Translate this page into:
Long COVID syndrome: An unfolding enigma
For correspondence: Dr Surendra Kumar Sharma, Department of Molecular Medicine, Jamia Hamdard Institute of Molecular Medicine, New Delhi 110062, & Professor of Practice, Faculty of Medicine, Datta Meghe Institute of Medical Sciences (DMIMS) (Deemed to be University), Sawangi (Meghe), Wardha 442 004, Maharashtra, & Formerly, Professor and Head, Department of Internal Medicine (WHO Collaborating Centre for Research & Training in Tuberculosis, Centre of Excellence for EPTB, Ministry of Health and Family Welfare, Government of India), & All India Institute of Medical Sciences; New Delhi 110 029, India e-mail: sksharma.aiims2@gmail.com
-
Received: ,
Abstract
Post-acute sequelae of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) disease (COVID-19), called as long COVID syndrome, is a major global public health issue after recovery from COVID-19. The disease occurs in symptomatic patients irrespective of illness severity. The symptoms continue after four wk of recovery from acute illness and persist beyond three months. Risk factors for long COVID include older age, female gender, multiple co-morbidities including diabetes mellitus, prior chronic respiratory illnesses, hospitalized patients with severe disease, especially receiving assisted ventilation, high viral load, reactivation of Epstein Barr (EB) virus and human herpes virus 6 (HH6), circulating auto antibodies against various organs and type I interferon. The prevalence varies from 10 to 20 per cent, and most data have been reported from high-income countries. Any system can get involved in long COVID. The symptoms include fatigue, cognition impairment, cough and dyspnoea, anosmia, hair loss and diarrhoea, among others. While there are no laboratory tests for confirmation of diagnosis, reduced complement C7 complexes at six months, and a two-gene biomarker including FYN and SARS-CoV-2 antisense ribonucleic acid (RNA) are emerging as potentially useful biomarkers for long COVID. There should be no alternative disease to explain various symptoms. Vaccination against SARS-CoV-2 and early use of oral antiviral nirmatrelvir within the first five days in patients with acute mild disease having various risk factors for progression to severe disease help in preventing long COVID. Several clinical trials are underway for the treatment of long COVID and the results of these are eagerly awaited. Physical and mental rehabilitation at home, at community level or in the hospital setting as appropriate is essential in patients with long COVID.
Keywords
Diagnosis
long COVID
post-acute sequelae of severe acute respiratory syndrome
prevalence
risk factors
treatment
Long-term clinical consequences have been described in survivors of severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) coronavirus infections. In a systematic review and a meta-analysis of 28 studies1, it was reported that lung function abnormalities (impaired diffusing capacity of the lung for carbon monoxide), reduced exercise capacity and psychological impairment (e.g., post-traumatic stress disorder, depression, anxiety) were common in SARS and MERS survivors following discharge from intensive care unit (ICU) or hospital.
Since the time of the first outbreak of SARS coronavirus-2 (SARS-CoV-2) disease (COVID-19), it was observed that, while the majority of COVID-19 patients had recovered fully within a few weeks, several patients still had persisting symptoms or developed newer symptoms for a long-time after recovery from the acute illness, or new symptoms had appeared in asymptomatic cases and there was no alternative disease to explain these symptoms. The existence of this entity was first documented in 2020 in a patient-led research collaborative survey to describe various manifestations experienced by the patients once they had recovered from acute COVID-192. Later, publications started appearing in the medical literature regarding the long-term consequences of COVID-19. Several names have been given to this entity (Table I)3-7, presently, being referred to simply as ‘long COVID’.
|
Chronic COVID syndrome (symptoms extending >12 wk from the onset of first symptoms) Chronic post-COVID syndrome (symptoms >12 wk) COVID long-haulers COVID-19 long haulers Long-tail COVID Long-haul COVID Long-hauler COVID-19 Long-term effects of COVID Long COVID Post COVID syndrome Post COVID-19 sequelae Postacute COVID (symptoms extending >3 wk from the onset of first symptoms) Post-acute sequelae of SARS-CoV-2 infection (PASC) Post-COVID-19 condition (PCC) |
Source: Ref 3–6
The United Kingdom (UK) National Institute for Health and Care Excellence (NICE), Royal College of General Practitioners (RCGP), Scottish Intercollegiate Guidelines Network (SIGN)8, World Health Organization (WHO)9, US Centers for Disease Control (CDC) and Prevention10, National Institutes of Health (NIH)11 and the Ministry of Health and Family Welfare of Government of India12 have coined their own terminology and definitions for symptomatic phases and long-term consequences of COVID-19 (Table II).
| NICE, RCGP, and SIGN guidelines8 | WHO Clinical case definition9 | CDC and IDSA guidelines10 | NIH definition11 | ||
|---|---|---|---|---|---|
| Acute COVID-19 | Ongoing symptomatic COVID-19 | Post-COVID-19 syndrome | Post-COVID-19 condition | Post-COVID conditions | Long COVID |
| Signs & symptoms of COVID-19 for up to 4 wk | Signs & symptoms of COVID-19 from 4 up to 12 wk |
Signs & symptoms that develop during or after an infection consistent with COVID-19, continue for more than 12 wk & are not explained by an alternative diagnosis. It usually presents with clusters of symptoms, often overlapping, which can fluctuate & change over time & can affect any system in the body. Post-COVID-19 syndrome may be considered before 12 wk while the possibility of an alternative underlying disease is also being assessed |
Continuation or development of new symptoms 3 months after the initial SARS-CoV-2 infection, with these symptoms lasting for at least 2 months with no other explanation | An umbrella term that encompasses a wide range of health consequences that can be present for 4 or more wk after infection with SARS-CoV-2 | Symptoms lingering for weeks, months or even years after their initial diagnosis, return of symptoms or development of new symptoms after full recovery within a few months & development of symptoms later in people who had no symptoms when they were infected initially |
SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; COVID-19, corona virus disease-19; NICE, National Institute for Health and Care Excellence; RCGP, Royal College of General Practitioners; SIGN, Scottish Intercollegiate Guidelines Network; WHO, World Health Organization; CDC, Centers for Disease Control and Prevention, USA; IDSA, Infectious Diseases Society of America; NIH, National Institutes of Health
Source: Ref 8-11
The term long COVID includes both ongoing symptomatic COVID-19 (from 4 to 12 wk) and post-COVID-19 syndrome (12 wk or more)7-12. However, a clear-cut consensus is still lacking on the definition of ‘long COVID’ for the purposes of clinical diagnosis, prognostic monitoring, disease surveillance and research, and this entity remains an evolving enigma. In this review, we describe the risk factors, pathogenesis, pathophysiology, clinical presentation, course and management of long COVID in adults.
Disease burden
Methodological issues
Several methodological issues hamper meaningful comparison of the epidemiological data of long COVID across various studies. The variations in case definitions employed, differences in the denominator used (e.g., hospitalized vs. non-hospitalized, mixed patients; community-based vs. hospital-based), study design (online/telephone surveys, patient re-evaluation in clinic/hospital setting, laboratory re-testing done/not done) should be kept in mind while interpreting results of various published studies. Moreover, most clinical data are self-reported symptoms (not verified by their physicians), obtained via text messages, social media, postal mail and telephonic/online survey.
