Translate this page into:
Methylenetetrahydrofolate reductase A1298C genetic variant & risk of schizophrenia: A meta-analysis
Reprint requests: Dr. Vandana Rai, Department of Biotechnology, Human Molecular Genetics Laboratory, VBS Purvanchal University, Jaunpur 222 003, Uttar Pradesh, India e-mail: raivandana@rediffmail.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Methylenetetrahydrofolate reductase (MTHFR) is an important enzyme of folate metabolism, whose role in schizophrenia is debatable. Numerous case-control studies have investigated the association of MTHFR A1298C polymorphism with schizophrenia, but results are controversial. The aim of the present study was to find the association between MTHFR A1298C gene polymorphism and schizophrenia.
Methods:
PubMed, Google Scholar, Science Direct and Springer link databases were searched for case-control association studies in which MTHFR A1298C polymorphism was investigated as a risk factor for schizophrenia. In all, 19 studies with 4049 cases and 5488 controls were included in this meta-analysis. Odds ratios (ORs) with 95 per cent confidence intervals (CIs) were used as an association measure.
Results:
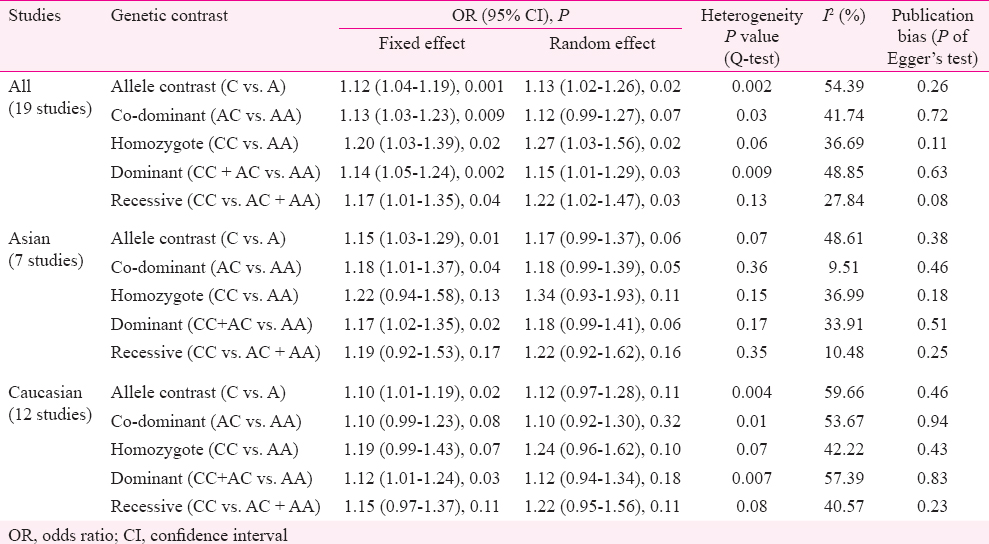
The results of meta-analysis reported a significant association between A1298C polymorphism and schizophrenia risk in overall comparisons in all genetic models (C vs. A: OR=1.13, 95% CI=1.01-1.27, P=0.02; CC vs. AA: OR=1.20, 95% CI=1.03-1.39, P=0.02; AC vs. AA: OR=1.13, 95% CI=1.03-1.23, P=0.009; AC+CC vs. AA: OR=1.14, 95% CI=1.02-1.24, P=0.002; CC vs. AA+AC: OR=1.17, 95% CI=1.01-1.35, P=0.04).
Interpretation & conclusions:
MTHFR A1298C polymorphism was found to be a risk factor for schizophrenia and might have played a significant role in the pathogenesis of schizophrenia.
Keywords
A1298C
folate
homocysteine
meta-analysis
methylenetetrahydrofolate reductase
polymorphism
schizophrenia
Schizophrenia is a psychiatric disorder with the prevalence rate of 1 per cent12. According to the Diagnostic and Statistical Manual of Mental Disorders IV criteria (DSM-IV)3, the diagnosis is based on symptoms such as delusions, hallucinations and affective flattening and alogia. The most widely considered hypothesis of schizophrenia is neurodevelopment hypothesis which integrates influences of environment and genes. The neurodevelopment hypothesis of schizophrenia postulates that altered biochemical pathways and defective neural circuitry during foetal brain development lead to cognitive and emotional defects in later part of life4. In the brain of schizophrenics, several neurotransmitter network systems have been found to be defective. Numerous schizophrenia candidate genes have been reported4567.
Folate is an essential nutrient required for de novo nucleotide synthesis and DNA methylation and it has been associated with DNA hypomethylation8910 and DNA strand breaks11. Methylenetetrahydrofolate (MTHFR) is an important enzyme of folate/homocysteine metabolism which catalyzes the synthesis of 5-methyltetrahydrofolate. 5-methyltetrahydrofolate donates methyl group for remethylation of homocysteine to methionine, which is a precursor of S-adenosylmethionine (SAM). SAM is the main methyl donor of several cellular methylation reactions. MTHFR polymorphism raises the dietary requirement for folic acid to maintain normal re-methylation of homocysteine to methionine12; consequently, low folate status in individuals with the MTHFR polymorphism results in an increase in homocysteine and a decrease in methionine levels. A deficiency in cellular folates and methyl-donors may be associated with abnormal methylation of DNA, proteins and neurotransmitters and DNA strand breaks1314151617. If an adequate amount of folate is not available concentration of intracellular homocysteine is increased, and consequently, methionine synthesis is reduced18. SAM is the only source of methyl group for DNA methylation reactions in the brain, and this forms a plausible biologic explanation for potential associations between MTHFR gene polymorphism and schizophrenia risk19.
In MTHFR gene, more than 40 polymorphisms have been reported, of which two mutations C677T20 and A1298C2122 have been extensively studied. It has been reported that the C677T mutation decreases MTHFR activity and increases the plasma homocysteine level while the second polymorphism A1298C results in reduced enzymatic activity but to a lesser extent than the C677T mutation22. The MTHFR A1298C polymorphism is associated with a reduction in the bioavailability of cellular folate23. The mutant genotype (CC) prevalence ranges from 7 to 12 per cent in Caucasian, 4-5 per cent in Hispanic and 1-4 per cent in Asian populations2425.
