Translate this page into:
Polymorphisms of UGT1A1*6, UGT1A1*27 & UGT1A1*28 in three major ethnic groups from Malaysia
Reprint requests: Professor Dr Mohd Zaki Salleh, Pharmacogenomics Centre (PROMISE), Faculty of Pharmacy, Universiti Teknologi MARA 42300 Puncak Alam, Selangor DE, Malaysia e-mail: zakisalleh@puncakalam.uitm.edu.my
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Genetic polymorphisms of uridine diphosphate glucuronyltransferase 1A1 (UGT1A1) have been associated with a wide variation of responses among patients prescribed with irinotecan. Lack of this enzyme is known to be associated with a high incidence of severe toxicity. The objective of this study was to investigate the prevalence of three different variants of UGT1A1 (UGT1A1*6, UGT1A1*27 and UGT1A1*28), which are associated with reduced enzyme activity and increased irinotecan toxicity, in the three main ethnic groups in Malaysia (Malays, Chinese and Indians).
Methods:
A total of 306 healthy unrelated volunteers were screened for UGT1A1*28, UGT1A1*6 and UGT1A1*27. Blood samples (5 ml) were obtained from each subject and DNA was extracted. PCR based methods were designed and validated for detection of UGT1A1*6, UGT1A1*27 and UGT1A1*28. Direct DNA sequencing was performed to validate the results of randomly selected samples.
Results:
Malays and Indian have two-fold higher frequency of homozygous of UGT1A1*28 (7TA/7TA) which was 8 and 8.8 per cent, respectively compared to the Chinese (4.9%). However, the distribution of UGT1A1*6 and UGT1A1*27 showed no significant differences among them. UGT1A1*27 which has not been detected in Caucasian and African American population, was found in the Malaysian Malays (3.33%) and Malaysian Chinese (2.0%).
Interpretation & conclusions:
There was interethnic variability in the frequency of UGT1A1*28 in the Malaysian population. Our results suggest that genotyping of UGT1A1*6, UGT1A1*28 and UGT1A1*27 need to be performed before patients are prescribed with irinotecan due to their high prevalence of allelic variant which could lead to adverse drug reaction.
Keywords
Genetic polymorphism
interethnic
irinotecan
UGT1A1
The Food Drug Administration (FDA), USA had first approved irinotecan label with pharmacogenetics information in 20051. In 2010, new pharmacogenetic information was added to the label regarding the risk of neutropenia in patients who had genetic defect of uridine diphosphate glucoronosyltransferase 1A1 (UGT1A1)2. Irinotecan is used either in combination with 5-fluorouracil (5-FU) and leucovorin or as a monotherapy for the second-line therapy in the treatment of colorectal cancer3. After intravenous (iv) administration, irinotecan is converted to its active metabolite, 7-ethyl-1 o-hydroxycamptothecin (SN-38) by a carboxylesterase. SN-38 is then detoxified by UGT1A1 enzyme to its inactive form SN-38 glucuronide (SN-38G) which is excreted into the bile and urine4. UGT1A1 is found to be polymorphic and resulted in wide inter-individual variation in patient's response as well as toxic side effects45.
More than 30 genetic variants in the promoter region and exon 1 of UGT1A1 have been reported to decrease enzyme activities and were related to diseases such as Criegler-Najjar (CN) and Gilbert syndrome67. Under-expression of UGT1A1 enzyme impaired the metabolism of SN-38 to its inactive form (SN-38G) and caused an excessive accumulation of toxic SN-38. Polymorphism of UGT1A1 is found responsible for the large inter-individual differences in the pharmacokinetics of irinotecan and risk of severe toxicity89.
UGT1A1*28 is a genetic polymorphism caused by an insertion repetitions of (TA)s in the (TA)6 TAA-box (-53 to -38 insertion of TA) at the promoter region. An increased number of TA repeats may reduce transcription efficiency, lower the enzyme concentrations and thus lead to the accumulation of SN-38 and irinotecan-induced toxicity8. UGT1A1*28 has been reported to cause approximately 70 per cent reduction of UGT1A enzyme activity8. It has been reported that patients with homozygous UGT1A1*28 had severe toxicities of irinotecan compared to either wild type UGT1A1*1 or heterozygous UGT1A1*288.
