Translate this page into:
Newer direct-acting antivirals for hepatitis C virus infection: Perspectives for India
Reprint requests: Dr. Anil Arora, Institute of Liver, Gastroenterology, & Pancreatico-Biliary Sciences, Ganga Ram Institute for Postgraduate Medical Education & Research, Sir Ganga Ram Hospital, Rajinder Nagar, New Delhi 110 060, India e-mail: dranilarora50@gmail.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Approximately three per cent of the world's population (170-200 million people) is chronically infected with hepatitis C virus (HCV) and almost 500,000 people die each year (mostly in lower middle-income countries) from complications secondary to HCV infection. In India, HCV infection imposes a considerable burden of mortality, morbidity and healthcare costs. In the last two decades, the treatment of HCV has evolved from interferon (IFN)-based therapies with or without ribavirin (RBV) to pegylated-IFN (PEG-IFN) and RBV-based therapies that were better tolerated by patients. However, the introduction of oral drugs, which specifically target virus-specific proteins, has now revolutionized the treatment of chronic HCV. These agents are known as direct-acting antivirals (DAAs). These drugs have resulted in very high HCV cure rates even with reduced treatment duration and an excellent tolerability by the patients compared to PEG-IFN- and RBV-based therapies. In India, sofosbuvir (SOF), one of the most effective DAAs, has been made available at a compassionate price; thus only those DAA-based management strategies, which contain SOF are adopted in India. Here, we review different DAAs and their possible roles in different genotypes and stages of liver disease, stressing upon the role of SOF. An attempt has also been made to devise strategies using SOF for the most prevalent genotypes in our country (genotypes 3 and 1) and cirrhosis.
Keywords
Antiviral therapy
chronic hepatitis
cirrhosis
direct-acting antivirals
hepatitis C virus
hepatocellular carcinoma
sofosbuvir
Introduction
Advances in hepatitis C virus (HCV) drug development in the last few years have taken a new turn and the evolution of antiviral therapy for HCV has rapidly progressed from plain interferon (IFN), to pegylated IFN (PEG-IFN) plus ribavirin (RBV), to PEG-IFN-based combinations with direct-acting antivirals (DAAs) and finally to IFN-free combinations of DAAs1. HCV infection can now be cured in almost all patients with these effective, safe and tolerable combinations of oral DAAs. Now even those patients who due to advanced liver disease, psychiatric conditions, anaemia or autoimmune disease were not eligible for treatment by PEG-IFN-based regimens, or those who had treatment failure with previous therapies, now have excellent choice of treatment options2.
In March 2015, sofosbuvir (SOF), the first DAA was launched in India at a compassionate price. This review article provides information on different DAAs and their possible roles in different genotypes and stages of liver disease. Since in India one can currently adopt only those DAAs containing management regimens which include SOF, considering the international23 and Indian recommendations4, an attempt has been made to devise strategies for the treatment of the most prevalent genotypes in our country (genotypes 3 and 1) using SOF.
Burden of the problem
Approximately three per cent of the world's population (170-200 million people) is chronically infected with HCV5, and almost 500,000 people die each year, mostly in lower middle-income countries, from complications secondary to HCV infection6. There are limited data on HCV prevalence from India with only a few population-based studies and blood bank data. It has been estimated that the prevalence of HCV in India is between 0.5 and 1.5 per cent7, but it can be presumed that this is just the tip of iceberg and many more HCV-infected patients remain undiagnosed.
Data from the US also suggest that only about 50 per cent of HCV patients are actually identified and 40 per cent of them receive specialist evaluation and ultimately only about 5-6 per cent are treated successfully8. Data from a tertiary care centre in north India on 777 HCV patients showed that 56 per cent of patients already had cirrhosis and seven per cent had HCC at the time of presentation. Only about 45 per cent were eligible for IFN-based antiviral treatment, and of these, only 24 per cent could receive antivirals and 14 per cent achieved a sustained viral response (SVR), which is considered as equivalent to cure. Hence, a large majority of HCV patients were left deprived of a cure to HCV infection9. Since HCV infection remains asymptomatic till the development of decompensated cirrhosis, an early diagnosis at this asymptomatic stage is required for timely intervention and to prevent progression to advanced liver disease and death.
Evolution of hepatitis c virus (HCV) treatment
In the last 20-25 yr, the treatment of HCV has emerged from IFN-based therapies with or without RBV, to PEG-IFN plus RBV combination, which was better tolerated by patients10. IFNs instead of targeting the HCV virus activate the immune mechanisms, which clears the virus. This activation of immune system by IFNs also leads to multiple unfavourable side effects leading to poor patient compliance to therapy. Thus, the goal of HCV researchers was to find shorter duration and IFN-free, orally administered drug combinations with minimum toxicity.