Among non-hospitalized, only hospitalized, or a combination of hospitalized and non-hospitalized COVID-19 patients, the overall pooled prevalence of long COVID has been computed to be 43 per cent [95% confidence intervals (CI): 39–46]13. The figure is higher in hospitalized (54%, 95% CI: 44–63) compared to non-hospitalized (34%, 95% CI: 25–46) patients. Females appear to be more frequently affected by long COVID compared to males (49%, 95% CI: 35–63) vs. 37 per cent (95% CI: 24–51)13. It has been estimated that 1 in 814 to 1 in 1015 patients will have persistence of symptoms, or will report new onset of symptoms after recovery from acute COVID-19 infection (≥4 wk).
Compared with the pre-delta era, the cumulative incidence of long COVID (cases/100 persons at 1 yr) has decreased from 10.42 to 3.5 among vaccinated persons who had SARS-COV-2 infection during the omicron era. This suggests that vaccination and emergence of mutated viral variants significantly reduced the risk of long COVID16.
Pathogenesis & pathophysiology
Current understanding of risk factors, pathogenesis and pathophysiology of long COVID is described in Figure 117-22.
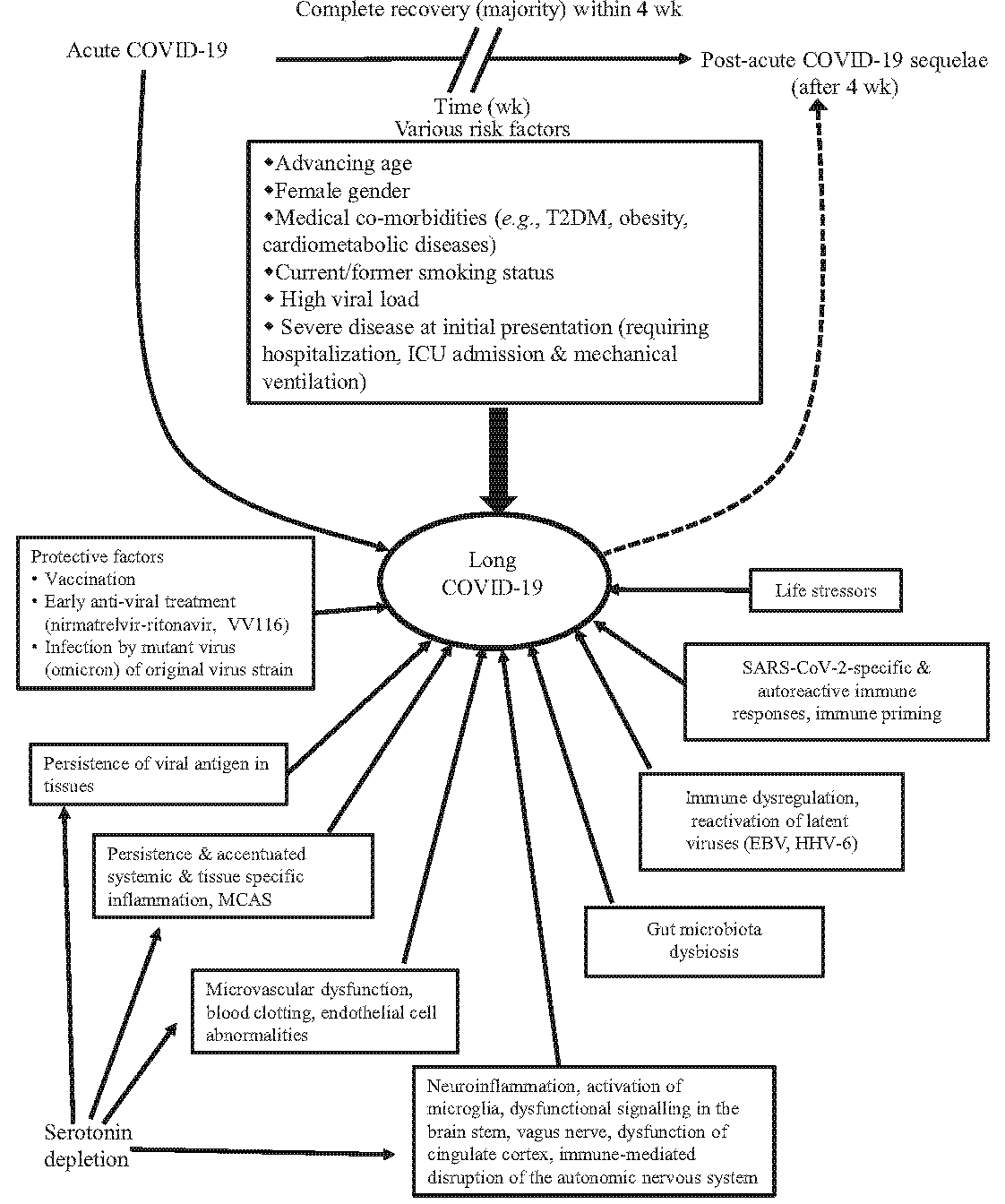
- Risk factors and pathogenesis of long COVID. Here, solid line indicates definitive consequence and interrupted line indicates tentative causation. SARS-Cov-2, severe acute respiratory syndrome coronavirus-2; COVID-19, corona virus disease 19; ICU, intensive care unit; MCAS, mast cell activation syndrome; HHV-6, human herpes virus-6; EBV, Ebstein-Barr virus. Source: Ref 17-20.
Risk factors for the development of long COVID
Data regarding potential risk factors for the development of long COVID are accumulating, and the evidence is inconsistent. Overall, advancing age [odds ratio (OR) 1.08, 95% CI: 1.02–1.15], female gender (OR 1.65, 95% CI: 1.41–1.92), current/former smoking status (OR 1.26, 95% CI: 1.04–1.54) and severity of COVID-19 at initial presentation (OR 1.4, 95% CI: 1.02–1.91) have been consistently documented to be risk factors for the development of long COVID23. Long COVID can occur even in patients with mild disease who do not require oxygen. There is no concrete evidence linking the duration of mechanical ventilation, and the use of sedatives on neurocognitive function in long COVID.
The reason for female gender predilection for long COVID has been postulated to be their hormonal influence, excessive inflammatory response, and stronger production of immunoglobulin G (IgG) antibodies activated by the virus24,25. Also, the ‘unmasking’ of an autoimmune reaction, perhaps due to molecular mimicry with some components of the female host, might play a vital role (Fig. 1)17-22. Other postulated factors include higher antibody responses against viruses, rates of adverse reactions to vaccinations and antiviral drugs, the influence of X-chromosome-linked genes on susceptibility to viral infections and hence to autoimmune processes; higher exposures to the virus in certain occupations such as nursing and education, use of ill-fitting personal protection equipment (PPE), which can predispose them to a higher incidence of the disease, among others. Further, increased awareness of the female gender of their body and related distress could also be a contributing factor.