Ando et al10 showed that 80 per cent of patients who suffered from life-threatening toxicities have variant sequences due to UGT1A1*6 (211G>A) and UGT1A1*27 (686C>A). Patients with non-small-cell lung cancer and homozygous UGT1A1*6 had significantly lower tumour response rates and shorter progression-free as well as overall survival when treated with irinotecan-based chemotherapy9. It was also found that Japanese patients with heterozygous UGT1A1*27 experienced severe toxicities such as leucopenia (grade 4) and/or diarrhoea (grade 3 or worst)10.
In this study, polymerase chain reaction (PCR) based methods of allele specific PCR (ASPCR) and denaturing high performance liquid chromatography (dHPLC) were used to investigate the distribution of allele frequencies of UGT1A1*28 (-53 to -38 insertion of TA) in the promoter region as well as UGT1A1*6 (211G>A) and UGT1A1* 27 (686C>A) located at the coding region of exon 1 among the three major ethnic groups in Malaysia and other populations.
Material & Methods
The study was conducted in the Pharmacogenomics Centre, Universiti Tecknologi MARA, Malaysia during a period of three years from 2008-2010. This study was approved by the Research Ethics Committee of Universiti Tecknologi MARA. Blood samples (5 ml) were collected from unrelated healthy volunteers after obtained a written informed consent. The participants were recruited from staff and students of Univesiti Teknologi MARA and others, who were residents of Klang Valley, Malaysia at the point of participation. An advertisement was put up to recruit subjects and participation was voluntary. The participants were inquired about their ancestry up to 3 generations and classified according to Malaysian-Malays, Malaysian-Chinese or Malaysian-Indian. Those unsure of originality were excluded. Genomic DNA was extracted using alkaline lysis method described previously11. DNA was stored at -20°C until analysis.
Genotyping of UGT1A1*28 by denaturing high performance liquid chromatography (dHPLC): dHPLC method was developed to determine the insertion of extra (TA) in the (TA)6 TAA-box at the promoter region. PCR was carried out to amplify the promoter region using designed primers (forward: 5’ GTG AAC TCC CTG CTA CCT TTG 3’ and reverse: 5’ CTC TGC TGC AGC TGC TGG ATG GC 3’) [RefSeq Gene: NG - 002601.2 (Genebank)].
PCR master mix consisted of detergent free buffer (20 mM Tris-HCl (tris-hydrochloride), 100 mM EDTA (ethylenediaminetetraacetic acid), 0.5 mM PMSF (phenylmethylsulfonyl fluoride), 1 mM DTT (DL-dithiothreitol) and 50% glycerol), 0.2 μM of each primers (forward and reverse), 0.2 mM of each dNTPs (deoxyribonucleotide triphosphate) and 1 unit of GoPhorIT™ Taq DNA Polymerase (Mbiotech Inc., Seoul, Korea) in a total reaction volume of 25 μl. The preparation of reaction mixtures for PCR was conducted on ice to reduce the chances of primers mis-annealing and artifacts formation.
Touch-down PCR was performed as follows: initial denaturation at 94°C for 3 min followed by 8 cycles of denaturation at 94°C for 1 min; annealing at 66°C with decrement of 1°C each cycle for 1 min and extension at 72°C for 1 min. Another 25 cycles at 94°C for 1 min, 58~C for 1 min, 72°C for 1 min were performed.
Prior to dHPLC analysis, the amplicons from touch-down PCR were subjected to post-PCR denaturing and reannealing steps to ensure the formation of homoduplexes or heteroduplexes. This process was started at 95°C for 3 min, which slowly decreased at a rate of 1.0°C every cycle until a temperature of 65°C was reached. The samples were stabilised at 25°C for 30 sec. Then, 3 μl of the samples were injected to dHPLC Varian system (Variant Inc., San Jose, California, US) at the optimal partial denaturing temperatures. Homozygous samples were re-analysed by mixing the samples with wild-type known sequence which was the positive control at a ratio of 1 : 1. This produced a mix population of heteroduplexes of wild type-mutant or homoduplexes of wild type-wild type during the reannealing step. This is an essential step to differentiate homozygous wild type and homozygous mutant type samples which have different and unique retention times.