HCV is a small, enveloped, single-stranded, positive-sense RNA virus. The size of HCV genome is 9.6 kb with only one long open reading frame (ORF). This ORF encodes a polyprotein of 3000 amino acids or more, which undergoes a complex series of co- and post-translational cleavages, mediated by both host and viral proteinases to yield the individual HCV proteins which are required for replication11. The N-terminal portion of the genome codes for the core and structural proteins (C, p7, E1 and E2) while the nonstructural proteins (NS2-NS5) are coded by the remaining genomes. The newer DAAs act on these HCV-encoded proteins that are required for viral replication. Protease inhibitors such as boceprevir and telaprevir were the first-generation DAAs, which targeted the NS3/4A serine protease responsible for cleaving the HCV polyprotein at four sites10. Both these drugs were not tested in Indian population.
Within a couple of years, many newer DAAs were introduced and licensed in the West and many other countries and are believed to be the ‘game changers’ in HCV management. With the introduction of DAAs, there has been a remarkable improvement in SVR rates in spite of shorter duration of treatment, and with better tolerability in comparison to PEG-IFN- and RBV-based therapies used till now10.
The most prevalent HCV genotype in India is genotype 3, and the standard therapy for this genotype till now had been a combination of PEG-IFN and RBV for 24 wk, with an SVR rate of 70 per cent or more7. The SVR rate for those infected with genotype 1 (the second most common HCV genotype in India) was 40-60 per cent, with a treatment duration of 48 wk121314. Traditionally, HCV-genotypes 2 and 3 were considered to be easy to treat and 1 and 4 were considered to be difficult to treat with conventional PEG-IFN–RBV therapy12. With the advent of DAAs, genotype 1 has switched its position with genotype 3 to become the new easy-to-treat genotype. Most of these newer DAAs have shown excellent results with genotypes 1 and 4, in the initial trials with or without PEG-IFN. Combinations of various DAAs without PEG-IFN may also enhance opportunities for intervention even in the settings of advanced cirrhosis.
Newer guidelines have been published by various liver societies such as European Association for the Study of Liver (EASL)2 and American Association for the Study of Liver Diseases3 in 2014-2015 for HCV management and have included DAAs as the main therapeutic option for HCV. The guidelines have approved the combination of DAAs and IFN-free regimens. American and European regulatory authorities (FDA and EMA) have approved the following second-generation DAAs to be used in clinical practice: (i) Simeprevir - approved for genotypes 1 and 4; (ii) Combination pill containing SOF and ledipasvir - approved for genotypes 1, 4, 5 and 6; (iii) Combination pill containing ombitasvir, paritaprevir and ritonavir tablets co-packaged with dasabuvir tablets - approved for genotype 1; (iv) Daclatasvir - approved for all genotypes; and (v) SOF - approved for all genotypes.
Since in India only SOF is available, the Indian guidelines published recently4 have incorporated only SOF-containing regimens.
Results of DAA-based regimens
The results of the DAA-based regimens are shown in (Figure).
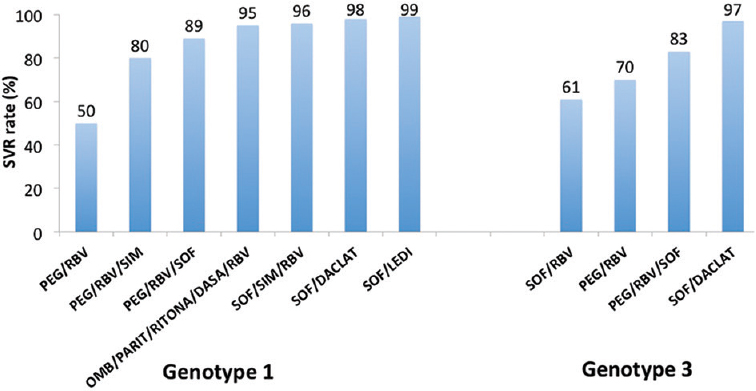
- Sustained viral response (SVR) rates with various regimens in genotypes 1 and 3. PEG, Peg-interferon; RBV, Ribavirin; SIM, Simeprevir; SOF, Sofosbuvir; OMB, Ombitasvir; PARIT, Paritaprevir; RITONA, Ritonavir; DASA, Dasabuvir; DACLAT, Daclatasvir; LEDI, Ledipasvir.
Genotype 1
SOF causes chain termination during replication of the HCV genome, thus leading to selective inhibition of the RNA-dependent RNA polymerase (the NS5B protein). It is capable of acting on all the genotypes of HCV15. In NEUTRINO phase 3 trial16, 291 treatment-naïve genotype 1 patients were treated for 12 wk with SOF plus PEG-IFN plus RBV. SVR12 (SVR after 12 wk of therapy completion) rates were 91 per cent. Even with patients having underlying cirrhosis, a SVR12 of 81 per cent was achieved in genotype 1.
Simeprevir is a NS3/4A protease inhibitor and is predominately active against genotypes 1, 2, 4, 5 and 6. In QUEST-1 and QUEST-2 trials, treatment-naïve patients with genotype 1 HCV were treated with simeprevir in combination with PEG-IFN α-2a or α-2b plus RBV1718. These patients received 12 wk of simeprevir plus PEG-IFN and were later followed up with treatment with PEG-IFN and RBV alone for 12 or 36 wk. A total of 521 patients received simeprevir in both the trials and achieved an SVR12 of 80.4 per cent.