Risk factors have also been described for certain specific manifestations of long COVID. The risk factors for fatigue/muscle weakness include female gender (OR 1.29, 95% CI: 1.1–1.52, P<0.0022); severe disease (OR 1.45, 95% CI: 1.06–1.98, P<0.021); corticosteroid usage (OR 1.36, 95% CI: 1.12–1.64, P<0.0016); and college or higher education (OR 1.22, 95% CI: 1.03–1.45, P=0.022)23. For pulmonary diffusion impairment for carbon dioxide, the risk factors were older age (OR 1.33,95% CI: 1.14–1.54, P=0.0003); diffusion impairment 33 per cent higher per 10-yr increase of age; female gender (OR 2.86, 95% CI: 1.92–4.26, P=0.0001); severe disease (OR 3.14, 95% CI: 1.77–5.59, P=0.0001); and college or higher education (OR 1.64, 95% CI: 1.11–2.41, P=0.013)]23. Anxiety/depression has been described in the female gender (OR 1.94, 95% CI: 1.59–2.37, P=0.0001), and severe disease (OR 1.54, 95% CI: 1.06–2.22, P=0.022)23.
A recent systematic review and meta-analysis26 (41 articles, n=8,60,783 patients) reported that female gender (OR 1.56, 95% CI: 1.41–1.73), age (OR 1.21, 95% CI: 1.11–1.33), high body mass index (BMI) (OR 1.15, 95% CI: 1.08–1.23), and smoking (OR 1.1, 95% CI: 1.07–1.13) were associated with an increased risk of developing post-COVID-19 condition (PCC)26. Other risk factors included the presence of comorbidities and previous hospitalization or ICU admission.
Symptoms & signs
The term long COVID describes patients with persistent, worsening, or new appearance of symptoms beyond 12 wk after recovery from acute COVID-19. Figure 2 describes the involvement of various organs with several manifestations of long COVID. These include dyspnoea, palpitations, chest pain, fatigue, body aches, sleep disturbances, inability to concentrate and severe cognitive impairment, among others27,28.

- Myriad clinical manifestations of long COVID. Here, red circle indicates very common, blue circle indicates less common and violet circle indicates least common. PTSD, post-traumatic stress disorder; OCS, obsessive-compulsive spectrum; POTS, postural tachycardia syndrome.
Dyspnoea has been reported to be a debilitating symptom that has affected the quality of life in patients with long COVID29,30. In a recently reported systematic review and meta-analysis31on prevalence, risk factors and treatments for long COVID, the authors observed the persistence of self-reported symptoms of breathlessness beyond four wk (27% in hospitalized vs. 17% non-hospitalized patients and 26% in patients with severe illness vs. 16% in non-severe illness). This pooled prevalence decreased significantly over the next 7–12 months. Female gender was a risk factor for breathlessness. It is a debilitating symptom whose exact prevalence, risk factors, mechanisms and treatments are poorly understood; several mechanisms, including systemic inflammation, deconditioning, prior or existing ventilatory defects and impaired mental health, have been proposed, but none are proven. However, systematic mechanistic research is required to find out the exact mechanisms of breathlessness in long COVID.
A mouse model study32 has revealed neuroinflammation, activation of microglia and brain dysfunction due to the involvement of subcortical white matter and hippocampus in mild respiratory SARS-CoV-2 infection. These effects are mediated by elevated levels of cytokines and chemokines, including CCL11 in the serum and cerebrospinal fluid. Other frequently reported clinical manifestations of long COVID include anxiety or depression, nausea, abdominal discomfort, skin rash, sore throat, tinnitus, ear pain, headache, peripheral neuropathy and joint and muscle pain, among others13,33-38. In are cent systematic review, meta-analysis13 of 41 studies, authors assessed 23 symptoms reported across 36 of the studies and reported the estimated pooled symptom-specific prevalence of most commonly documented long COVID manifestations were fatigue (23%, 95% CI: 17–30), memory problems (14%, 95% CI: 10–19), breathlessness (13%, 95% CI: 11–15); sleep disturbances (11%, 95% CI: 5–23); and joint pains (10%, 95% CI: 4–22).
In a population-based, cross-sectional study39 from southern Germany conducted by postal mail among 18–65 yr old COVID-19 survivors (n=11,710 respondents), symptom clusters such as fatigue (37.2%, 95% CI: 36.4–38.1%), neurocognitive impairment (31.3%, 95% CI: 30.5–32.2%), chest symptoms (30.2%, 95% CI: 29.4-31), smell or taste disorder (23.6%, 95% CI: 22.9–24.4), and anxiety/depression (21.1%, 95% CI: 20.4–21.9), among others had persisted at 6-12 months after acute infection.
The long-COVID in Scotland study (Long-CISS)40,41 had evaluated COVID-19 survivors (n=33,281) and never-infected individuals (n=62,957). Previous symptomatic infection was associated with poorer quality of life, impairment of all daily activities. Salient persistent symptoms observed included dyspnoea (OR 3.43, 95% CI: 3.29–3.58), palpitations (OR 2.51, 95% CI: 2.36–2.66), chest pain (OR 2.09, 95% CI: 1.96–2.23) and confusion (OR 2.92, 95% CI: 2.78–3.07) among others. Lack of recovery from acute COVID-19 was associated with more severe infection, older age, female gender, white ethnic group, social deprivation, pre-existing respiratory disease and multi-morbidity40,41.
In a longitudinal cohort study42 conducted in all army bases in Switzerland [n=501, 464 (94%) males], it was reported that while young, previously healthy individuals, largely recovered from COVID-19, the presence of higher BMI (kg/m2), dyslipidaemia, and lower aerobic threshold 180 days after COVID-19 resulted in a higher risk of developing metabolic disorders and cardiovascular complications. These observations warrant long-term follow up of COVID-19 survivors.
Postural tachycardia syndrome (POTS) due to the involvement of the autonomic nervous system has been reported in long COVID43. POTS is diagnosed by the presence of orthostatic tachycardia (increase in heart rate of >30 beats/min within 10 min of assuming upright posture in adults) without orthostatic hypotension, for at least 3 months with associated symptoms of orthostatic intolerance. In long COVID, brain fog (memory problems, lack of mental clarity, poor concentration and an inability to focus) has been a disabling symptom. Following COVID-19, an increased occurrence of the following, namely, diabetes mellitus and cerebrovascular events (especially acute ischaemic stroke), deep vein thrombosis, pulmonary embolism, bleeding and coagulopathies have also been described13,33-44.