The optimised melting temperatures allowed us to detect variations of insertion of extra (TA) in the (TA)6 TAA-box at the promoter region of UGT1A1. Changes in the profiles of the peaks were observed (Fig 1 A to D). The peaks were verified using the Star Reviewer® software (Variant Inc., San Jose, California, US). The peaks were analyzed according to their unique chromatographic patterns of the DNA elution peaks and retention times (Fig. 2). All samples were screened and these showing different profiles were confirmed with direct sequencing.
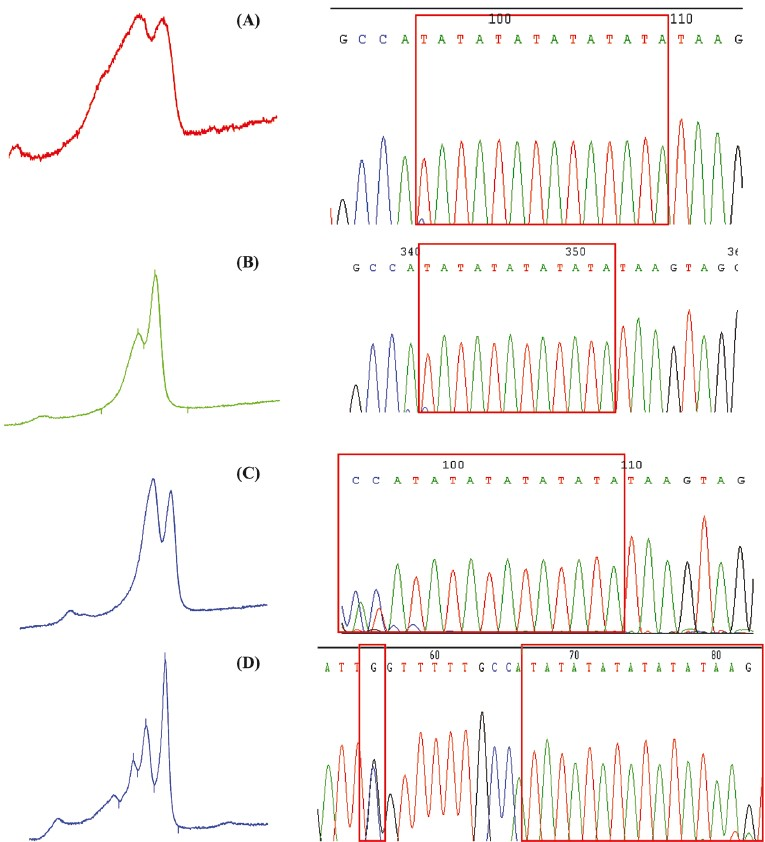
- dHPLC peak profiles and sequencing results for the confirmation of insertion of TA box at promoter region of UGT1A1*28. (A) Homozygous for UGT1A1*28; (B) wild-type for UGT1A1*1; (C) heterozygous for UGT1A1*1/*28 (TA6/TA7), (D) combination of heterozygous for UGT1A1*28TA6/TA8 and SNPs at -63 (G>C) known as UGT1A1*68.

- DNA sequencing results confirm the variation sites. (A) Sample identified as wild-type UGT1A1*1/*1; (B) Sample identified as heterozygous UGT1A1*1/*6; (C) Sample identified as mutant UGT1A1 *6/*6; (D) Control identified as heterozygous UGT1A1*1/*6 heterozygous UGT1A1*1/*27. (L= 100 bp ladder, WT= wild-type and MUT= mutant).
Genotyping of UGT1A1*6 and UGT1A1*27 by allele specific polymerase chain reaction (ASPCR): Genetic variations of UGT1A1*6 and UGT1A1*27 were determined using ASPCR method. Allele specific primers which enable the detection of UGT1A1*6 and UGT1A1*27 in two different tubes, were designed. PCR amplification was performed in two separate tubes with multiplex for wild-type of UGT1A1*6 and UGT1A1*27 in one, and allele specific primers for detection of variant in another. The expected sizes for UGT1A1*6 and UGT1A1*27 are 658 and 180 bp, respectively and visualisation of wild type and variant bands in separate lanes enabled the determination of genetic variation. An example of the results is shown in Fig. 2A – D.