In the PROMISE trial19, simeprevir's efficacy was examined in patients who had relapsed after previous therapy with PEG-IFN plus RBV. Of the relapsers, 79 per cent of patients treated with simeprevir in combination with PEG-IFN plus RBV achieved SVR12, and among patients given simeprevir, 93 per cent met the response-guided therapy criteria and were eligible to complete PEG-IFN plus RBV at week 24. Within the genotypes, 1b had higher SVR12 rates of 86 per cent in comparison to 70 per cent SVR12 achieved by 1a.
In the ASPIRE trial20, simeprevir's efficacy was evaluated in treatment-experienced patients with HCV genotype 1. Patients were randomized into 12, 24 or 48 wk of simeprevir at a dose of 100 mg daily or 150 mg daily plus PEG-IFN and RBV for 48 wk or to placebo which consisted of 48 wk of PEG-IFN and RBV. SVR rates were significantly higher in the simeprevir-containing group; it ranged from 60.6 to 80.0 per cent in comparison to 22.7 per cent in the placebo group.
With treatment with simeprevir, a naturally occurring mutation at NS3/4A protease (Q80K polymorphism) was identified and is considered to be a good predictor of treatment response in genotype 1a patients. The effect of simeprevir nullifies with the presence of the Q80K mutation in these patients. Thus, Q80K mutation should be tested in genotype 1a patients before starting therapy with simeprevir3.
The progress of DAAs in HCV treatment is moving from IFN-based regimens to IFN-free regimens, which is the current standard of care in most western countries. Different DAAs have been combined with or without RBV in different regimes that were free of IFN. SOF plus ledipasvir with interferons have been used in ION 121, ION 222 and ION 323 trials. In ION-1 trial, a very high SVR12 of 99 and 97 per cent was achieved in treatment-naïve genotype 1 patients with and without RBV, respectively, with 12 wk of therapy. When the treatment duration was extended to 24 wk, SVR 12 rates were 99 and 98 per cent in both groups, respectively21. In ION2 trial, genotype 1 treatment-experienced patients were randomized to receive SOF and ledipasvir with or without RBV for either 12 or 24 wk, respectively. The SVR12 rates were 96 and 94 per cent with and without RBV in patients who were treated for 12 wk, respectively, while SVR12 rates were 99 per cent in both the RBV-containing and RBV-free groups if treated for 24 wk22. In ION 3 study, similar SVR12 rates of 94, 93 and 95 per cent were seen in patients who received ledipasvir and SOF alone for 8 wk, ledipasvir and SOF with RBV for 8 wk and ledipasvir and SOF for 12 wk, respectively23. Thus, ION-3 trial confirmed that treatment efficacy was not reduced if shorter treatment duration was chosen for non-cirrhotic treatment-naïve HCV genotype 1 patients.
Various three-drug combinations of DAAs have been studied using ombitasvir (NS5A inhibitor), paritaprevir (NS3/4a inhibitor) and dasabuvir (NS5B inhibitor). In SAPPHIRE-I24, non-cirrhotic treatment-naïve patients with HCV genotype 1 received the three-drug combination with RBV for 12 wk in comparison to SAPPHIRE-II25 trial which included treatment-experienced genotype 1 patients. Both studies demonstrated SVR12 rates above 95 per cent.
Genotypes 2 and 3
It is estimated that HCV genotype 3 accounts for 54.3 million (30.1%) cases globally and is the second most common genotype in the world. Out of this, approximately 75 per cent occur in South Asia including India26. With the use of new IFN-free therapy using DAAs, the genotype 3 is now considered to be the difficult-to-treat genotype. The new DAAs have excellent efficacy for genotype 1 with SVR rates well above 90 per cent, but with genotype 3, the results are not superior to PEG-IFN plus RBV regimens.
Gane et al27 demonstrated a 100 per cent SVR at 24 wk in all genotypes 2 and 3 patients who received SOF plus RBV with or without PEG-IFN for 12 wk. These initial results with DAA-containing IFN-free regimen in HCV-3 patients looked promising27. Due to these encouraging early results, different phase III studies in HCV-2 and 3 were carried out in treatment-naïve patients (Fission), treatment-experienced patients (Fusion) and IFN-intolerant or IFN-unwilling patients (Positron), to assess the effectiveness of SOF plus RBV. In a non-inferiority trial (Fission), 499 patients with HCV genotypes 2 and 3 were treated with SOF plus RBV alone for 12 wk or PEG-IFN alfa-2a plus RBV for 24 wk16. In genotypes 2 and 3, response rates were only 67 per cent in both SOF plus RBV and PEG-IFN plus RBV groups. Genotype 3 showed a lower response rates than among those with genotype 2 infection (56 vs. 97%, respectively) treated with SOF and RBV. SVR12 rates were also lower in cirrhotics and only 47 per cent could achieve an SVR12 in the SOF and RBV groups compared with 38 per cent in the PEG-IFN and RBV groups. In Positron trial28, SOF and RBV were given to genotypes 2 and 3 patients, who were either ineligible or intolerant to IFN-containing therapy or who did not opt for IFN-containing regimens. SOF plus divided RBV according to weight for 12 wk was given to 109 patients of genotype 2 and 98 patients of genotype 3 (totally 207 patients). The overall SVR12 rate including both genotypes 2 and 3 was 78 per cent; however, patients of genotype 3 demonstrated much lower SVR12 rates in comparison to genotype 2 patients (61 vs. 93%, respectively). The SVR12 achieved in genotype 3 cirrhotic patients was only 21 per cent. In the Fusion trial, patients with HCV genotype 2 or 3, who previously had no response to Peg-IFN-based regimen, were assessed for efficacy of SOF and RBV28. The SVR12 achieved in HCV genotype 2 patients was 86 and 94 per cent after 12 and 16 wk, respectively, compared with much lower SVR12 of 30 and 62 per cent in genotype 3 patients.