The clinical manifestations of long COVID are shown in Figure 2. Health outcomes in patients surviving COVID-19 in some of the published studies from different parts of the world34,38,39,41,42,44 and India37,45-51 are summarized in Tables III and IV, respectively.
| Variable | Zhao (2021)34 | Darcis (2021)38 | Deuel (2022)42 | Peter (2022)39 | Hastie (2022)40 | Gottlieb (2023)44 |
|---|---|---|---|---|---|---|
| Design | Cross-sectional | Prospective | Cross-sectional | Population-based, cross-sectional study | Prospective |
Multicentre prospective cohort study (the Innovative Support for Patients with SARS-CoV-2 Infections Registry [INSPIRE]) |
| Place of study | Henan Province, China | Liege, Belgium | Army bases in Switzerland | Four administratively & geographically defined regions in the Federal State of Baden-Württemberg in south-western Germany | Scotland | In 8 centres across the USA |
| Period of COVID-19 diagnosis | Jan 16–Feb 6, 2021 |
2 Mar–Oct 1, 2020 |
Mar 1, 2020–Dec 31, 2020 | Oct 2020–Mar 2021 | From Apr 2020 | Pre-Delta (Dec 11, 2020–Jun 4, 2021), Delta (Jul 24, 2021–Dec 17, 2021), and Omicron (Jan 8, 2022–Jun 25, 2022) |
| Population studied | Hospitalized, survivors | Hospitalized, survivors | Non-hospitalized, unvaccinated survivors adults with recent military service | Hospitalized survivors |
Survivors who were never infected, asymptomatic infection, symptomatic infection |
Hospitalized & non-hospitalized survivors |
| Method | Face-to-face interviews, battery of tests | Face-to-face interviews, battery of tests | Comprehensive test battery administered during a single full day of testing | Postal mail, questionnaire | Questionnaire survey | Questionnaire survey |
| No. of patients studied (n/N) | 94/94 |
1 month 59/199 3 months 101/199 6 months 78/199 |
2500 contacted 530 signed up 501 examined |
50,457 invited 12,053 (24%) responded 11,710 included for analysis |
14% (90,578/ 625,315), 9% (21,963/242,412) & 11% (934/8625) for the 6, 12 & 18 month follow up questionnaires |
8298 screened, 5475 enrolled & completed a baseline survey, 3223 (58.9%) with complete baseline and 3 months data for analysis included |
| Age (yr) | 48.1† | 60.5±13.9† | 21(21–23)‡ | 44.1±13.7 |
Never infected 45 (31–56) Asymptomatic infection (n=1795) 43 (28–57) Symptomatic infection (n=31,486) 44 (30–56) |
>18 yr |
| Male:Female | 54:40 | 126:73 | 472:29 | 4829:6821 |
Never infected M:F =59:41 Asymptomatic infection M:F = 53:47 Symptomatic infection M:F = 64:36 |
M=750 F =1554 Transgender = 29 |
| Study participants |
Mild (n=3) Moderate (n=48) Severe (n=41) Critical (n=2) Control group: No |
ICU (n=52; 26.1%) Tracheal intubation (n=33; 16.6%) Required oxygen (n=160; 80.4%) Control group: No |
Control group (no clinical or serological evidence of past infection with SARS-CoV-2; n=251), asymptomatic group (no clinical evidence but positive serology; n=46); & patients after confirmed COVID-19 (n=196) (data missing n=8) |
n=11602 No medical care = 8988 (77.5) Outpatient care = 2202 (19.0) Inpatient non-ICU care = 315 (2.7) ICU care = 97 (0.8) |
Never infected (n=62,957) Asymptomatic infection (n=1795) Symptomatic infection (n=31,486) |
Positive COVID-19 result (n = 2402; 74.5%) Negative COVID-19 result (n = 821; 25.5%) Among COVID-positives, 463 (19.3%) were pre-Delta, 1198 (49.9%) Delta, & 741 (30.8%) Omicron |
| Follow up duration (days) | 365 | 180 (150–221)* | >180 since COVID-19 (n=177); <180 days since COVID-19 (n=19) | 6–12 months post-acute infection | 180, 365, 545 | 90 |
| Vaccinated | ND | ND | 0 | 220/11431 | Never infected, 0 vaccine (96%), 1 vaccine (3.8%), 2–4 vaccines (0.6%) Asymptomatic infection 0 vaccine (94%), 1 vaccine (5.3%), 2–4 vaccines (0.7%) Symptomatic infection 0 vaccine (97%), 1 vaccine (3.1%), 2–4 vaccines (0.2%) | 1357 |
| Chief conclusions | One yr after hospitalization for COVID-19, a cohort of survivors were mainly troubled with muscle fatigue & insomnia. Pulmonary structural abnormalities & pulmonary diffusion capacities were highly prevalent in surviving COVID-19 patients. It is necessary to intervene in the main target population for long-term recovery | The prevalence of persistent symptoms following hospitalisation with COVID-19 is high & stable for up to 6 months after discharge. However, biological, functional & iconographic abnormalities improved significantly over time | Young, previously healthy, individuals largely recover from SARS-CoV-2 infection. However, the constellation of higher BMI, dyslipidaemia, & lower physical endurance 180 days after COVID-19 is suggestive of a higher risk of developing metabolic disorders and possible cardiovascular complications. These findings will guide future investigations & follow up management |
A considerable burden of self-reported post-acute symptom clusters & possible sequelae, notably fatigue & neurocognitive impairment, 6 to 12 months after acute SARS-CoV-2 infection with a substantial impact on general health & working capacity |
Of the 31,486 symptomatic infections,1856 (6%) had not recovered &13,350 (42%) only partially. No recovery was associated with hospitalized infection, age, female sex, deprivation, respiratory disease, depression & multimorbidity. Previous symptomatic infection was associated with poorer quality of life, impairment across all daily activities & 24 persistent symptoms including breathlessness (OR 3.43, 95% CI: 3.29–3.58), palpitations (OR 2.51, OR 2.36–2.66), chest pain (OR 2.09, 95% CI:1.96–2.23), & confusion (OR 2.92, 95% CI: 2.78–3.07). Asymptomatic infection was not associated with adverse outcomes. Vaccination was associated with a reduced risk of 7 symptoms |
More prolonged severe fatigue (16.7% vs. 11.5% vs. 12.3%; P=0.017) & presence of ≥3 prolonged symptoms (28.4% I. 21.7% I. 16%; P<0.001)were evident in pre-delta COVID-positive cohort exhibited compared with the delta & omicron cohorts |
N, total study population; n, number of study participants included at that period of last follow-up visit; ND, not described; OR, odds ratio; ICU, intensive care unit
| Variable | Sathyamurthy et al37 (2021) | Naik et al 51 (2021) |
Arjun et al 45 (2022) |
Yadav et al 46 (2022) |
Goel et al 47 (2023) |
Jain et al 48 (2023) | Nair et al 49 (2023) | Nair et al 50 (2023) |
|---|---|---|---|---|---|---|---|---|
| Design | Cross-sectional | Prospective observational | Retrospective cohort | Prospective observational | Prospective observational | Cross-sectional | Prospective cohort | Prospective observational |
| Place of study | Chennai | New Delhi | Bhubaneswar | Pune | Delhi | Agra | Kochi | Kochi |
| Period of COVID-19 diagnosis | Aug 1, 2020– Nov 30, 2020 | Oct 2020–Feb 2021 | January 2022– February 2022 | April 2021– June 2021 | May 2020– December 2020 | July 2020 & September 2020 | February to July 2021 | October 2021– December 2021 |