Several case-control studies investigating the association between MTHFR gene A1298C polymorphism and schizophrenia have been published, but the results were conflicting. Some reported positive association262728293031 but others did not find any association between A1298C polymorphism and schizophrenia32333435. To shed some light on these controversial results, we performed a meta-analysis of published case-control studies, and a pooled estimate of the A1298C polymorphism as risk for schizophrenia was obtained for the allele contrast, homozygote, dominant, co-dominant and recessive models.
Material & Methods
Selection of studies: PubMed, Google Scholar, Science Direct and Springer Link databases were searched for the studies which investigated A1298C polymorphism as risk factor for schizophrenia up to June 2014. The articles were searched using the following terms - ’methylenetetrahydrofolate reductase,’ ’MTHFR’, ’A1298C’ and ’Schizophrenia’. Studies published between 2003 and 2012 were included in the present meta-analysis. Eligible studies met the following criteria: (i) the study should be a case-control association study, (ii) patients were diagnosed by psychiatrists according to the DSM-IV, (iii) the study should report the sample size, distribution of MTHFR A1298C genotypes, or other information necessary for estimation of the odds ratio (OR) and 95 per cent confidence interval (CI), and (iv) the studies should be published. The following exclusion criteria were used: (i) studies containing duplicate data, (ii) no usable data reported, and (iii) case reports, letter to editor, book chapters or reviews.
Data extraction: The following information was extracted from each study - first author's family name, journal name, year of publication, country name, number of cases and controls and the distribution of genotypes. If any author reported the results on different populations, each population was included as an independent study. The allele numbers were calculated from the corresponding genotype distributions.
Statistical analysis: Pooled OR with its corresponding 95 per cent CI were calculated to investigate the association between MTHFR A1298C polymorphism and risk of schizophrenia for the allele contrast (C vs. A), homozygote (CC vs. AA), heterozygote (AC vs. AA), recessive (CC vs. AC+AA) and dominant (CC+AC vs. AA) models. The degree of heterogeneity between the studies was assessed by the Q-test based on Chi-squared statistic36. We also measured the effect of heterogeneity by another measure, I2=100%×(Q−df)/Q37. I2 ranges between 0 and 100 per cent, and I2 values of 25, 50 and 75 per cent were defined as low, moderate and high estimates of heterogeneity, respectively. The pooled OR was estimated using both fixed effects (FE) and random effects models3839. Whether the genotype distributions of controls were in Hardy–Weinberg equilibrium (HWE) was evaluated in control genotypes of each study.
Publication bias: Publication bias was estimated by funnel plot and Egger's linear regression test40. All statistical analyses were done by software MIX version 1.741.
Results
Characteristic analysis of eligible studies: A total of 49 studies were retrieved after PubMed, Google Scholars, Science Direct and Springer Link databases. After reviewing abstracts of all retrieved studies, 24 were excluded and full text of the remaining 25 articles was considered. Of the 25 studies, four were review articles and one was not relevant for present meta-analysis, so five studies were excluded. A total of 19 studies (Fig. 1) that investigated the association between A1298C polymorphism and schizophrenia were found suitable for the inclusion in the present meta-analysis26272829303132333435424344454647. Two authors2633 studied different populations; we included data from each population as an independent study. All 19 studies were performed in different countries - Korea294546, China263047, Romania3444, Turkey4243, Bulgaria35, Denmark33, Germany27, Norway33, Poland32, Scotland26, Spain28, Sweden33 and Syria31 Table I. Except two studies2729, genotype distribution of control population in all studies was in HWE.
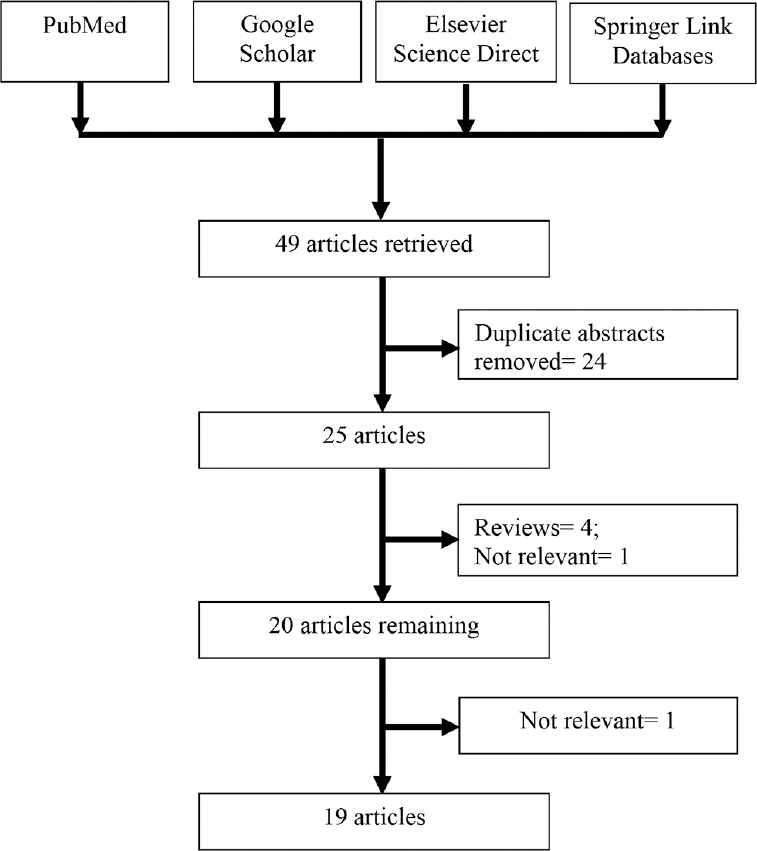
- Flow diagram showing selection of studies.
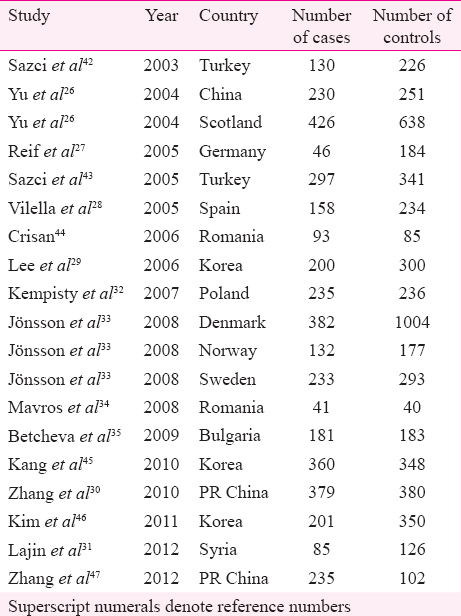
In all 19 studies, total cases were 4049 with AA (2127), AC (1518) and CC (404), and controls were 5488 with AA (2950), AC (2045) and CC (493) genotypes. In controls’ genotypes, the percentage of AA, AC and CC were 53.75, 37.26 and 8.98 per cent, respectively. In total cases, genotype percentages of AA, AC and CC were 52.53, 37.49 and 9.98 per cent, respectively (Table II).