The final PCR protocol comprised 25 μl of reaction mixture of 1 × PCR buffer (Biotools®, B & M Labs, S.A., Madrid, Spain), 2.0 mM MgCl2, 0.20 mM of each dNTPs (Promega Corporation Madison, WI, USA), 0.6 μM of common reverse primer and 0.2 μM of wild type and variant type primers (patented primer sequences, A PCR Genotyping Kit for Detection of UGT1A1*6 and UGT1A1*27”- PI 20094933), 100 to 200 ng (2 μl) genomic DNA as template and 1.0 U DNA Taq polymerase (Biotools®, B & M Labs, S.A., Madrid, Spain). PCR amplification was performed with initial denaturation of 80°C for 5 min then 94°C for 2 min; followed by 10 cycles of Touch-down PCR of denaturation at 94°C for 45 sec, annealing and elongation at 72 to 62°C (decrements of 1°C/cycle) for 1 min. The PCR was continued with another 25 cycles of denaturation at 94°C for 45 sec, annealing 62°C for 45 sec, and elongation at 72°C for 1 min. Finally, the PCR process was run at 72°C for 3 min. The PCR was performed using GeneAmp® PCR System 2700 Perkin Elmer (Applied Biosystem, Roche Molecular System Inc., Branchburg, NJ, USA).
The PCR products were documented using 2 per cent agarose gel stained with ethidium bromide (LE, analytical grade; Promega Corporation, USA) in 1 × TBE buffer (Tris, Borate, EDTA) at 110 Volt for 30 min.
Positive control was generated by cloning the homozygous wild-type and homozygous mutant DNA samples into a plasmid. The positive controls were used in each run to avoid false positive results.
Direct DNA sequencing: Results obtained from both the dHPLC analysis and ASPCR were validated by direct DNA sequencing. The DNA samples were purified using QIAquick® PCR Purification Kit (Qiagen, Hilden, Germany) and sequenced on ABI 3700 using BigDye® Terminator Cycle Sequencing Ready Reaction Kit (Applied Biosystems, Foster City, CA, USA). The DNA sequences were aligned and compared with the gene sequence (accession number: NC_000002.) from NCBI GenBank.
Results
A total of 306 healthy unrelated volunteers from three major ethnic groups (Malay= 100; Chinese= 104 and Indian= 102) in Malaysia were recruited for the determination of UGT1A1*6, *27 and *28.
UGT1A1*28 variants were detected in all three Malaysian races. About 24.5 to 39.2 per cent of Malaysian were heterozygous of UGT1A1*28 (6TA/7TA) variant. In this study, Malays and Indian have two fold higher frequency of homozygous of UGT1A1*28 (7TA/7TA) which is 8 and 8.8 per cent, respectively compared to only 4.9 per cent in the Chinese. The predicted frequencies of UGT1A1*28 genotypes among the three major ethnic groups in Malaysia were calculated according to Hardy-Weinberg equation with 95% confidence intervals (95% CI) (Table). All the genotypes were found to follow Hardy-Weinberg equilibrium (HWE). In comparison to the allele frequencies reported among the Caucasians and some other ethnic groups in Asian countries such as Taiwan and Hong Kong14–16, UGT1A1*28 variant was found to be higher in the Malays and Indians with allele frequency of 25 and 28 per cent, respectively.

Interestingly, a combination of heterozygous insertion of 8TA genotype and also a SNPs located at -63 (G>C) or known as UGT1A1*68 was detected in 0.02 per cent of the Malaysian-Chinese population. UGT1A1*68 have been reported previously in Caucasian, Indian and African-American population717. Sample with combination of these 2 alleles showed different peak profile (Fig. 1).
The genotype and allele frequencies of UGT1A1*6 were higher than UGT1A1*27 in the studied populations (Table). The genotype and allele frequencies of UGT1A1*6 and UGT1A1*27 were not statistically significant among the Malaysian Malay, Chinese and Indian. UGT1A1*27 was found in this population where the frequencies were less than 3 per cent in Malay and Chinese Malaysian. However, it was not detected in Indian subjects in this study.