To improve SVR rates in genotype 3 patients receiving SOF, it was postulated that either treatment duration could be extended or another drug can be added to SOF plus RBV regimens. Extended treatment of SOF and RBV for 24 wk was demonstrated in Valence trial29 which demonstrated a SVR12 of 85 per cent in genotype 3 infections. Ninety three per cent of treatment-naïve genotype 3 HCV patients achieved an SVR12 after 24 wk of treatment. This trial indicated that eradication of HCV with the new DAAs, even in genotype 3 patients, is possible and can be successful. The Lonestar-2 study30 tested triple therapy with PEG-IFN plus SOF plus RBV in treatment-experienced HCV genotypes 2 and 3 patients for 12 wk and achieved an SVR of 83 per cent in both cirrhotic as well as non-cirrhotic patients.
SOF in combination with daclatasvir has been studied in treatment-naïve patients with HCV genotype 2 or 331. The trial randomized patients to one of the three treatment arms. In the first arm, where SOF was given for seven days, which was followed by SOF plus daclatasvir for 23 wk, a SVR rate of 88 per cent was achieved. Higher SVR rates of 93 and 100 per cent were achieved in arms with SOF plus daclatasvir with or without RBV for 24 weeks. Thus, a total of 89 per cent of 18 patients with genotype 3 infections had a SVR at week 12.
Compensated and decompensated cirrhosis
In patients with cirrhosis or advanced fibrosis, PEG-IFN showed increased incidence of side effects and much lower SVR rates as compared to patients without advanced fibrosis or cirrhosis12. The ION-1 (treatment naïve) and ION 2 (treatment experienced) trials were phase III studies which assessed the efficacy of SOF and ledipasvir for 12 or 24 wk with and without RBV in HCV genotype 1 patients22. Among the total study population, 16 per cent of patients in the ION-1 study and 20 per cent in the ION-2 study had cirrhosis. The ION-1 study results demonstrated that SVR12 rates among treatment-naïve patients with liver cirrhosis in any of the different treatment arms (94-100%) were comparable to patients without cirrhosis (97-99%), and there was no significant difference. Even in ION-2 study, cirrhotic patients when treated for 12 wk with ledipasvir plus SOF with and without RBV achieved an SVR12 of 82 and 86 per cent, respectively. If the duration of treatment was extended for 24 wk, the SVR12 rates achieved were 99 per cent in both the RBV-containing and RBV-free groups.
The Cosmos study32 assessed the effectiveness of SOF and simepravir with and without RBV. In this study in the subgroup of treatment-naïve or treatment-experienced patients with F3-F4 fibrosis, the SVR rates achieved were 85 per cent in the 12 wk arm and 100 per cent in the 24 wk arms, without any significant difference achieved by adding additional RBV. Turquoise-II study33 included only cirrhotic patients (Child class A) and treated them with three new antiviral agents and RBV. This resulted in high rates of SVR in both treatment-naïve and treatment-experienced patients. SVR12 rate achieved after 12 wk of therapy was 92 per cent comparable to 96 per cent after 24 wk of therapy. Thus, these studies have indicated that oral, IFN-free regimens are effective in patients with HCV genotype 1 cirrhosis, and thus one can achieve high SVR rates in these difficult HCV patients and can ultimately cause disease regression34.
With HCV genotype 3 cirrhosis, results are not as encouraging and lag much behind that of genotype 1. Extended therapies for 24 wk with SOF/RBV29 or adding PEG-IFN to SOF/RBV regimen30 can improve outcomes to a certain extent. Many HCV patients present to healthcare system only after decompensation. The real challenge of DAAs now would be to treat these decompensated cirrhotic patients. The first available data showed a SVR4 rate of 89 per cent in 20 patients with HCV genotype 1 decompensated cirrhosis with Child B status who received ledipasvir plus SOF for 12 wk35. All patients in this study achieved a 100 per cent end-of-treatment-response.