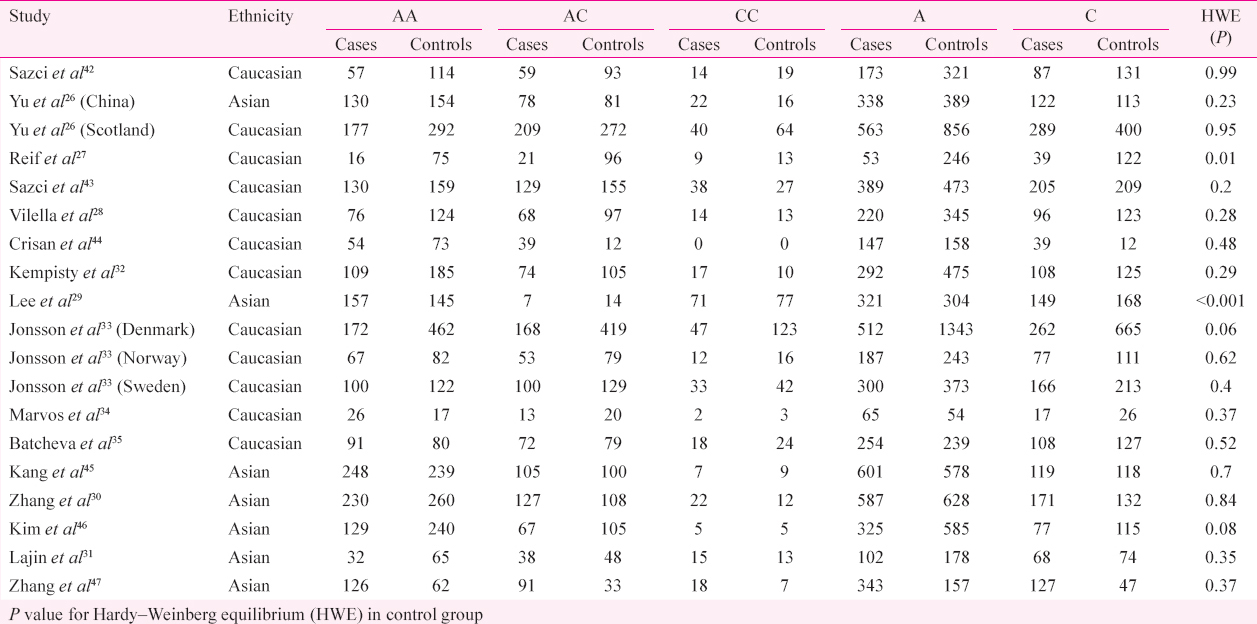
The results of the overall meta-analysis and the heterogeneity test are shown in Table III. A1298C polymorphism was significantly associated with schizophrenia under all five models [C vs. A (allele contrast model): OR=1.13, 95% CI=1.02-1.26, P=0.02 (Fig. 2); CC vs. AA (homozygote model): OR=1.20, 95% CI=1.03-1.39, P=0.02 (Fig. 3); CC vs. AA+AC (recessive model): OR=1.17, 95% CI=1.01-1.35, P=0.04; AC vs. AA (co-dominant model): OR=1.13, 95% CI=1.03-1.23, P=0.009; CC+AC vs. AA (dominant model): OR=1.14, 95% CI=1.05-1.24, P=0.002) (Fig. 4)].
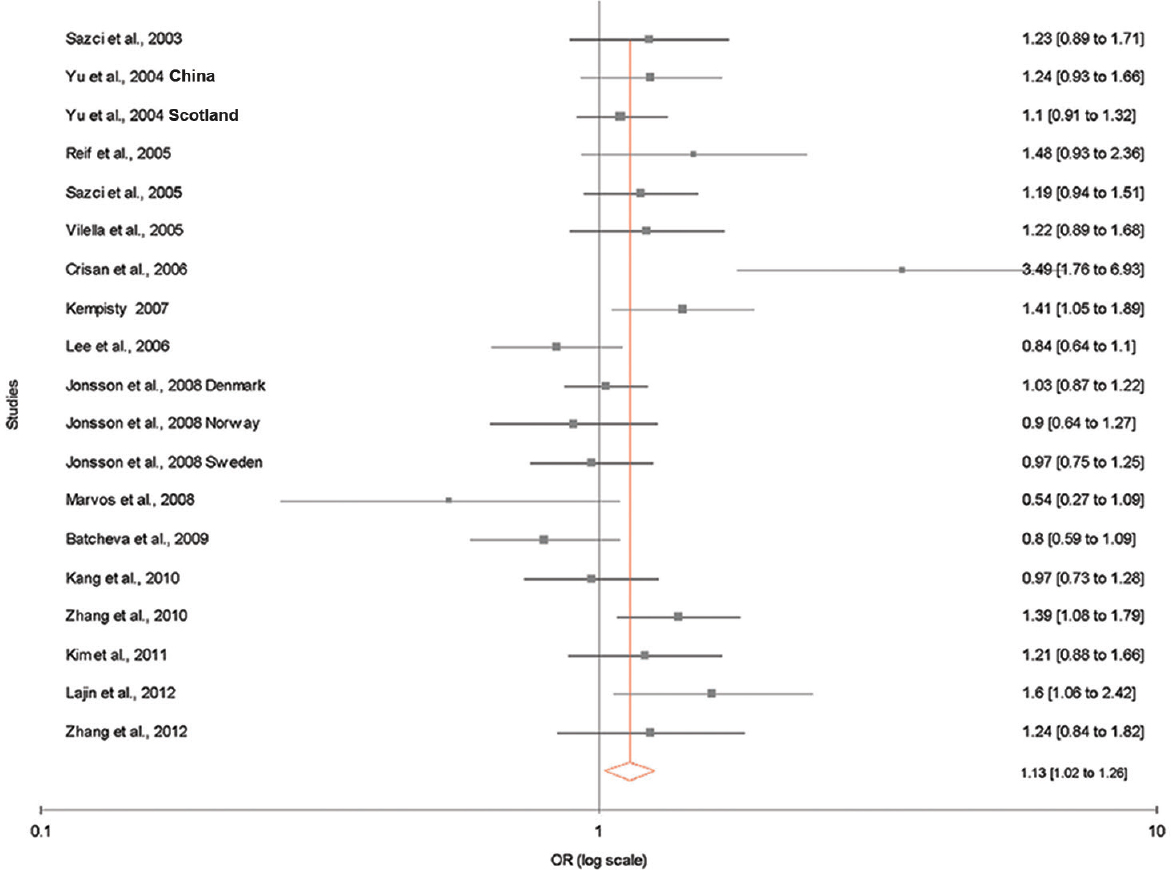
- Random effect forest plot showing a significant association between methylenetetrahydrofolate reductase (MTHFR) A1298C polymorphism and risk of schizophrenia using allele contrast model.