| Population studied | Hospitalized, survivors | Hospitalized & non-hospitalized survivors | Hospitalized & non-hospitalized survivors | Hospitalized survivors | Non-hospitalized survivors | Hospitalized survivors | Hospitalized survivors | Hospitalized & non-hospitalised survivors |
| Method | Telephonic interview | Questionnaire survey, telephonic interviews | Telephonic interview | Telephonic interview | Questionnaire survey | Questionnaire survey | Questionnaire survey | Questionnaire survey |
| No. of patients studied (n/N) | 279/284 (5 could not complete interview) | 272/1234 (150 had ongoing symptomatic COVID-19 + 122 post-COVID-19) | 535/524 (11 duplicates & errors) | 345 | 150 | 568 called for intervention; 491 included for intervention & analysis | 454/120 | 414 |
| Age (yr)* | 71±5.6 | 44.3±13 | 34±14 | 52.7±15.1 | 46.6±16 | 57.2 ± 8.5 | 55.0 ± 17.5 | 44.4±17.7 |
| Male:Female | 178:101 | 191:81 | 312:212 | 228:117 | 100:50 | 304:187 | 66:54 | 202:212 |
| Study participants |
Mild–moderate (n=163; 58.4%) Severe/critical (n= 116; 41.6%) Control group: No |
Overall (n=1234), mild =1059 (85.8%); moderate= 135 (10.9%); severe = 40 (3.3%) |
Mild–moderate (n=505; 96.4%) Severe (n=17 3.2%) Critical (received invasive ventilation) (n=2; 0.4%) |
Disease severity details ND | mild (n = 118; 78.7%) moderate (n22; 14.7%) severe (n = 10; 6.7%) |
Distribution according to duration of ICU stay <3 days n=98 3–7 days n=266 >7 days n=127 |
Mild (n=70; 58.4%) Moderate (n=20; 16.6%) Severe (n=30; 25%) | Disease severity details ND |
| Follow up duration (days) | 90 | 276 patients (26%) for more than 6 months, 264 (24%) for 3–6 months | 28 | 90 & 180 | 26 (10–71)† | 561(548–580)† | 14 & 42 | 14, 42 & 84 |
| Vaccinated | ND | ND |
3 doses (2 doses + a precautionary dose; n=15, 2.9%) 2 doses (n=466, 88.9%) 1 dose (n=17, 3.2%) Not received vaccine (n=26, 5%) |
2 doses (n=50, 14.4%) One dose (n=146, 42.3%) |
ND | ND | 1 dose (n=6) |
2 doses (n=395, 95.5%) 1 dose (n=19, 4.5%) |
| Chief conclusions | In older adults post-COVID-19 syndrome occurred in 9.3%. After 90 days following recovery, fatigue, cough & breathlessness were common symptoms. | 272/1234 (22%) had long COVID. Of these (n=272) 150 (12.1%) had symptoms till 12 wk, & 122 (9.9%) had symptoms >12 wk | During the omicron wave, 8.2% had self-reported long COVID (compared to 29.2% during the Delta wave) | 117/345 (33.9%) & 33/75 (44%) of the patients studied had long COVID at the end of 3 & 6 months, respectively. Commonly reported symptoms included fatigue & body aches, dyspnoea, alopecia & new-onset diabetes mellitus | Long-COVID manifestations were significantly higher in patients presenting with severe COVID-19 compared to those with mild & moderate disease |
Severe COVID-19 survivors had new-onset psychological disorders & sleep disturbances; long-term quality of life & work ability remained poor; & prevalence of diabetes mellitus, hypertension & obesity decreased significantly after 1.5 yr after discharge |
High prevalence of post-COVID-19 symptoms at a minimum of 6 wk post-discharge among hospitalized survivors with COVID-19. Incidence of post-COVID-19 syndrome was similar in mild, moderate & severe COVID-19 survivors | Long COVID occurred in 161 (38.9%), 42 (10.1%) patients at 6 & 12 wk, respectively. Of these 42, 39 (9.4%) had persistent symptoms from 6-12 wk post-COVID-19 & 3 patients had developed new onset of long COVID symptoms at the end of 12 wk. 122 patients (29.4%) had symptoms at 6 wk without symptoms persisting up to 12 wk of testing negative for COVID-19 |
N, total study population; n, number of study participants included at that period of last follow up visit
Effect of COVID-19 vaccination
The impact of COVID-19 vaccination on long COVID is still not fully understood and sparse published data are available on this subject. A systematic review52 (11 peer-reviewed studies and 6 preprints) reported that overall, vaccination was linked to lower risks or odds of long COVID (low level of evidence, grade III, case-control, cohort studies), with preliminary evidence indicating that two doses are more effective than one.
A Scottish population cohort study40,41 has also documented that pre-infection vaccination was associated with reduced risk of some persistent symptoms40,41. A recent systematic review meta-analysis26 showed that patients who had been vaccinated against COVID-19 with two doses had a significantly lower risk of developing PCC compared with patients who were not vaccinated (OR 0.57, 95% CI: 0.43–0.76). Further, recent data suggest that administration of a booster dose, compared to one or two doses, reduced long COVID risk by 74 per cent (95% CI: 56-92%)53.
A study54 evaluated the occurrence of long COVID in a cohort of individuals from the US Department of Veterans Affairs national healthcare databases (n=33,940) with vaccine breakthrough infection (BTI) and contemporary (n=49,83,491), historical (n=57,85,273) and vaccinated (n=25,66,369) SARS-CoV-2 subjects without evidence of SARS-CoV-2 infection (controls). Six months after infection, individuals with BTI had a higher incidence of post-acute sequelae [hazard ratio (HR)=1.5, 95% CI: 1.46–1.54]. These post-acute sequelae included cardiovascular, coagulation and haematologic, gastrointestinal, kidney, mental health, metabolic, musculoskeletal and neurologic disorders. Similar observations were noted in comparisons with the historical and vaccinated controls.
Differential diagnosis
Long COVID must be differentiated from certain other disease entities like myalgic encephalomyelitis or chronic fatigue syndrome (ME/CFS), mastcell activation syndrome (MCAS) and POTS due to other causes55. The ME/CFS lasts for ≥6 months after possible triggers such as stress or viral infection. It is characterized by fatigue associated with the occurrence of at least four of the following, namely, headache, myalgia, joint pain, post-exertional malaise (PEM), sore throat, tender lymph nodes, unrefreshing sleep, cognitive impairment or orthostatic intolerance55.
The MCAS is a recurrent and chronic condition. It is characterized by multi-organ involvement, such as, respiratory (wheezing), cardiovascular (hypotensive syncope, near syncope or tachycardia), naso-ocular (conjunctival injection, pruritus or nasal stuffiness), cutaneous (urticaria, angioedema or flushing), gastrointestinal (diarrhoea, nausea, vomiting or abdominal cramp), musculoskeletal (muscle or joint pain) systems, neuropsychiatric (headache, anxiety, sleeplessness or cognitive impairments), and occurrence of systemic or constitutional symptoms (fatigue, aesthenia or fever). There is increased serum total tryptase (or other suitable biomarkers for MCAS)55.
