Translate this page into:
Multi-drug resistance in clinical isolates of Gram-negative bacilli in a tertiary care hospital of Assam
*For correspondence: reema_44@rediffmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Sir,
The widespread use of β-lactam antibiotics has lead to the development of resistance to this group of antibiotics in bacterial pathogens due to various types of β lactamase production. Amongst the mechanisms of resistance to third generation cephalosporins, production of extended spectrum β-lactamases (ESBLs) and AmpC β lactamases is the most common1. Since both ESBL and AmpC β-lactamase are encoded on plasmids and confer a selective advantage to strains harbouring these in a hospital setting, it is important to know the occurrence of ESBL and AmpC producing strains as well as their antibiotic susceptibilities to newer agents to guide empirical therapy. This laboratory based study was carried out over a period of one year from May 2011 to April 2012 at the department of Microbiology, Assam Medical College and Hospital, Dibrugarh, Assam, India to determine the occurrence of multi-drug resistant Gram-negative bacilli producing AmpC β-lactamases and ESBL causing infection in hospitalized patients.
A total of 150 non repeat, non enteric consecutive clinical isolates of different Gram-negative bacilli obtained from various clinical specimens like blood, wound, swab, pus, CSF, urine, sputum suction tube aspirate, tracheal aspirate peritoneal fluid of hospitalized patients were identified biochemically by the standard methods2. Isolates from mixed culture were excluded from the study. The antibiogram of the isolates was determined by the standard Kirby Bauer's disc diffusion method3 and Clinical Laboratory Standards Institute (CLSI) recommendations4.
Isolates that yielded a cefoxitin zone diameter of less than 18 mm and resistant to 3rd generation cephalosporins (screen positive) were tested for AmpC enzyme production by AmpC disc test1 and modified three-dimensional test as described by Manchanda and Singh5. In modified three-dimensional test, fresh overnight growth of test organism from Mueller-Hinton agar (MHA) plate was taken in a micro centrifuge tube. Peptone water was added and centrifuged at 900 g for 15 min. Crude enzyme extract was prepared by repeated freeze thawing at -80°C seven times. A lawn culture of Escherichia coli ATCC 25922 (Hi-media microbiogics) was prepared on MHA plates and cefoxitin (30 µg) discs were placed on the plate. Linear slits were cut using a sterile surgical blade 3 mm away from the cefoxitin disc; 30 to 40 µl of the enzyme extract was added to a well made at the outer edge of the slit, without overflowing. The plates were kept upright for 5 to 10 min until the liquid dried and were incubated at 37°C for overnight. Clear distortion of zone of inhibition of cefoxitin was taken as AmpC β lactamase producer.
Isolates that fall in the sensitivity range to cefotaxime, ceftazidime or ceftriaxone (3GC) and cefoxitin as per the CLSI4 were subjected to disk antagonism test67 for inducible AmpC detection. Dicks of inducing agent cefoxitin (CX) and cephalosporins [cefpirome (CPM), ceftazidime(CZ), ceftriaxone(CTR) and cefotaxime (CTX)] were placed on the surface of the test bacterial lawn on MHA plates on a lawn of bacterial culture of the suspected inducible AmpC β-lactamase producers separated by 15 mm. The plates were examined after overnight incubation at 37 °C. If blunting of the cephalosporin discs adjacent to the cefoxitin discs occurred, the organisms were considered to produce inducible AmpC β lactamase.
Screen positive isolates for possible ESBL production were confirmed by combined disc method (CLSI)4.
With modified disc diffusion method 108 isolates were identified as possible ESBLs producers and 113 isolates as possible AmpC producers resistant to cefoxitin. Multi-drug resistance (resistance to 3 or more antibiotics) was detected with commonest phenotype being ampicillin, co-trimoxazole and ceftazidime resistance phenotype (50.6%).
AmpC β-lactamase (both plasmid mediated and inducible chromosomal) was detected in 48 (32%) Gram-negative isolates which was significantly higher than studies elsewhere8910. Maximal occurrence of AmpC β-lactamase was found among non fermenting Gram-negative bacilli as shown in the Table. Modified three Dimensional test was found to be more sensitive in detection of plasmid mediated AmpC as it could detect three more isolates to be positive which were negative by AmpC disc test. Thirty eight (95%) of these isolates were cefoxitin resistant whereas the remaining two isolates were cefoxitin sensitive. This finding reiterates the limited usefulness of using cefoxitin as a screening disc to detect plasmid mediated AmpC beta lactamase producing isolates510.
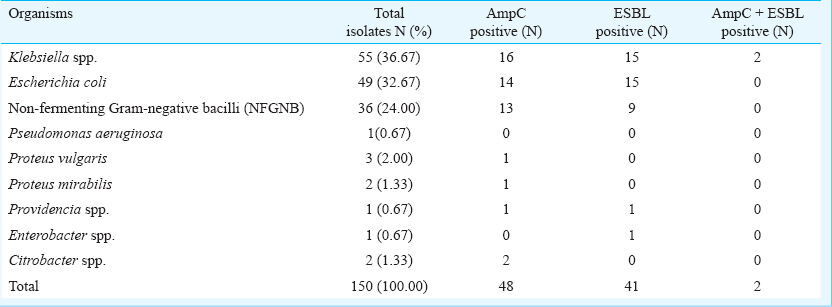
Inducible expression of chromosomal AmpC β-lactamases, although rare in E. coli and Klebsiella pneumoniae, is associated with a significant risk of therapeutic failure with all β-lactam drugs except carbapenems11. Disc antagonism test for chromosomal inducible AmpC β-lactamase was positive in eight isolates including four K. pneumoniae isolates and four non fermenting Gram-negative bacilli. Since earlier studies have not reported presence of this type of resistance in Klebsiella isolates12, confirmation of this finding by molecular methods will be needed.