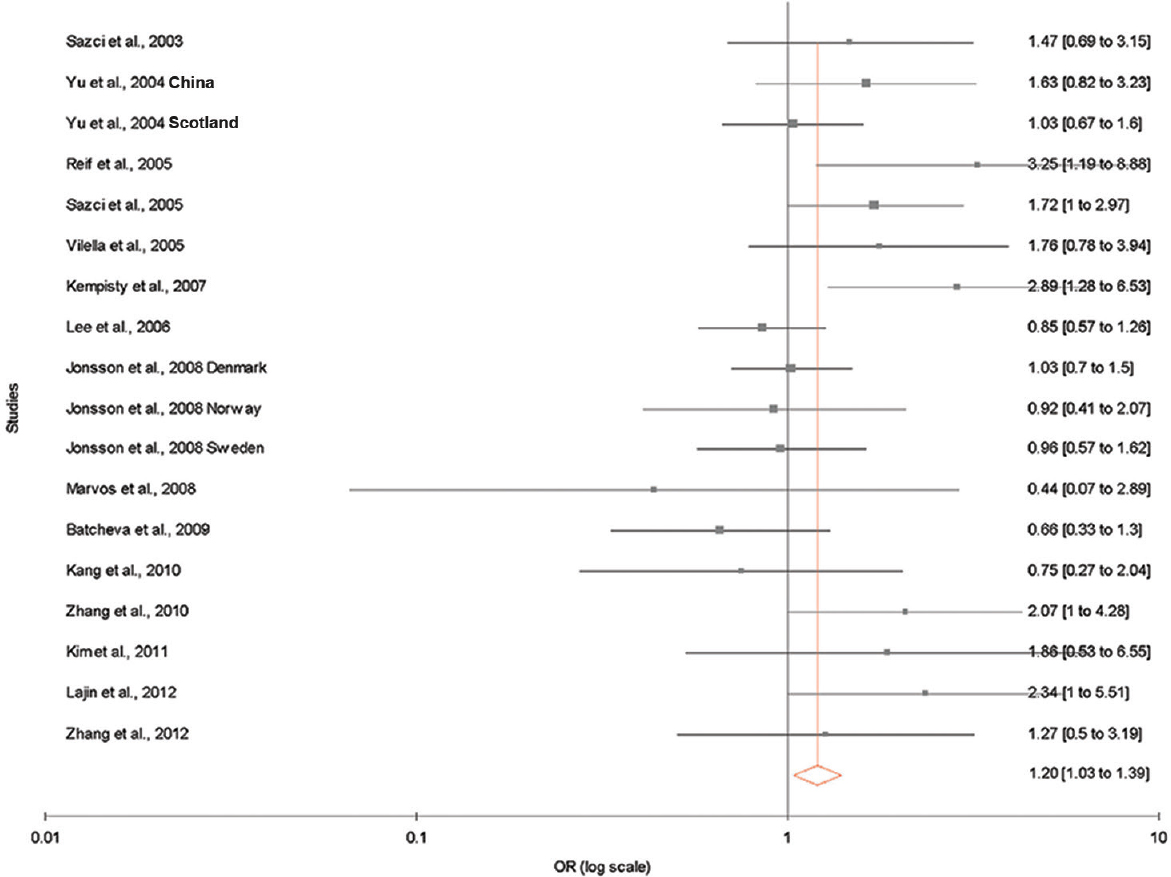
- Fixed effect forest plot showing a significant association between methylenetetrahydrofolate reductase (MTHFR) A1298C polymorphism and risk of schizophrenia using homozygote model. Only 18 studies that had homozygous mutants were included.