A multicentre randomized controlled study36 in decompensated cirrhotic HCV genotypes 1 and 4 patients (The Solar-2 study) included 104 patients with Child-Turcotte-Pugh (CTP) class B cirrhosis or CTP class C who were treatment naive or treatment experienced. They were randomly assigned to receive daily combination of ledipasvir (90 mg) and SOF (400 mg) and RBV (initial dose of 600 mg, increased as tolerated) for 12 or 24 weeks. The SVR12 in the 12 wk treatment course was achieved in 87 per cent of patients and in the 24 wk treatment course was achieved in 89 per cent of patients36. The study also revealed improvement in baseline CTP and Model for End-Stage Liver Disease (MELD) scores in >50 per cent of the treated patients. This study suggested that a 12 wk course of ledipasvir plus SOF and RBV was an appropriate regimen for HCV genotype 1 or 4 patients who had decompensated cirrhosis.
These are preliminary findings and these results need validation including more patients with different genotypes, as patients with cirrhosis are less able to clear or cure infected cells. There is scarcity of data on the efficacy of IFN-free regimens in patients with decompensated cirrhosis, and the key question of improvement at this advanced stage of the disease even after achieving SVR has not been fully addressed.
HCV in end-stage renal disease (ESRD) and renal transplant recipients
HCV infection has a high prevalence rate in patients on haemodialysis and causes an increased risk for liver-related and all-cause mortality. If the patient is a candidate for renal transplantation, then antiviral therapy is indicated for all patients on haemodialysis as HCV-associated liver damage may be accelerated by immunosuppression being given after transplant. RBV usage is problematic in this setting and an individualized RBV dosing of 200 mg/day or 200 mg/every other day or 200 mg thrice weekly after haemodialysis is recommended, and substantial hematopoietic support is mandatory. According to the EASL guidelines2, patients who are on haemodialysis should receive an IFN-free regimen and preferably a RBV-free regimen, for duration of 12 wk in patients without cirrhosis and for 24 wk in patients with cirrhosis. DAAs such as simeprevir, daclatasvir and the combination of ritonavir-boosted paritaprevir, ombitasvir and dasabuvir are cleared by hepatic metabolism and can be safely used in patients with severe renal disease. It is recommended to not use SOF in patients with an eGFR <30 ml/min/1.73 m2 or with end-stage renal disease as there is paucity of safety data at this stage of renal disease. Since DAAs that are safe in patients with severe renal disease (simeprevir, daclatasvir, the combinations of paritaprevir, ritonavir, ombitasvir and dasabuvir or grazoprevir and elbasvir) are not available in India, currently, the only treatment option available for these patients is Peg-IFNα as monotherapy or with very low dose of RBV4. IFNα-based therapy cannot be given after renal transplantation as it may lead to graft rejection.
Patients with ESRD and chronic hepatitis C (CH-C) without cirrhosis are counselled to proceed to renal transplantation while still viraemic, with or without prior treatment of HCV infection for 12 wk, in the expectation that therapy with SOF /RBV or other SOF-based regimens can be started after renal functions improve following successful renal transplantation4. However, results with this approach have not yet been reported in any large case series.
HCV-HIV co-infection
HIV co-infection results in early occurrence of advanced liver fibrosis and cirrhosis in patients with HCV. Individuals co-infected with HIV-HCV are at three times greater risk of progression to cirrhosis or decompensated liver disease in comparison to patients who are only infected with HCV alone4. The indications for HCV treatment in co-infected patients are similar to those in patients with HCV monoinfection. With DAA therapy, strong immune system is less important and treatment is less likely to be affected by the CD4 count. However, for IFN-based regimens, in patients with CD4 cell count <350 cells/μl, antiretroviral therapy (ART) should be initiated first, beginning anti-HCV treatment only after improving CD4 cell count to >500/μl4.
In patients on antiretroviral drugs, drug interactions between DAA and HIV antiviral drugs need to be looked at critically when initiating therapy in HIV/HCV co-infected patients. SOF is an ideal drug as there are very few significant drug interactions. Simeprevir has significant drug interactions with efavirenz and darunaprevir/ritonavir. The concomitant administration of RBV and didanosine may result in mitochondrial toxicity leading to hepatomegaly, hepatic steatosis, pancreatitis and lactic acidosis4. Concomitant zidovudine use enhances the risk of RBV-associated anaemia and should be avoided. SOF is not recommended with tipranavir as this drug induces P-gp. For Indian patients, the choice of SOF and RBV with or without Peg-IFNα is based on the same principles as in HCV mono-infected patients, keeping the availability of DAAs and drug interactions in mind4.
Suggested regimens for India
In this section, the potential use of SOF in the management of HCV as a solo available DAA in India is discussed, with stress on the management of genotype 1 and genotype 3 which together account for >95 per cent of HCV-infected patients of India7. Other DAAs that are likely to be approved in near future, will also be discussed. The suggested SOF-based regimen for Indian patients is summarized in Table I.

Genotype 1
For non-cirrhotic genotype 1, treatment-naïve patients who can tolerate IFN, a combination of weekly PEG-IFN, daily weight-based RBV (1000 and 1200 mg in patients <75 and ≥75 kg, respectively) and daily SOF (400 mg) for 12 wk is the best regimen. In treatment-experienced patients in whom prior PEG-IFN plus RBV treatment has failed, daily SOF (400 mg) and weight-based RBV [1000 mg (<75 kg) to 1200 mg (>75 kg)] plus weekly PEG-IFN for 24 wk can be tried. Treatment-naïve genotype 1 patients who are IFN-intolerant or ineligible for IFN can be treated with daily weight-based RBV (1000 and 1200 mg in patients <75 and ≥75 kg, respectively) and daily SOF (400 mg) for 24 wk4.
