zebrafish embryo model. Further investigations on structural characterization and clinical pathophysiological studies in rat models may lead to the development of a novel drug against MRSA.
Acknowledgment
Authors are acknowledge the Manonmaniam Sundaranar University, Xpression Biotek Pvt Ltd and University Grants Commission, Government of India [F. No. 41-519/2012(SR)] for the facilities and financial assistance.
References
- Evaluation of traditional chinese medicinal plants for anti-MRSA activity with reference to the treatment record of infectious diseases. Molecules. 2012;17:2955-67.
- [Google Scholar]
- MRSA prevalence in European healthcare settings: a review. BMC Infect Dis. 2011;11:138.
- [Google Scholar]
- Increased prevalence of methicillin-resistant Staphylococcus aureus nasal colonization in household contacts of children with community acquired disease. BMC Infect Dis. 2012;12:45.
- [Google Scholar]
- Isolation and screening of soil actinomycetes as source of antibiotics active against bacteria. Int J Microbiol Res. 2010;2:12-6.
- [Google Scholar]
- Isolation of a small molecule with anti-MRSA activity from a mangrove symbiont Streptomyces sp. PVRK-1 and its biomedical studies in Zebrafish embryos. Asian Pac J Trop Biomed. 2011;1:341-7.
- [Google Scholar]
- The evolving role of natural products in drug discovery. Nat Rev Drug Discov. 2005;4:206-20.
- [Google Scholar]
- Arenimycin, an antibiotic effective against rifampin-and methicillin-resistant Staphylococcus aureus from the marine actinomycete Salinispora arenicola. J Antibiot (Tokyo). 2010;63:37-9.
- [Google Scholar]
- Abyssomicin C– A polycyclic antibiotic from a marine Verrucosispora strain as an inhibitor of the p-aminobenzoic acid/tetrahydrofolate biosynthesis pathway. Angew Chem Int Ed Engl. 2004;43:2574-6.
- [Google Scholar]
- Fijimycins A–C, three antibacterial etamycin-class depsipeptides from a marine-derived Streptomyces sp. Bioorg Med Chem. 2011;19:6557-62.
- [Google Scholar]
- Zebrafish – an in vivo model for drug screening. Innov Pharmaceut Technol 2003:38-45.
- [Google Scholar]
- Zebrafish: an emerging technology for in vivo pharmacological assessment to identify potential safety liabilities in early drug discovery. Br J Pharmacol. 2008;154:1400-13.
- [Google Scholar]
- Use of 16S rRNA and rpoB genes as molecular markers for microbial ecology studies. Appl Environ Microbiol. 2007;73:278-88.
- [Google Scholar]
- Optimization of the fermentation process of actinomycete strain Hhs.015T. J Biomed Biotechnol 2010 2010:141876.
- [Google Scholar]
- Laminaria japonica extract, an inhibitor of Clavibater michiganese Subsp. Sepedonicum. PLoS One. 2014;9:e94329.
- [Google Scholar]
- Performance standards for antimicrobial susceptibility testing; Seventeenth informational supplement. In: CLSI document M100-S17. Wayne, Pennsylvania, USA: CLSI; 2007.
- [Google Scholar]
- Cynodon dactylon and Sida acuta extracts impact on the function of the cardiovascular system in zebrafish embryos. J Biomed Res. 2012;26:90-7.
- [Google Scholar]
- Screening of herbal extracts influencing hematopoiesis and their chemical genetic effects in embryonic Zebrafish. Asian Pac J Trop Biomed. 2012;2:S1002-9.
- [Google Scholar]
- Perspectives on daptomycin resistance, with emphasis on resistance in Staphylococcus aureus. Clin Infect Dis. 2007;45:601-8.
- [Google Scholar]
- MRSA Working Party of the British Society for Antimicrobial Chemotherapy. Guidelines (2008) for the prophylaxis and treatment of methicillin - resistant Staphylococcus aureus (MRSA) infections in the United Kingdom. J Antimicrob Chemother. 2009;63:849-61.
- [Google Scholar]
- Increased mortality associated with methicillin-resistant Staphylococcus aureus (MRSA) infection in the intensive care unit: results from the EPIC II study. Int J Antimicrob Agents. 2011;38:331-5.
- [Google Scholar]
- Arenamides A-C, cytotoxic NFkappaB inhibitors from the marine actinomycete Salinispora arenicola. J Nat Prod. 2009;72:396-402.
- [Google Scholar]
- Enumeration, isolation and some physiological properties of actinomycetes from sea water and sediment. Syst Appl Microbiol. 1987;10:85-91.
- [Google Scholar]
- Bioassay directed isolation of active compounds with antiyeast activity from a Cassia fistula seed extract. Molecules. 2011;16:7583-92.
- [Google Scholar]
- TPU-0037-A, B, C and D, novel lydicamycin congeners with anti-MRSA activity from Streptomyces platensis TP-A0598. J Antibiot (Tokyo). 2002;55:873-0.
- [Google Scholar]
- The marinopyrroles, antibiotics of an unprecedented structure class from a marine Streptomyces sp. Org Lett. 2008;10:629-31.
- [Google Scholar]
- Zebrafish: a preclinical model for drug screening. Assay Drug Dev Technol. 2002;1:41-8.
- [Google Scholar]
- A star with stripes: zebrafish as an infection model. Trends Microbiol. 2004;12:451-7.
- [Google Scholar]
- Establishment of multi-site infection model in zebrafish larvae for studying Staphylococcus aureus infectious disease. J Genet Genomics. 2012;39:521-34.
- [Google Scholar]