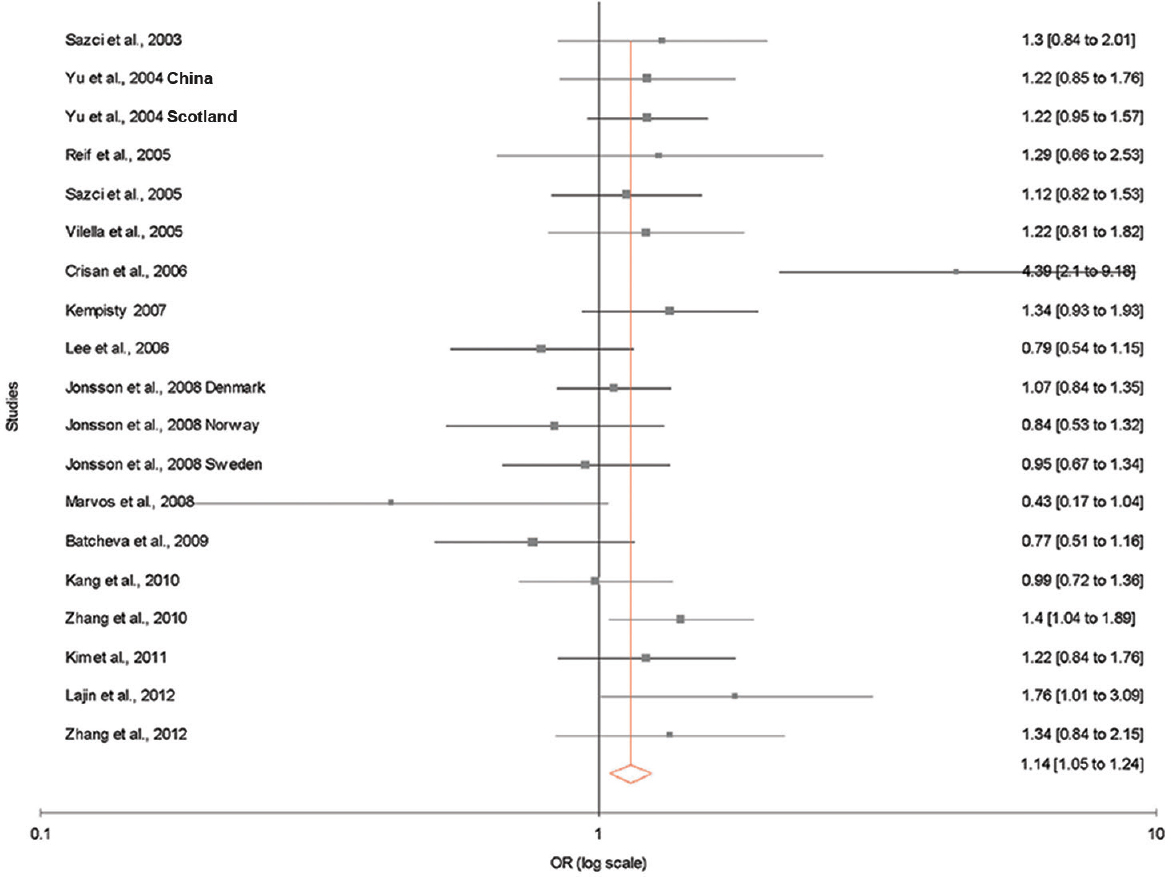
- Forest plots (random effect) showing no association between methylenetetrahydrofolate reductase A1298C polymorphism and risk of schizophrenia using dominant model (CC+AC vs. AA).
Subgroup analysis: Subgroup analysis based on ethnicity was performed. Of the 19 studies included in the present meta-analysis, seven were from Asian, and 12 from Caucasian populations. In Asian population, allele contrast meta-analysis showed significant association adopting fixed (OR=1.15; 95% CI=1.03-1.29; P=0.01; I2=48.61%; Pheterogeneity=0.07; PPb=0.38) effect model. Combined mutant genotypes also showed significant association with fixed (OR=1.17; 95% CI=1.02-1.35; P=0.02) and random (OR=1.18; 95% CI=0.99-1.41; P=0.06) effect models. In this subgroup, heterogeneity between studies (I2=33.91%; Pheterogeneity=0.17) was moderate and publication bias (PPb=0.51) was absent (Table III).
In Caucasian population, allele contrast meta-analysis showed significant association with FE model (OR=1.10; 95% CI=1.01-1.19; P=0.02) with significant heterogeneity (I2=59.66%; Pheterogeneity=0.004). The combined mutant genotype showed significant association with FE model (OR=1.12; 95% CI=1.01-1.24; P=0.03) with moderate heterogeneity (I2=57.39%; Pheterogeneity=0.007) and no publication bias (PPb=0.23) (Table III).
Publication bias: Publication bias was not observed as funnel plots were symmetrical (Fig. 5A–F). In addition, results of Eggers also did not suggest any evidence of publication bias (P=0.77 for C vs. A with additive model; P = 0.93 for AC vs. AA with co-dominant model; P=0.41 for CC vs. AA with homozygote model; P=0.66 for CC+AC vs. AA with dominant model; P=0.26 for CC vs. AA+AC with recessive model).
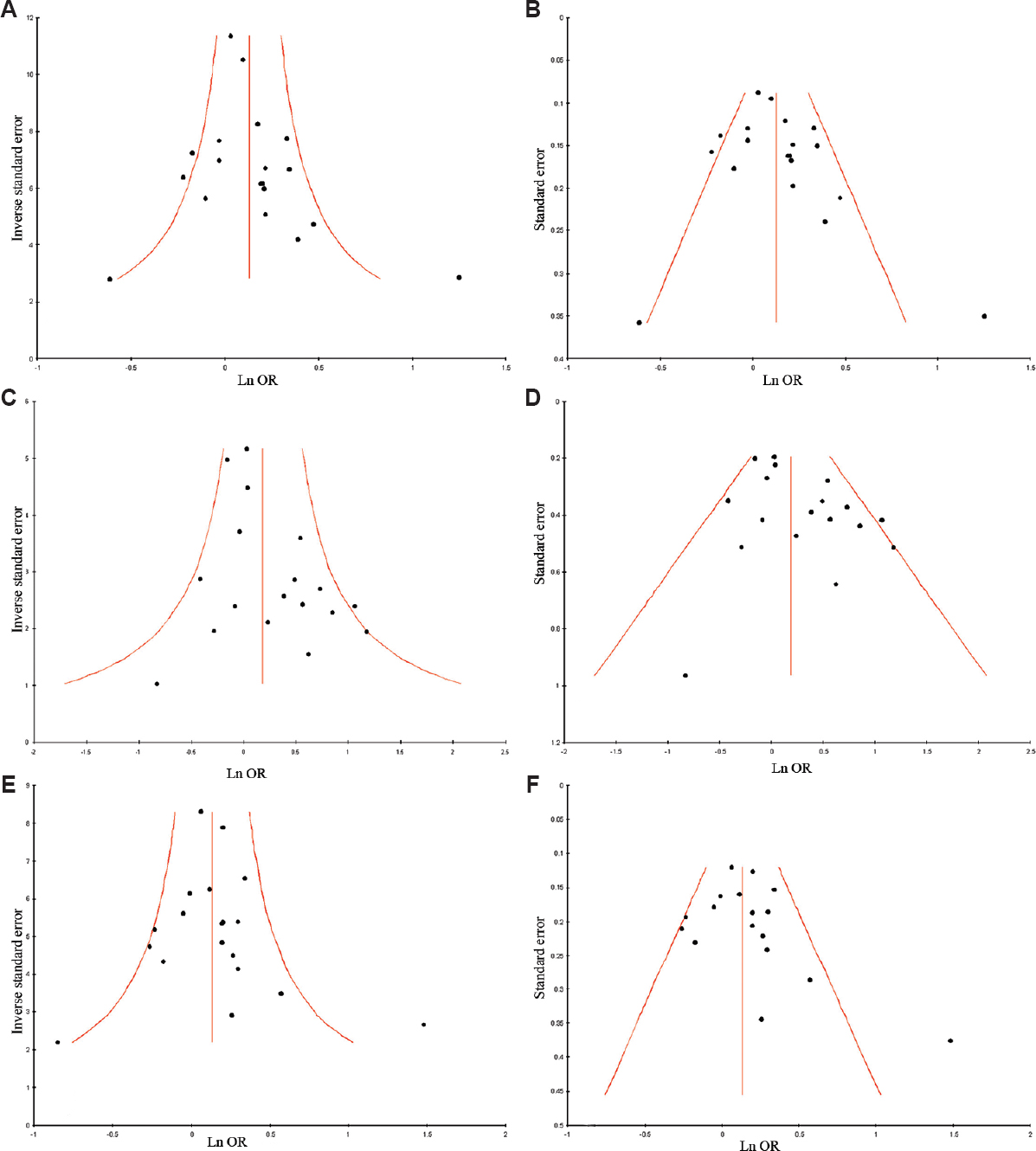
- (A) Funnel plot of precision by log odds ratio (allele contrast model); (B) Funnel plot of standard error by log odds ratio (allele contrast model); (C) Funnel plot of precision by log odds ratio (homozygote model); (D) Funnel plot of standard error by log odds ratio (homozygote model); (E) Funnel plot of precision by log odds ratio (dominant model); (F) Funnel plot of standard error by log odds ratio (dominant model).