Genotype 3
With the available data, the answer to HCV genotype 3 in India would be either a triple therapy with PEG-IFN/SOF/RBV for 12 wk or SOF/RBV for 24 wk. Treatment-naïve genotype 3 patients can be treated with weekly PEG-IFN, daily weight-based RBV (1000 and 1200 mg in patients <75 and ≥75 kg, respectively) and daily SOF (400 mg) for 12 wk; while in treatment-experienced patients in whom prior PEG-IFN plus RBV treatment has failed previously, retreatment with daily SOF (400 mg) and weight-based RBV [1000 mg (<75 kg) to 1200 mg (>75 kg)] plus weekly PEG-IFN for 12 wk can be tried. In genotype 3 patients, who are intolerant to IFN, daily weight-based RBV (1000 and 1200 mg in patients <75 kg and ≥75 kg, respectively) and daily SOF (400 mg) for 24 wk is a good option. This can also be tried in those patients in whom prior PEG-IFN plus RBV treatment has failed4.
Another DAA, which is likely to be launched in Indian market, is daclatasvir. It also promises to be a good drug for genotype 3, but will cause an enormous increase in cost of therapy if used in combination with SOF. Future studies with DAAs are needed in genotype 3 patients, to validate available data in larger cohorts and to test promising new DAA combinations, particularly in countries like India, where genotype 3 population is in a majority.
Cirrhosis
With the advent of DAAs, it is now reasonable that most of the patients with compensated cirrhosis should be treated if there are no contraindications and will prevent short- to mid-term complications of cirrhosis. However, HCV treatment of cirrhotics should be given by only experienced practitioners31.
Compensated cirrhotic patients with good liver function and without cytopaenia tolerate a 12-24 wk IFN-based DAA regimen well and these cirrhotic patients can be treated as recommended above across all genotypes. Daily SOF (400 mg) and weight-based RBV [1000 mg (<75 kg) to 1200 mg (>75 kg)] for up to 48 wk are recommended for patients with HCV genotype 3 who have decompensated cirrhosis (CTP class B or C)4. No regimen without ledipasvir can be given for decompensated HCV genotype 1 patients4. This group will have to wait for newer DAAs to be approved in India.
Adverse effects, interactions and precautions with the use of sofosbuvir
The most common adverse effects of SOF in combination with RBV were fatigue and headache (incidence ≥20%). When SOF is used in combination with PEG-IFN and RBV, the most common adverse events observed are fatigue, headache, nausea, insomnia and anaemia4. Cardiac patients who are taking amiodarone can develop severe bradycardia when SOF is taken in combination with another DAA. This effect increases in patients who are also receiving beta-blockers and have advanced liver disease. Thus, co-administration of amiodarone with SOF is not recommended3. If there are no alternative and viable treatment options, a strict cardiac monitoring is recommended.
Since many regimens contain RBV and PEG-IFN, care must be taken before their administration. RBV can lead to foetal death or birth defects, and animal studies have shown that IFNs have abortifacient effects. Thus, it is advisable to avoid pregnancy in female patients who are on these drugs. Pregnancy should be ruled out prior to initiating therapy, and couples should be counselled for using contraception and to have monthly pregnancy tests4.
RBV cannot be recommended for patients with severe renal impairment or end-stage renal disease4.
Recommendations of Indian National Association for Study of the Liver (INASL) guidelines
The Indian National Association for Study of the Liver (INASL) published their guidelines for antiviral therapy against HCV infection in 20154. According to the INASL, the current limitations for the treatment of HCV in India include the poor response of genotype 3, non-availability of many of the DAAs recommended by other guidelines and the high cost of therapy that cannot be afforded by the population of India. Since only one DAA, SOF, is available in India, only two SOF-based regimens are possible: either dual drug therapy in combination with RBV alone for six months or triple drug therapy in combination with RBV and PEG IFN for three months. The utility of these regimens in various situations has been discussed by the INASL4. The recommended regimens by INASL are summarized in Table II.

Limitations of DAAs in Indian context
There are certain limitations of DAAs, especially in Indian context, which must be addressed:
-
Treatment of genotype 3: The genotype 3 is the most common HCV genotype followed by genotype 17. The genotype 3 is now considered difficult-to-treat genotype as far as DAAs are concerned. Till newer DAAs are launched in India, which have better efficacy for genotype 3, we will have to continue with SOF plus PEG-IFN and RBV.
-
Treatment of patients with compensated and decompensated cirrhosis: In India, >50 per cent of HCV patients present to healthcare system only after decompensation. The real challenge of DAAs now would be to treat these decompensated cirrhotic patients. More robust data are urgently needed to decide treatment regimens for these patients with advanced disease.