Laboratory abnormalities
As such there are no laboratory abnormalities which can confirm long COVID. The perturbations that are encountered during the acute stage (like increased neutrophils, low lymphocyte count, increased D-dimer; anaemia, thrombocytosis, coagulation abnormalities, dyslipidaemia, abnormal liver function tests, hypoalbuminaemia (<3.5g/dl), electrolyte abnormalities), may take variable time to revert to normal depending upon host-immune response. These do not constitute to be part of long COVID. Other laboratory abnormalities evident in long COVID include increased lipocalin-2 (LCN-2), matrix metalloprotease-7 (MMP-7) and hepatocyte growth factor (HGF). It is yet to be established whether these manifestations are cause or consequence of long COVID13,24,33-51. New onset diabetes mellitus or uncontrolled hyperglycaemia due to pancreatic involvement and elevated glycosylated haemoglobin (HbA1c) all need to be carefully monitored. The role of complement biomarkers in the diagnosis of long COVID is being explored56,57. Recent studies56 have demonstrated that a combination of four complement biomarkers, namely the activation fragments iC3b, terminal complement complex (TCC), Ba and C5a, had a predictive power of 0.785 diagnosing long COVID. In light of these findings, the potential of currently available/newly discovered inhibitors of complement activation in treating long COVID needs to be explored. A recent study of blood transcriptomics has revealed the persistence of SARS-CoV-2 RNA and candidate biomarkers in long COVID patients. A two-gene biomarker, including FYN and SARS-CoV-2 antisense RNA, correctly classified long COVID (48 patients vs. 12 controls with 93.8% sensitivity and 91.7% specificity). Specific immune transcripts and immune-metablosim scores correlated with systemic viral load and patient-reported anxiety and depression, providing mechanistic links and therapeutic target sin long COVID58.
Management
There is still a significant gap in the management of long COVID despite the frequent issuance of guidelines by several organizations. Patients with long COVID can be clinically categorized as mild, moderate and severe59 depending upon their disease severity, and appropriate diagnostic testing, including imaging and follow up, should be done. These patients should be offered an integrated management plan with targeted intervention based on clinical and appropriate laboratory assessment. Three-tier rehabilitation service model has been proposed depending on patients’ requirements. This model includes a hospital-based multidisciplinary specialist team (for evaluating of moderate and severe long COVID patients) and a community therapy team. The community therapy team facilitates regular follow up of stable patients and self-management at home.
Clinical assessment for organ involvement in long COVID and management algorithmis described in Supplementary Material: Box60 and Figure 37,61,62, respectively. Careful attention should be given to underlying lung conditions (e.g., post-tuberculosis sequelae, interstitial lung disease) as these have a bearing on long-term domiciliary oxygen therapy requirements. Ensuring optimum control of co-morbid conditions (e.g., diabetes mellitus, hypertension) is equally important.

- Algorithm for long COVID management.CBC, complete blood count; LFT, liver function tests; KFT, kidney function tests; CRP, C-reactive protein; DLCO, diffusing capacity of the lungs for carbon monoxide; NT-pro-BNP, N-terminal prohormone of brain natriuretic peptide. Source: Ref 7,59,60.
Several clinical drug trials are underway [40 research projects sponsored by the NIH, USA and 4 by the National Institute for Health and Care Research (NIHCR), UK] and their results are eagerly awaited. At present, no effective drug treatment is available for long COVID63. A list of potential drugs for symptomatic treatment is provided in Table V64,65. The role of antifibrotic treatment for lung fibrosis is not established. Naltrexone, along-lasting opioid antagonist, has been repurposed in a recent single-centre pre-post interventional study (n=52) and has shown some promise in long COVID patients66. Oral low-dose naltrexone (LDN) prescribed for 2 months (1 mg daily for the first month, followed by 2 mg daily in the second month) resulted in significant improvement in recovery from COVID-19, limitation in activities of daily living (ADL), energy, pain, concentration levels and sleep disturbance (all P≤0.001) without showing significant improvement in mood (P=0.054).
| Drug | Drug class | Proposed mechanism of action |
|---|---|---|
| Antivirals | ||
| Favipiravir* | SARS-CoV-2 RNA-dependent RNA polymerase inhibitor | Viral clearance or reduction in inflammation |
| Nirmatrelvir/ritonavir (Paxlovid)* | Protease inhibitor | Viral clearance or reduction in inflammation |
| Remdesivir VV116 |
SARS-CoV-2 RNA-dependent RNA polymerase inhibitor Oral analogue of remdesivir (deuterated remdesivir hydrobromide) |
Viral clearance Viral clearance |
| Cardiac agents | ||
| Ivabradine | HCN channel blocker | Controls heart rate in POTS |
| Metoprolol succinate | Beta-1receptorantagonist | Improves cardiac function |
| Anti-inflammatory agents | ||
| Cannabinoid-containing formulations* | Cannabinoids | Anti-inflammatory |
| Imatinib* | TKI | Anti-inflammatory |
| Infliximab* | TNF-alpha inhibitor | Anti-inflammatory |
| Low dose naltrexone | Opioid antagonist | Anti-inflammatory |
| Pentoxifylline | Xanthine derivative haemorrheologic agent | Anti-inflammatory/immunomodulator vasodilator |
| Ibudilast* | Phosphodiesterase inhibitor | Blocks inflammatory pathways |
| Fluvoxamine | SSRI | Improves parosmia |
| Vitamin D | Dietary supplement/immunomodulator | For post-COVID-19 vitamin D deficiency for immunomodulation |
| Respiratory agents | ||
| LYT-100 (deupirfenidone)* | Antifibrotic | Antifibrotic/anti-inflammatory |
| Montelukast | Leukotriene receptor antagonists | Improves respiratory symptoms of long COVID |
| S-1226* | Bronchodilator | Improves respiratory symptoms of long COVID symptoms |
| Others | ||
| AXA1125* | Endogenous metabolic modulator | Improves muscle function |
| TNX-102 (cyclobenzaprine)* | Muscle relaxant | Reduces multisite pain |
| Lithium carbonate | Antimanic agent | Improves fatigue & brain fog |
| RSLV-132* | RNase-Fc fusion protein | Lessens fatigue |
| Temelimab* | Monoclonal antibody | Improves cognitive functioning |
| Vortioxetine | SSRI | Improves cognitive functioning |
| Pimozide | Dopamine receptor antagonist | Treats tinnitus |
| Somatropin | Growth hormone | Resolves associated growth hormone secretion disorder |
HCN, hyperpolarisation-activated cyclic nucleotide-gated; RNA, ribonucleic acid; POTS, postural orthostatic tachycardia syndrome; TKI, tyrosine kinase inhibitor; SSRI, selective serotonin reuptake inhibitor; RNase, ribonuclease; S-1226, a mixture of perfluorooctylbromide (PFOB) nebulized with a medical gas mixture containing CO2; AXA1125, a mixture of five specific amino acids (leucine, isoleucine, valine, arginine & glutamine) & N-acetyl-L-cysteine; RSLV-132, human RNase fused to the Fc domain of human IgG1
Source: Ref 61, 62
A personalized rehabilitation plan should be worked out for long COVID patients. Early institution of rehabilitation has been observed to be a key factor for functional independence and long-term recovery of patients with long COVID.