Discussion
Several obstetric complications such as foetal hypoxia, maternal stress, infection, bleeding during pregnancy and pre-eclampsia are the risk factors for psychiatric disorders in offspring484950515253. In addition, perinatal folate deficiency and higher plasma concentration of homocysteine in mother have also been reported as a risk factor for schizophrenia54. Folate supplies the substrate for MTHFR reaction. During reduced serum folate and dysfunctional MTHFR, there is a profound deficit in MTHFR-dependent cellular and physiological processes. MTHFR enzyme activity is reduced in heterozygous (AC) and homozygous (CC) individuals, which leads to hyperhomocysteinemia22.
Folate deficiency along with MTHFR polymorphism leads to DNA hypomethylation55565758, and abnormal embryogenesis, which results into developmental malformation in foetus. The neurons in the developing brain are vulnerable to the effect of folate deficiency59. Two main metabolic pathways of homocysteine elimination, i.e., betaine remethylation and transsulfuration are not found in brain6061. Hence, MTHFR hypofunction/folate deficiency increases the concentration of homocysteine in brain which is toxic to neurons and blood vessels and can induce DNA strand breakage, oxidative stress and apoptosis61626364656667.
Several meta-analyses have been published accessing folate pathway genes polymorphism as risk factor for various diseases/disorders such as neural tube defects68, cleft lip and palate69, Down syndrome70, hyperurecemia71, autism72, bipolar disorder73, depression74, Alzheimer's disease75, recurrent pregnancy loss76 and cancer7778. Twelve meta-analyses have been published regarding MTHFR polymorphisms and schizophrenia334672798081828384858687. Of these, in only four meta-analyses MTHFR A1298C polymorphism has been investigated as a risk factor for schizophrenia72798185. Zintzaras81 pooled nine studies for A1298C meta-analysis and reported that the A1298C association with schizophrenia was only marginally significant with a fixed effect (FE) OR of 1.16 (95% CI=1.09-1.331). Gilbody et al72 performed a meta-analysis of only two studies examining the association between polymorphisms A1298C and schizophrenia and reported an association with fixed-effects [OR (CC vs. AA)=1.64 95% CI=1.04-2.54], with low heterogeneity (I2=0%). Peerbooms et al85 conducted a meta-analysis of 10 published case-control studies investigating associations between MTHFR A1298C and schizophrenia and reported that schizophrenia was not significantly associated with A1298C polymorphism. Hu et al86 pooled 12 studies and reported a significant association between A1298C polymorphism and schizophrenia (OR=1.13, 95% CI 1.03-1.24).
The present meta-analysis had several strengths along with limitations. The main strength of the present meta-analysis was that the publication bias was not detected and pooled number of cases and controls from different studies significantly increased the statistical power of the analysis. The limitations of the present meta-analysis were (i) use of crude OR, (ii) small sample size (9537 samples), (iii) inclusion of studies with small sample size, (iv) only one gene MTHFR and one polymorphism (A1298C) of folate pathway considered, and (v) gene-gene interactions not considered.
In conclusion, the present meta-analysis indicated that A1298C polymorphism was associated with schizophrenia risk. In future, several case-control studies with larger samples size are required to investigate gene-gene and gene-environment interactions involving the MTHFR A1298C polymorphism to determine the susceptibility for schizophrenia.
Acknowledgment
Authors acknowledge Leon Bax (Chief Scientific Officer at BiostatXL, UMC Utrecht, The Netherlands) for his valuable suggestions.
Conflicts of Interest: None.
References
- Twin studies of schizophrenia: From bow-and-arrow concordances to star wars Mx and functional genomics. Am J Med Genet. 2000;97:12-7.
- [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. (4th ed). Washington, DC: American Psychiatric Press; 1994.
- [Google Scholar]
- The genetics of adult-onset neuropsychiatric disease: Complexities and conundra? Science. 2003;302:822-6.
- [Google Scholar]
- Schizophrenia, psychiatric genetics, and Darwinian psychiatry: An evolutionary framework. Schizophr Bull. 2008;34:722-33.
- [Google Scholar]
- Folate deficiency causes uracil misincorporation into human DNA and chromosome breakage: Implications for cancer and neuronal damage. Proc Natl Acad Sci U S A. 1997;94:3290-5.
- [Google Scholar]
- Mechanisms of DNA damage, DNA hypomethylation, and tumor progression in the folate/methyl-deficient rat model of hepatocarcinogenesis. J Nutr. 2003;133(11 Suppl 1):3740S-7S.
- [Google Scholar]
- Genomic hypomethylation is specific for preneoplastic liver in folate/methyl deficient rats and does not occur in non-target tissues. Mutat Res. 2004;548:53-9.