Discussion
UGT1A1*6 and UGT1A1*27 alleles were commonly reported in Asians10121718. We failed to validate an ASPCR method initially to determine the insertion of extra (TA) in the (TA)6TAA-box at the promoter region of UGT1A1 using the same platform. The detection of UGT1A1*28 was however, successful with the use of dHPLC. The results from both ASPCR and dHPLC were re-confirmed by direct DNA sequencing.
UGT1A1*6 was found to be present at high frequencies among the three major ethnics in Malaysia. This is in accordance with the 5 to 15 per cent frequencies reported on other studies in Asian population1415. The Caucasians have a lower frequency of UGT1A1*6 (5%) and reported to be rare variant among this population19. Previous study showed a correlation between UGT1A1*6 genotype and adverse reaction of irinotecan whereby patients with UGT1A1*6 variant had higher level of SN-3813. The occurrence of adverse effects have been reported in patients with UGT1A1*6 genotypes in Asians91314. The distribution of UGT1A1*6 and UGT1A1*27 was found to be similar among the three major ethnic groups in Malaysia. Similar results were observed among the Malay, Chinese and Indian in Singapore14. UGT1A1*27 was found to be higher in Malaysian-Malays (3.0%) followed by Malaysian-Chinese (2.0%). Lower allele frequency of UGT1A1*27 was found in the healthy Japanese population 0.67 per cent13. None of the Malaysian-Indians had this genotype. Similarly, UGT1A1*27 was not detected in Caucasian and African-American population19.
Patients with UGT1A1*28 genotype have been reported to have seven times higher risk of side effects from irinotecan than people who do not have it10. Some studies showed that combination of two polymorphic variations in the same gene may increase the risk of adverse effects. Higher incidence of neutropenia has been observed in patients who had combination of UGT1A1*28 and UGT1A1*6 genotype with lower glucuronidation capacity for SN-38 thus leading to the accumulation of SN-38 toxic metabolites20. This suggests a gene dose effect and patients are recommended to be genotyped for the commonly encountered variants.
The allelic types and frequency of UGT1A1*28 among the Malaysian Indians in this study showed similarity to Caucasian population19. On the other hand, lower allele frequency of UGT1A1*28 was observed in Malaysian Chinese (17%), similar to those reported in Chinese in Singapore, Taiwan and Japan at 16, 14.3 and 9.7 per cent, respectively141519. In our previous studies on genetic polymorphism of CYP2C9 and CYP2D6, the Malaysian Indians showed similar pattern of high frequencies for alleles that are found common in Caucasians2122; while the Malaysian Malays and Chinese were intermediate between East Asians and Caucasian with respect to allelic frequencies of CYP2D611. In this study, combination of TA8 genotype and UGT1A*68 (G>C) was found in 0.02 per cent of Malaysian Chinese population. Interestingly, UGT1A*68 (G>C) has been reported in Indian population7. This suggests heterogeneity and inter-ethnic difference for UGT1A*28 in Malaysia.
UGT1A1 variants with reduced functionalities are highly prevalent in Asia and thus irinotecan-induced adverse effects among the patients are expected. Genotyping of this variant prior to initiation of irinotecan among patients is relevant in our local population.
With the high prevalence of genetic variants of UGT1A1 among the three ethnic groups in Malaysia and increasing colorectal cancer cases in Malaysia23, genotyping of UGT1A1*28, UGT1A1*6 and UGT1A1*27 is relevant among patients prescribed with irinotecan in Malaysia. As there may be inter-ethnic differences in the distribution of allelic variant in UGT1A1, it is important to know its prevalence to predict the population likely to be at risk of adverse effects.
Acknowledgment
This research was supported by a grant from the Ministry of Higher Education Malaysia (FRGS 5/3/1175) and Universiti Teknologi MARA (UiTM).
References
- U.S. Food and Drug Administration. Hepatic dysfunction, pancreatitis, UGT1A1. 2005. Final Label FDA approval drug label. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2005/020571s024,027,028lbl.pdf
- [Google Scholar]
- U.S. Food and Drug administration. 2010. Camptosar (irinotecan hydrochloride) injection: FDA approval drug label. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020571s031s032s033s036s037lbl.pdf
- [Google Scholar]
- Pharmacogenetic profiling across the irinotecan pathway in Asian patients with cancer. Br J Clin Pharmacol. 2005;59:415-24.