Research gaps
There are no systematic well-planned, designed research studies on long COVID with adequate control subjects. These are all descriptive observational studies conducted in a convenient sample. Most of these are from high-income countries34,38,39,41,42,44 that have evaluated long COVID in survivors among hospitalized populations with fewer studies37,45,51,67 from low and middle- income countries, including India. Sparse data are available on long COVID stratified on ethnicity. The therapeutic potential of interventions for clinical phenotypes of long COVID that have been defined recently, namely chronic fatigue-like syndrome, headache, and memory loss; respiratory syndrome, characterized by cough and dyspnoea; chronic pain; and neurosensorial syndrome (altered taste and smell) and their impact on quality of life merits further study68.
Challenges ahead
A recently published meta-analysis69 reported the occurrence of post-COVID symptoms at two years after SARS-CoV-2 infection in 30 per cent of patients. Fatigue (28%, 95% CI: 12–47), cognitive impairments (27.6%, 95% CI: 12.6–45.8) and pain (8.4%, 95%CI: 4.9–12.8) were the most prevalent post-COVID symptoms. SARS-CoV-2 has been rapidly evolving with the emergence of multiple variants with increased transmissibility and less virulence and/or efficiency to evade vaccination protection. Identifying predictors for early recognition of the subset of individuals, likely to develop long COVID is needed as early institution of appropriate rehabilitation will ensure a good quality of life and facilitate recovery of functional independence.
There is a need to set up multidisciplinary, single-stop clinics to monitor and follow-up COVID-19 survivors for the development long COVID. For developing a standardized diagnostic algorithm, a systematic referral system from primary to secondary, tertiary and quaternary levels of care is crucial for prioritization and optimal management of high-risk patients. Development of standardized treatment and rehabilitation guidelines are urgently required for long COVID. A standardized reporting system is also required for long COVID.
Outbreaks of previous viral infections like SARS, MERS, etc., also have left long-term sequelae before the COVID-19 era. But, these have lasted albeit for a shorter period. In the initial period of the current COVID-19 pandemic, the variants of concern were lethal and caused high mortality, with a significant proportion of patients developing long COVID. As the pandemic evolved, the availability of vaccines and the emergence of mutant strains of the virus later in the pandemic produced less severe disease. COVID-19 survivors need to be prospectively followed up in long-term studies to ascertain the long COVID prevalence.
Financial support & sponsorship
None.
Conflicts of Interest
None.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing of the manuscript and no images were manipulated using AI.
References
- Long-term clinical outcomes in survivors of severe acute respiratory syndrome and Middle East respiratory syndrome coronavirus outbreaks after hospitalisation or ICU admission: A systematic review and meta-analysis. J Rehabil Med. 2020;52:jrm00063.
- [Google Scholar]
- Report: What does COVID-19 recovery actually look like?. Available from: https://patientresearchcovid19.com/research/report-1/, accessed on November 16, 2022.
- Long COVID definitions and models of care : a scoping review. Ann Intern Med. 2024;177:929-40.
- [Google Scholar]
- Chronic COVID syndrome: Need for an appropriate medical terminology for long-COVID and COVID long-haulers. J Med Virol. 2021;93:2555-6.
- [Google Scholar]
- COVID-19: From an acute to chronic disease? Potential long-term health consequences. Crit Rev Clin Lab Sci. 2021;58:297-310.
- [Google Scholar]
- COVID-19 rapid guideline: managing the long-term effects of COVID-19. London: National Institute for Health and Care Excellence (NICE); 2020.
- Post COVID-19 condition (Long COVID). Available from: https://www.who.int/europe/news-room/fact-sheets/item/post-covid-19-condition, accessed on January 29, 2024.
- Post-COVID conditions: Information for healthcare providers. Available from: https://archive.cdc.gov/#/details?url=https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/post-covid-conditions.html, accessed on August 10, 2024.
- COVID-19 research. What is Long COVID?. Available from: https://public4.pagefreezer.com/browse/NIH%20COVID-19%20Research/18-07-2024T11:42/https://covid19.nih.gov/covid-19-topics/long-covid, accessed on January 29, 2024.
- National comprehensive guidelines for management of post-COVID respiratory sequelae. Available from: https://www.mohfw.gov.in/pdf/NationalComprehensive GuidelinesforManagementofPostCovidSequelae.pdf, accessed on January 29, 2024.
- Global prevalence of post COVID-19 condition or long COVID: A meta-analysis and systematic review. J Infect Dis. 2022;226:1593-607.
- [Google Scholar]
- Persistence of somatic symptoms after COVID-19 in the Netherlands: an observational cohort study. Lancet. 2022;400:452-61.
- [Google Scholar]
- Prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK: 1 April 2021. Available from: https://www.ons.gov.uk/ peoplepopulationandcommunity/healthandsocialcare/conditions anddiseases/bulletins/prevalenceofongoingsymptomsfollowing coronaviruscovid19infectionintheuk/1april2021, accessed July 22, 2023.
- Post acute sequelae of SARS-CoV-2 infection in the pre-delta, delta, and omicron eras. N Engl J Med. 2024;391:515-25.
- [Google Scholar]
- Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21:133-46.
- [Google Scholar]
- Serotonin reduction in post-acute sequelae of viral infection. Cell. 2023;186:4851-4867.e20.
- [Google Scholar]
- Risk of long COVID associated with delta versus omicron variants of SARS-CoV-2. Lancet. 2022;399:2263-4.
- [Google Scholar]
- Early clues regarding the pathogenesis of long-COVID. Trends Immunol. 2022;43:268-70.
- [Google Scholar]
- Health outcomes in people 2 years after surviving hospitalisation with COVID-19: a longitudinal cohort study. Lancet Respir Med. 2022;10:863-76.
- [Google Scholar]
- A comparison study of SARS-CoV-2 IgG antibody between male and female COVID-19 patients: A possible reason underlying different outcome between sex. J Med Virol. 2020;92:2050-4.
- [Google Scholar]
- Female gender is associated with long COVID syndrome: A prospective cohort study. Clin Microbiol Infect. 2022;28:611.e9-611.e16.
- [Google Scholar]
- Risk factors associated with post-COVID-19 condition: A systematic review and meta-analysis. JAMA Intern Med. 2023;183:566-80.
- [Google Scholar]
- Long COVID is associated with severe cognitive slowing: a multicentre cross-sectional study. EClinicalMedicine. 2024;68:102434.