- [Google Scholar]
- Characterization of a pseudogene for murine methylenetetrahydrofolate reductase. Mol Cell Biochem. 2003;252:391-5.
- [Google Scholar]
- Metabolism of monocarbons and trisomy 21: Sensitivity to methotrexate. Ann Genet. 1986;29:16-9.
- [Google Scholar]
- Leucocyte ultrastructure and folate metabolism in Down's syndrome. S Afr Med J. 1977;51:369-74.
- [Google Scholar]
- Lack of checkpoint control at the metaphase/anaphase transition: A mechanism of meiotic nondisjunction in mammalian females. J Cell Biol. 1997;139:1611-9.
- [Google Scholar]
- ’Spontaneous’ genetic damage in man: Evaluation of interindividual variability, relationship among markers of damage, and influence of nutritional status. Mutat Res. 1997;377:125-35.
- [Google Scholar]
- Homocysteine metabolism in children with Down syndrome: In vitro modulation. Am J Hum Genet. 2001;69:88-95.
- [Google Scholar]
- Polymorphisms in genes involved in folate metabolism as maternal risk factors for Down syndrome. Am J Hum Genet. 2000;67:623-30.
- [Google Scholar]
- Treatment of depression: Time to consider folic acid and Vitamin B12. J Psychopharmacol. 2005;19:59-65.
- [Google Scholar]
- A candidate genetic risk factor for vascular disease: A common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995;10:111-3.
- [Google Scholar]
- A second common mutation in the methylenetetrahydrofolate reductase gene: An additional risk factor for neural-tube defects? Am J Hum Genet. 1998;62:1044-51.
- [Google Scholar]
- A second genetic polymorphism in methylenetetrahydrofolate reductase (MTHFR) associated with decreased enzyme activity. Mol Genet Metab. 1998;64:169-72.
- [Google Scholar]
- Thermolabile variant of 5,10-methylenetetrahydrofolate reductase associated with low red-cell folates: Implications for folate intake recommendations. Lancet. 1997;349:1591-3.
- [Google Scholar]
- 5,10-Methylenetetrahydrofolate reductase gene variants and congenital anomalies: A HuGE review. Am J Epidemiol. 2000;151:862-77.
- [Google Scholar]
- 5,10-Methylenetetrahydrofolate reductase polymorphisms and leukemia risk: A HuGE minireview. Am J Epidemiol. 2003;157:571-82.
- [Google Scholar]
- No association between polymorphisms of methylenetetrahydrofolate reductase gene and schizophrenia in both Chinese and Scottish populations. Mol Psychiatry. 2004;9:1063-5.
- [Google Scholar]
- Homocysteinemia as well as methylenetetrahydrofolate reductase polymorphism are associated with affective psychoses. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1162-8.
- [Google Scholar]
- Further evidence that hyperhomocysteinemia and methylenetetrahydrofolate reductase C677T and A1289C polymorphisms are not risk factors for schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1169-74.
- [Google Scholar]
- Serum homocysteine, folate level and methylenetetrahydrofolate reductase 677, 1298 gene polymorphism in Korean schizophrenic patients. Neuroreport. 2006;17:743-6.
- [Google Scholar]
- Further evidence that methylenetetrahydrofolate reductase A1298C polymorphism is a risk factor for schizophrenia. J Neural Transm (Vienna). 2010;117:1115-7.
- [Google Scholar]
- Association between MTHFR C677T and A1298C, and MTRR A66G polymorphisms and susceptibility to schizophrenia in a Syrian study cohort. Asian J Psychiatr. 2012;5:144-9.
- [Google Scholar]
- Distribution of 1298A>C polymorphism of methylenetetrahydrofolate reductase gene in patients with bipolar disorder and schizophrenia. Eur Psychiatry. 2007;22:39-43.
- [Google Scholar]
- Two methylenetetrahydrofolate reductase gene (MTHFR) polymorphisms, schizophrenia and bipolar disorder: An association study. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:976-82.
- [Google Scholar]
- The genetic polymorphism of MTHFR gene in schizophrenia. Rev Med Chir Soc Med Nat Iasi. 2008;112:76-82.
- [Google Scholar]
- Case-control association study of 59 candidate genes reveals the DRD2 SNP rs6277 (C957T) as the only susceptibility factor for schizophrenia in the Bulgarian population. J Hum Genet. 2009;54:98-107.
- [Google Scholar]
- The combination of estimates from different experiments. Biometrics. 1954;10:101-29.
- [Google Scholar]
- Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719-48.
- [Google Scholar]
- Development and validation of MIX: Comprehensive free software for meta-analysis of causal research data. BMC Med Res Methodol. 2006;6:50.
- [Google Scholar]
- Methylenetetrahydrofolate reductase gene polymorphisms in patients with schizophrenia. Brain Res Mol Brain Res. 2003;117:104-7.
- [Google Scholar]
- Association of the C677T and A1298C polymorphisms of methylenetetrahydrofolate reductase gene with schizophrenia: Association is significant in men but not in women. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1113-23.
- [Google Scholar]
- P.1.a.009 Methylenetetrahydrofolate reductase gene polymorphisms (C677T and A1298C) in patients with schizophrenia. Eur Neuropsychopharmacol. 2006;16(Suppl 4):S213.
- [Google Scholar]
- No association between functional polymorphisms in COMT and MTHFR and schizophrenia risk in Korean population. Epidemiol Health. 2010;32:e2010011.
- [Google Scholar]
- No association of functional polymorphisms in methlylenetetrahydrofolate reductase and the risk and minor physical anomalies of schizophrenia in Korean population. J Korean Med Sci. 2011;26:1356-63.
- [Google Scholar]
- Association between the MTHFR gene polymorphisms and refractory schizophrenia. J Clin Psychiatry. 2012;22:293-7.
- [Google Scholar]
- Obstetrical complications and subsequent schizophrenia in adolescent and young adult offsprings: Is there a relationship? Eur J Obstet Gynecol Reprod Biol. 2004;114:130-6.
- [Google Scholar]
- Obstetric conditions and risk of first admission with schizophrenia: A Danish national register based study. Schizophr Res. 2007;97:51-9.
- [Google Scholar]
- Gene-environment interaction and covariation in schizophrenia: The role of obstetric complications. Schizophr Bull. 2008;34:1083-94.
- [Google Scholar]
- Schizophrenia, “Just the Facts”: What we know in 2008 part 1: Overview. Schizophr Res. 2008;100:4-19.