-
Availability of DAAs in India: SOF is the first DAA to be launched in India. It can take much more time before other DAAs can be launched here.
-
Cost: Although SOF is cheaper than PEG-IFN, the cost of triple therapy using PEG-IFN/RBV would be much higher, and not affordable to most Indian patients.
Newer promising regimens with validation in larger populations, which have genotype 3 predominant population, are needed. Moreover, good, safe combinations of DAAs for cirrhotic patients are required to achieve a cure for all. In India, access to these new DAAs remains a limiting factor. SOF has been made available in Indian market since March 20154. For other DAAs to be available, it will take much longer. How much SOF alone can be beneficial without any other DAAs in India is debatable. Efforts should be made to introduce other DAAs in India soon.
The most profound barrier to accessing treatment for hepatitis C globally, including in high-income countries, is the exorbitant prices of the new DAAs. The wholesale list price for SOF in the West costs around $1000 per 400 mg pill37. This adds up to the total cost for the SOF 12-wk treatment course to be around $84,00037. The cost adds up even further when multiple DAAs are used for complete treatment. In India, many of our patients remain deprived of HCV treatment with PEG-IFN therapy due to its high cost. There is no government-run healthcare programme yet in India that takes care of the treatment of HCV patients. Perhaps public-private partnerships may help in this regard.
Is PEG-IFN plus RBV still an option for India?
The uncertainty of the timeline for DAAs other than SOF in India precluded the developers of Indian HCV guidelines to incorporate other DAAs in their recommendations. In India, only SOF regimens with PEG-IFN plus RBV for 12 or 24 wk can be offered to genotypes 1 and 3 patients. As the cost will be enormous, it has to be discussed whether using PEG-IFN/RBV without DAAs is still an option for easy-to-treat Indian patients.
Patients with IL28B CC genotype have higher SVR rates than those with either TC or TT genotypes38. Thus, patients can be identified using IL28B status and can be treated without the use of a DAA. In the Asian populations including India, there is higher prevalence of C allele and thus PEG-IFN plus RBV-based therapy can be a reasonable initial treatment strategy in the resource-limited countries.
Undetectable HCV RNA at week four of therapy, also known as the rapid virologic response (RVR), is one of the strongest predictors of SVR1439. Thus, a shorter duration of treatment of 24 wk of PEG-IFN plus RBV dual therapy can be given to patients who do not achieve an RVR40. Addition of DAAs can be reserved for only those patients who do not achieve an RVR. This strategy will be attractive in resource-limited countries or at places where an availability of DAAs is limited, especially in Asian countries where IL28B CC genotype proportion is high and approximately 50-65 per cent of genotype 1 patients achieve RVR with dual therapy of PEG-IFN plus RBV41. Thus, treatment with PEG-IFN plus RBV in a real-world setting can be highly cost-effective yet equally effective as PEG-IFN+SOF+RBV combination in well-selected treatment-naïve genotype 1/3 patients. Low-income population with genotype 1 or 3 who cannot afford SOF can still be started on PEG-IFN- and RBV-based strategies, but wherever possible, they should be provided financial assistance from governmental or non-governmental social groups.
Eradication of HCV: Is the dream achievable?
Eradicating HCV infection will reduce the incidence of liver decompensation and liver cancer42. Reducing the pool of infected people could significantly decrease the chances of spreading new infections in the community43. The aim of healthcare community should be to avoid missing any opportunity to diagnose HCV and timely intervene before advanced liver disease sets in when treatment has poorer outcome in addition to being costly.
If we wish to achieve 100 per cent SVR in infected patients, our aim should be to screen our populations for silent HCV-infected patients. It is the time to build health programmes including both governmental and non-governmental organizations for population screening of HCV and to give early cure to these patients and curb the transmission of this deadly virus.
Conclusions
With the availability of DAAs, highly effective, short duration and safe regimens have created better outcomes for patients with HCV infection, especially in those groups where SVR was low with prior therapies or in those where IFN-based treatment strategies were contraindicated. Having SOF and other DAAs will definitely benefit Indian HCV patients, but efforts should be made to make DAAs accessible to all patients.
Conflicts of Interest: None.
References
- A glass half full: Implications of screening for hepatitis C virus in the era of highly effective antiviral therapy. Hepatology. 2015;61:1455-8.
- [Google Scholar]
- European Association for Study of Liver. EASL clinical practice guidelines: Management of hepatitis C virus infection. J Hepatol. 2014;60:392-420.
- [Google Scholar]
- Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62:932-54.
- [Google Scholar]
- Indian National Association for Study of the Liver (INASL) guidance for antiviral therapy against HCV infection in 2015. J Clin Exp Hepatol. 2015;5:221-38.
- [Google Scholar]
- Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095-128.
- [Google Scholar]
- Consensus statement of HCV task force of the Indian National Association for Study of the Liver (INASL). Part I: Status report of HCV infection in India. J Clin Exp Hepatol. 2014;4:106-16.
- [Google Scholar]
- Late diagnosis of hepatitis C virus infection in the Chronic Hepatitis Cohort Study (CHeCS): Missed opportunities for intervention. Hepatology. 2015;61:1479-84.