- [Google Scholar]
- Long-term dyspnea, regional ventilation distribution and peripheral lung function in COVID-19 survivors: a 1 year follow up study. BMC Pulm Med. 2022;22:408.
- [Google Scholar]
- Health-related quality of life profiles, trajectories, persistent symptoms and pulmonary function one year after ICU discharge in invasively ventilated COVID-19 patients, a prospective follow-up study. Respir Med. 2021;189:106665.
- [Google Scholar]
- Prevalence, risk factors and treatments for post-COVID-19 breathlessness: A systematic review and meta-analysis. Eur Respir Rev. 2022;31:220071.
- [Google Scholar]
- Mild respiratory COVID can cause multi-lineage neural cell and myelin dysregulation. Cell. 2022;185:2452-2468.e16.
- [Google Scholar]
- Symptoms and health outcomes among survivors of COVID-19 infection 1 year after discharge from hospitals in Wuhan, China. JAMA Netw Open. 2021;4:e2127403.
- [Google Scholar]
- Follow-up study on COVID-19 survivors one year after discharge from hospital. Int J Infect Dis. 2021;112:173-82.
- [Google Scholar]
- 1-year outcomes in hospital survivors with COVID-19: A longitudinal cohort study. Lancet. 2021;398:747-58.
- [Google Scholar]
- Characterising the long-term clinical outcomes of 1190 hospitalised patients with COVID-19 in New York City: a retrospective case series. BMJ Open. 2021;11:e049488.
- [Google Scholar]
- Prevalence, pattern and functional outcome of post COVID-19 syndrome in older adults. Cureus. 2021;13:e17189.
- [Google Scholar]
- Long-term clinical follow-up of patients suffering from moderate-to-severe COVID-19 infection: A monocentric prospective observational cohort study. Int J Infect Dis. 2021;109:209-16.
- [Google Scholar]
- Post-acute sequelae of COVID-19 six to 12 months after infection: population based study. BMJ. 2022;379:e071050.
- [Google Scholar]
- Outcomes among confirmed cases and a matched comparison group in the Long-COVID in Scotland study. Nat Commun. 2022;13:5663.
- [Google Scholar]
- Author correction: Outcomes among confirmed cases and a matched comparison group in the long-COVID in Scotland study. Nat Commun. 2022;13:6540.
- [Google Scholar]
- Persistence, prevalence, and polymorphism of sequelae after COVID-19 in unvaccinated, young adults of the Swiss Armed Forces: a longitudinal, cohort study (LoCoMo) Lancet Infect Dis. 2022;22:1694-702.
- [Google Scholar]
- Post-COVID-19 tachycardia syndrome: A distinct phenotype of post-acute COVID-19 syndrome. Am J Med. 2021;134:1451-6.
- [Google Scholar]
- Severe fatigue and persistent symptoms at 3 months following severe acute respiratory syndrome coronavirus 2 infections during the pre-delta, delta, and omicron time periods: A multicenter prospective cohort study. Clin Infect Dis. 2023;76:1930-41.
- [Google Scholar]
- Long COVID following Omicron wave in Eastern India – A retrospective cohort study. J Med Virol. 2023;95:e28214.
- [Google Scholar]
- Long COVID among moderate to severe COVID-19 cases in India during second wave of COVID-19. Asia Pac J Public Health. 2022;34:846-8.
- [Google Scholar]
- Initial COVID-19 Severity and long-COVID manifestations: An observational analysis. Thorac Res Pract. 2023;24:22-8.
- [Google Scholar]
- Long-term quality of life and work ability among severe COVID-19 survivors: A multicenter study. Dialogues Health. 2023;2:100124.
- [Google Scholar]
- Incidence and characterization of post-COVID-19 symptoms in hospitalized COVID-19 survivors to recognize syndemic connotations in India: Single-center prospective observational cohort study. JMIR Form Res. 2023;7:e40028.
- [Google Scholar]
- Characterization and predictive risk scoring of long COVID in a south Indian cohort after breakthrough COVID infection; a prospective single centre study. BMC Infect Dis. 2023;23:670.
- [Google Scholar]
- Post COVID-19 sequelae: A prospective observational study from Northern India. Drug Discov Ther. 2021;15:254-60.
- [Google Scholar]
- Impact of COVID-19 vaccination on the risk of developing long-COVID and on existing long-COVID symptoms: A systematic review. E Clinical Medicine. 2022;53:101624.
- [Google Scholar]
- Long COVID prevalence and the impact of the third SARS-CoV-2 vaccine dose: A cross-sectional analysis from the third follow-up of the Borriana Cohort, Valencia, Spain (2020-2022) Vaccines (Basel). 2023;11:1590.
- [Google Scholar]
- Long COVID or post-COVID-19 syndrome: Putative pathophysiology, risk factors, and treatments. Infect Dis (Lond). 2021;53:737-54.
- [Google Scholar]
- Complement dysregulation is a prevalent and therapeutically amenable feature of long COVID. Med. 2024;5:239-253.e5.
- [Google Scholar]
- Persistent complement dysregulation with signs of thromboinflammation in active long covid. Science. 2024;383:eadg7942.
- [Google Scholar]
- Blood transcriptomic analyses reveal persistent SARS-CoV-2 RNA and candidate biomarkers in post-COVID conditions. Lancet Microbe. 2024;5:100849.
- [Google Scholar]
- The Post-COVID-19 Functional Status Scale: A tool to measure functional status over time after COVID-19. Eur Respir J. 2020;56:2001494.
- [Google Scholar]
- Developing services for long COVID: lessons from a study of wounded healers. Clin Med (Lond). 2021;21:59-65.
- [Google Scholar]
- COVID-19 rapid guideline: managing the long-term effects of COVID-19. Available from: https://www.nice.org.uk/guidance/NG188, accessed on January 29, 2024.
- Clinical trials on the pharmacological treatment of long COVID: A systematic review. J Med Virol. 2023;95:e28289.
- [Google Scholar]
- Therapeutic trials for long COVID-19: A call to action from the interventions taskforce of the RECOVER initiative. Front Immunol. 2023;14:1129459.
- [Google Scholar]
- VV116 versus nirmatrelvir-ritonavir for oral treatment of Covid-19. N Engl J Med. 2023;388:406-17.
- [Google Scholar]
- Safety and efficacy of low dose naltrexone in a long covid cohort: An interventional pre-post study. Brain Behav Immun Health. 2022;24:100485.
- [Google Scholar]
- Identification of spectrum of persistent post-COVID-19 symptoms and their duration in Central India: A pilot study. J Family Med Prim Care. 2022;11:7850-6.
- [Google Scholar]
- Clinical phenotypes and quality of life to define post-COVID-19 syndrome: A cluster analysis of the multinational, prospective ORCHESTRA cohort. EClinicalMedicine. 2023;62:102107.
- [Google Scholar]
- Persistence of post-COVID symptoms in the general population two years after SARS-CoV-2 infection: A systematic review and meta-analysis. J Infect. 2024;88:77-88.
- [Google Scholar]