- [Google Scholar]
- What we know: Findings that every theory of schizophrenia should explain. Schizophr Bull. 2009;35:493-508.
- [Google Scholar]
- Do maternal folate and homocysteine levels play a role in neurodevelopmental processes that increase risk for schizophrenia? Harv Rev Psychiatry. 2005;13:197-205.
- [Google Scholar]
- A common mutation in the 5,10-methylenetetrahydrofolate reductase gene affects genomic DNA methylation through an interaction with folate status. Proc Natl Acad Sci U S A. 2002;99:5606-11.
- [Google Scholar]
- 5,10-methylenetetrahydrofolate reductase (MTHFR) 677C→T and 1298A→C mutations are associated with DNA hypomethylation. J Med Genet. 2004;41:454-8.
- [Google Scholar]
- Homocysteine and folate metabolism in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1103-12.
- [Google Scholar]
- The metabolism of homocysteine: Pathways and regulation. Eur J Pediatr. 1998;157(Suppl 2):S40-4.
- [Google Scholar]
- Transcranial magnetic stimulation for the deficit syndrome of schizophrenia: A pilot investigation. Psychiatry Clin Neurosci. 2005;59:354-7.
- [Google Scholar]
- Schizophrenia and single-carbon metabolism. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1124-32.
- [Google Scholar]
- Homocysteine and schizophrenia: From prenatal to adult life. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1175-80.
- [Google Scholar]
- Homocysteine levels in adolescent schizophrenia patients. Eur Neuropsychopharmacol. 2006;16:588-91.
- [Google Scholar]
- Plasma homocysteine, folate and B12 in chronic schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1289-96.
- [Google Scholar]
- Folate deficiency inhibits proliferation of adult hippocampal progenitors. Neuroreport. 2005;16:1055-9.
- [Google Scholar]
- Synaptosomal plasma membrane transport of excitatory sulphur amino acid transmitter candidates: Kinetic characterization and analysis of carrier specificity. J Neurosci Res. 1992;32:60-8.
- [Google Scholar]
- Polymorphisms in folate metabolism genes as maternal risk factor for neural tube defects: An updated meta-analysis. Metab Brain Dis. 2015;30:7-24.
- [Google Scholar]
- Maternal methylenetetrahydrofolate reductase (MTHFR) gene A1298C polymorphism and risk of nonsyndromic cleft lip and/or Palate (NSCL/P) in offspring: A meta-analysis. Asian J Med Sci. 2014;6:16-21.
- [Google Scholar]
- Polymorphism in folate metabolic pathway gene as maternal risk factor for Down syndrome. Int J Biol Med Res. 2011;2:1055-60.
- [Google Scholar]
- The MTHFR C677T polymorphism and hyperuricemia risk: A meta-analysis of 558 cases and 912 controls. Metabolomics. 2016;6:2153-60.
- [Google Scholar]
- Methylenetetrahydrofolate reductase (MTHFR) genetic polymorphisms and psychiatric disorders: A HuGE review. Am J Epidemiol. 2007;165:1-13.
- [Google Scholar]
- Evaluation of methylenetetrahydrofolate reductase gene variant (C677T) as risk factor for bipolar disorder. Cell Mol Biol (Noisy-Le-Grand). 2011;57(Suppl-2):OL1558-66.
- [Google Scholar]
- Genetic polymorphisms of methylenetetrahydrofolate reductase (MTHFR) gene and susceptibility to depression in Asian population: A systematic meta-analysis. Cell Mol Biol (Noisy-Le-Grand). 2014;60:29-36.
- [Google Scholar]
- Methylenetetrahydrofolate Reductase (MTHFR) C677T Polymorphism and Alzheimer disease risk: A meta-analysis. Mol Neurobiol. 2017;54:1173-86.
- [Google Scholar]
- Methylenetetrahydrofolate reductase gene A1298C polymorphism and susceptibility to recurrent pregnancy loss: A meta-analysis. Cell Mol Biol (Noisy-Le-Grand). 2014;60:27-34.
- [Google Scholar]
- Methylenetetrahydrofolate reductase A1298C polymorphism and breast cancer risk: A meta-analysis of 33 studies. Ann Med Health Sci Res. 2014;4:841-51.
- [Google Scholar]
- Role of MTHFR A1298C gene polymorphism in the etiology of prostate cancer: A systematic review and updated meta-analysis. Egypt J Med Hum Genet. 2016;17:141-8.
- [Google Scholar]
- A meta-analysis of the MTHFR C677T polymorphism and schizophrenia risk. Am J Med Genet B Neuropsychiatr Genet. 2005;135B:2-4.
- [Google Scholar]
- Homocysteine, methylenetetrahydrofolate reductase and risk of schizophrenia: A meta-analysis. Mol Psychiatry. 2006;11:143-9.
- [Google Scholar]
- C677T and A1298C methylenetetrahydrofolate reductase gene polymorphisms in schizophrenia, bipolar disorder and depression: A meta-analysis of genetic association studies. Psychiatr Genet. 2006;16:105-15.
- [Google Scholar]
- Systematic meta-analyses and field synopsis of genetic association studies in schizophrenia: The SzGene database. Nat Genet. 2008;40:827-34.
- [Google Scholar]
- Genetic associations with schizophrenia: Meta-analyses of 12 candidate genes. Schizophr Res. 2008;104:96-107.
- [Google Scholar]
- Gene-wide association study between the methylenetetrahydrofolate reductase gene (MTHFR) and schizophrenia in the Japanese population, with an updated meta-analysis on currently available data. Schizophr Res. 2010;124:216-22.
- [Google Scholar]
- Meta-analysis of MTHFR gene variants in schizophrenia, bipolar disorder and unipolar depressive disorder: Evidence for a common genetic vulnerability? Brain Behav Immun. 2011;25:1530-43.
- [Google Scholar]
- Methylenetetrahydrofolate reductase (MTHFR) polymorphism susceptibility to schizophrenia and bipolar disorder: An updated meta-analysis. J Neural Transm (Vienna). 2015;122:307-20.
- [Google Scholar]
- Role of MTHFR C677T gene polymorphism in the susceptibility of schizophrenia: An updated meta-analysis. Asian J Psychiatr. 2016;20:41-51.
- [Google Scholar]