- [Google Scholar]
- Most patients of hepatitis C virus infection in India present late for interferon-based antiviral treatment: An epidemiological study of 777 patients from a North Indian tertiary care center. J Clin Exp Hepatol. 2015;5:134-41.
- [Google Scholar]
- Viral hepatitis and liver cancer: The case of hepatitis C. Oncogene. 2006;25:3834-47.
- [Google Scholar]
- Diagnosis, management, and treatment of hepatitis C: An update. Hepatology. 2009;49:1335-74.
- [Google Scholar]
- Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: A randomised trial. Lancet. 2001;358:958-65.
- [Google Scholar]
- Sustained virological response rates to antiviral therapy in genotype 1 and 3 chronic hepatitis C patients: A study from north India. J Clin Exp Hepatol. 2014;4:287-92.
- [Google Scholar]
- PSI-7851, a pronucleotide of beta-D-2’-deoxy-2’-fluoro-2’-C-methyluridine monophosphate, is a potent and pan-genotype inhibitor of hepatitis C virus replication. Antimicrob Agents Chemother. 2010;54:3187-96.
- [Google Scholar]
- Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878-87.
- [Google Scholar]
- Simeprevir with pegylated interferon alfa 2a plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-1): A phase 3, randomised, double-blind, placebo-controlled trial. Lancet. 2014;384:403-13.
- [Google Scholar]
- Simeprevir with pegylated interferon alfa 2a or 2b plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-2): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2014;384:414-26.
- [Google Scholar]
- Simeprevir with peginterferon and ribavirin leads to high rates of SVR in patients with HCV genotype 1 who relapsed after previous therapy: A phase 3 trial. Gastroenterology. 2014;146:1669-79.e3.
- [Google Scholar]
- Simeprevir increases rate of sustained virologic response among treatment-experienced patients with HCV genotype-1 infection: A phase IIb trial. Gastroenterology. 2014;146:430-41.e6.
- [Google Scholar]
- Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889-98.
- [Google Scholar]
- Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370:1483-93.
- [Google Scholar]
- Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370:1879-88.
- [Google Scholar]
- Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370:1594-603.
- [Google Scholar]
- Retreatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370:1604-14.
- [Google Scholar]
- Global distribution and prevalence of hepatitis C virus genotypes. Hepatology. 2015;61:77-87.
- [Google Scholar]
- Nucleotide polymerase inhibitor sofosbuvir plus ribavirin for hepatitis C. N Engl J Med. 2013;368:34-44.
- [Google Scholar]
- Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med. 2013;368:1867-77.
- [Google Scholar]
- Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med. 2014;370:1993-2001.
- [Google Scholar]
- Sofosbuvir with peginterferon-ribavirin for 12 weeks in previously treated patients with hepatitis C genotype 2 or 3 and cirrhosis. Hepatology. 2015;61:769-75.
- [Google Scholar]
- Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014;370:211-21.
- [Google Scholar]
- Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: The COSMOS randomised study. Lancet. 2014;384:1756-65.
- [Google Scholar]
- ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med. 2014;370:1973-82.
- [Google Scholar]
- Cirrhosis regression in hepatitis C patients with sustained virological response after antiviral therapy: A meta-analysis. Liver Int. 2015;35:30-6.
- [Google Scholar]
- O6 Sofosbuvir/ledipasvir fixed dose combination is safe and effective in difficult-to-treat populations including genotype-3 patients, decompensated genotype-1 patients, and genotype-1 patients with prior sofosbuvir treatment experience. J Hepatol. 2014;60:S3-4.
- [Google Scholar]
- Ledipasvir and sofosbuvir plus ribavirin in patients with genotype 1 or 4 hepatitis C virus infection and advanced liver disease: A multicentre, open-label, randomised, phase 2 trial. Lancet Infect Dis. 2016;16:685-97.
- [Google Scholar]
- Each of these Hepatitis C pills cost $1,000. That's actually a great deal. 2014. Vox. Available from: https://www.vox.com/2014/7/16/5902271/hepatitis-c-drug-sovaldi-price
- [Google Scholar]
- Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399-401.
- [Google Scholar]
- Sustained virological response based on the week 4 response in hepatitis C virus genotype 1 patients treated with peginterferons a-2a and a-2b, plus ribavirin. Eur J Gastroenterol Hepatol. 2013;25:1317-20.
- [Google Scholar]
- Peginterferon alfa-2a and ribavirin for 24 weeks in hepatitis C type 1 and 4 patients with rapid virological response. Gastroenterology. 2008;135:451-8.
- [Google Scholar]
- Pegylated interferon-alpha-2a plus ribavirin for treatment-naive Asian patients with hepatitis C virus genotype 1 infection: A multicenter, randomized controlled trial. Clin Infect Dis. 2008;47:1260-9.
- [Google Scholar]
- Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308:2584-93.
- [Google Scholar]
- Hepatitis C virus treatment for prevention among people who inject drugs: Modeling treatment scale-up in the age of direct-acting antivirals. Hepatology. 2013;58:1598-609.
- [Google Scholar]