- [Google Scholar]
- Irinotecan dosing: does the CPT in CPT-1 1 stand for “Can’t Predict Toxicity”. J Clin Oncol. 2002;20:7-8.
- [Google Scholar]
- Dihydropyrimidine dehydrogenase and the efficacy and toxicity of 5-fluorouracil. Eur J Cancer. 2004;40:939-50.
- [Google Scholar]
- Nomenclature update for the mamalian UDP glycosyltransferase (UGT) gene superfamily. Pharmacogenet Genomics. 2005;15:677-85.
- [Google Scholar]
- Gilbert's syndrome: high frequency of the (TA)7 TAA allele in India and its interaction with a novel CAT insertion in promoter of the gene for bilirubin UDP-glucuronosyltransferase 1 gene. World J Gastroenterol. 2006;12:2269-75.
- [Google Scholar]
- Comprehensive analysis of UGT1A polymorphisms predictive for pharmacokinetics and treatment outcome in patients with non-small-cell lung cancer treated with irinotecan and cisplatin. J Clin Oncol. 2006;24:2237-44.
- [Google Scholar]
- Polymorphisms of UDP-glucuronosyltransferase gene and irinotecan toxicity: a pharmacogenetic analysis. Cancer Res. 2000;60:6921-6.
- [Google Scholar]
- Heterogeneity of the CYP2D6 gene among Malays in Malaysia. J Clin Pharm Ther. 2001;26:205-11.
- [Google Scholar]
- The genetic basis of the reduced expression of bilirubin UDP-glucuronosyltransferase 1 in Gilbert's syndrome. N Engl J Med. 1995;333:1171-5.
- [Google Scholar]
- UGT1A1*6 polymorphism is most predictive of severe neutropenia induced by irinotecan in Japanese cancer patients. Int J Clin Oncol. 2009;14:136-42.
- [Google Scholar]
- Role of UGT1A1*6, UGT1A1*28 and ABCG2 c.421 C>A polymorphisms in irinotecan-induced neutropenia in Asian cancer patients. Cancer Sci. 2007;98:1461-7.
- [Google Scholar]
- Variations of the bilirubin uridine-diphosphoglucuronosyl transferase 1A1 gene in healthy Taiwanese. Pharmacogenetics. 2000;10:539-44.
- [Google Scholar]
- The global distribution of length polymorphisms of the promoters of the glucuronosyltransferase 1 gene (UGT1A1): hematologic and evolutionary implications. Blood Cells Mol Dis. 2003;31:98-101.
- [Google Scholar]
- Neonatal hyperbilirubinemia and a common mutation of the bilirubin uridine diphosphate- glucuronosyltransferase gene in Japanese. J Hum Genet. 1999;44:22-5.
- [Google Scholar]
- Pharmacogenetic impact of polymorphisms in the coding region of the UGT1A1 gene on SN-38 glucuronidation in Japanese patients with cancer. Cancer Sci. 2006;97:1255-9.
- [Google Scholar]
- Racial variability in haplotype frequencies of UGT1A1 and glucuronidation activity of a novel single nucleotide polymorphism 686 C>T (P229L) found in an African-American. (2005) Drug Metab Dispos. 2005;33:458-65.
- [Google Scholar]
- Irinotecan pharmacokinetics/pharmacodynamics and UGT1A genetic polymorphisms in Japanese: roles of UGT1A1*6 and *28. Pharmacogenet Genomics. 2007;17:497-504.
- [Google Scholar]
- Malaysian Indians are genetically similar to Caucasians: CYP2C9 polymorphism. J Clin PharmTher. 2006;31:187-91.
- [Google Scholar]
- Genetic polymorphism of CYP2D6: Malaysian Indians have the highest frequency for CYP2D6*4 in Asia. Eur J Clin Pharmaco. 2001;57:617-8.
- [Google Scholar]
- National Cancer Registry. 2004. Second Report of the Cancer incidence in Malaysia. Kuala Lumpur, Malaysia, National Cancer Registry: Ministry of Health Malaysia. Available from: http://www.radiologymalaysia.org/Archive/NCR/2ndNCR.pdf
- [Google Scholar]