ESBL production was detected in 27.33 per cent isolates (41/150). However, in a previous study from this institute detected ESBL in 33 per cent isolates with highest incidence in K. pneumoniae (42.5%)13. Their study included both in-and out-patients’ samples, whereas we included samples from hospitalized patients only. Increased occurrence AmpC β-lactamase in hospitalized patients may be a reason for relative decrease of ESBL in these isolates in our study. Co-existence of AmpC β-lactamase may give false negative result in detection of ESBL8.
Co-existence of AmpC and ESBL was demonstrated in two Klebsiella isolates; which could be because of dissemination of plasmid mediated AmpC enzyme among Entrerobacteriaceae sometimes in combination with ESBL14.
In the present study strong association was noticed among AmpC β-lactamase production and duration of hospitalization for more than 72 h (P< 0.01) using chi square test. This shows that at present AmpC harbouring isolates are largely restricted to hospitalized patients only. However, no such association was observed in ESBL producers. This is in accordance with studies by other authors from India515.
All the AmpC and ESBL positive isolates were multi-drug resistant. Concurrent resistance to amikacin and gentamicin was also significantly associated with production of AmpC β-lactamase and ESBL (P < 0.05) using Chi square test. These isolates also showed concurrent resistance to cefoxitin (96%), ampicillin (92%), ceftriaxone and cefotaxime (83%), ceftazidime (77%), co-trimoxazole (71%) and ciprofloxacin (69%), respectively. However, all the ESBL and AmpC producing isolates were sensitive to imipenem, thereby reiterating the continued efficacy of carbapenems as the first line agents for treatment of health care associated infections caused by the members of Enterobacteriaceae producing ESBL and AmpC β-lactamases.
In conclusion, we detected AmpC and ESBLs in the Gram-negative multi-drug resistant clinical isolates obtained from hospitalized patients. Strict antibiotic policies and measures to limit indiscriminate use of cephalosporins should be undertaken to minimize the emergence of such resistance.
References
- AmpC disk test for detection of plasmid-mediated AmpC β lactamases in Enterobacteriaceae lacking chromosomal AmpC- β-lactamases. J Clin Microbiol. 2005;43:3110-3.
- [Google Scholar]
- Tests for the identification of bacteria. In: Collee JG, Fraser AG, Marmion BP, Simmons A, eds. Mackie & McCartney practical medical microbiology (14th ed). Edinburgh, UK: Churchill Livingstone; 1996. p. :131-49.
- [Google Scholar]
- Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45:493-6.
- [Google Scholar]
- Clinical and Laboratory Standards Institutes (CLSI). Performance standards for antimicrobial susceptibility testing; twenty-second Informational Supplement. CLSI document M100-S22. Wayne, PA: Clinical and Laboratory Standard Institute; 2012.
- [Google Scholar]
- Occurrence and detection of AmpC β-lactamases among Gram-negative clinical isolates using a modified three-dimensional test at Guru Tegh Bahadur Hospital, Delhi, India. J Antimicrob Chemother. 2003;51:415-8.
- [Google Scholar]
- In vitro antagonism of β-lactam antibiotics by cefoxitin. Antimicrob Agents Chemother. 1982;21:968-75.
- [Google Scholar]
- AmpC beta-lactamase producing bacterial isolates from Kolkata hospital. Indian J Med Res. 2005;122:224-33.
- [Google Scholar]
- Evaluation of methods for AmpC β-lactamase in Gram negative clinical isolates from tertiary care hospitals. Indian J Med Microbiol. 2005;23:120-4.
- [Google Scholar]
- Detection of AmpC β lactamases production in Escherichia coli & Klebsiella by an inhibitor based method. Indian J Med Res. 2007;126:220-3.
- [Google Scholar]
- AmpC β-lactamases among Gram negative clinical isolates from a tertiary hospital, South India. Braz J Microbiol. 2010;41:596-602.
- [Google Scholar]
- Plasmid-determined AmpC type β-lactamases. Antimicrob Agents Chemother. 2002;46:1-11.
- [Google Scholar]
- Enterobacter bacteremia: clinical features and emergence of antibiotic resistance during therapy. Ann Intern Med. 1991;115:585-90.
- [Google Scholar]
- Extended spectrum β lactamase producing Gram negative bacilli in a tertiary referral hospital of Assam-experience with two methods. Indian J Pathol Microbiol. 2006;49:623-5.
- [Google Scholar]
- Development of resistance during antimicrobial therapy caused by insertion sequence interruption of porin genes. Antimicrob Agents Chemother. 1999;43:937-9.
- [Google Scholar]
- Multidrug-resistant Pseudomonas aeruginosa strains harbouring R-plasmids and AmpC β- lactamases isolated from hospitalised burn patients in a tertiary care hospital of North India. FEMS Microbiol Lett. 2003;228:181-6.
- [Google Scholar]





