Translate this page into:
Isolation of bacteria from diabetic foot ulcers with special reference to anaerobe isolation by simple two-step combustion technique in candle jar
Reprint requests: Dr Prasanta Kumar Maiti, Department of Microbiology, Institute of Post Graduate Medical Education & Research, Kolkata 700 020, West Bengal, India e-mail: pkmaitiipgmer@gmail.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Although polymicrobial infections involving both aerobic and anaerobic bacteria are very common in diabetic foot ulcers, in many centres of developing countries, anaerobes are rarely isolated due to technical difficulties. This can be overcome by using a new simple, innovative technique of a combination of candle combustion and use of acidified copper-coated steel wool, as reported here.
Methods:
In-house developed method was used in a prospective clinico-microbiological study for anaerobes from randomly selected 43 patients with diabetic foot ulcers along with conventional method of anaerobic culture in GasPak system and aerobic culture by standard laboratory procedures. For primary isolation of anaerobes, Brucella blood agar supplemented with hemin (5 μg/ml) and menadione (1 μg/ml) was used. Antibiotic sensitivity tests were performed by the standard disc diffusion method for aerobes and E-test method for anaerobes.
Results:
All the 43 samples were culture positive, of which aerobic Gram-negative bacteria (GNB) predominated, followed by Staphylococcus aureus, Enterococcus and diphtheroids. Anaerobes isolated from 21 samples were Peptostreptococcus, Bacteroides, Porphyromonas, Veillonella spp. and Clostridium perfringens by both GasPak and in-house developed and modified candle jar techniques. Imipenem and metronidazole were most sensitive while clindamycin, penicillin and cefoxitin were least sensitive drugs for anaerobes. Aerobic GNB were found to be multidrug resistant, especially to penicillin and cephalosporins. The most sensitive drug was piperacillin-tazobactam.
Interpretation & conclusions:
For isolation of anaerobes from clinical specimens such as diabetic foot ulcers, modified candle jar technique was found to be as reliable as GasPak system. This modified technique needs to be tested for many other clinical materials which are not yet evaluated.
Keywords
Anaerobiosis
diabetic foot ulcer
modified candle jar technique
oxygen reduction
Wounds of diabetic foot very often get infected due to several factors including high blood sugar level, suppressed immunity, inadequate blood supply and neuropathy1. Usually, such infections are polymicrobial where anaerobic bacteria co-exist with aerobic organisms2. In these cases, anaerobes often complicate the long-standing ulcers by producing necrotic materials and foul odour3. Yet from the clinical specimens, usually only aerobic organisms are isolated due to technical difficulties and limited resources. Modified candle jar technique, developed and validated for culture of anaerobes4, can be a simpler alternative for such cases. This study was undertaken to apply modified candle for method to isolate anaerobes from the samples of diabetic foot ulcer.
Material & Methods
In collaboration with the Endocrinology department of Institute of Post Graduate Medical Education & Research, Kolkata, a tertiary care hospital in India, this clinico-microbiological study on diabetic foot infection was carried out from May to October 2013 in the Microbiology department. Forty three patients with diabetes and deep ulcers, osteomyelitis or severe infections on the feet were randomly selected. The grading of ulcers was done based on Wagner's classification5. Samples were collected for microbiological studies during debridement of the wounds so that deep tissue samples could be collected. Bone and tissue pieces were also obtained wherever possible. If these were not available, swabs from the deeper tissues, were collected.
Samples were inoculated immediately at the bedside, on pre-reduced Brucella blood agar (Hi-Media, India) plates enriched with 5 μg/ml hemin and 1 μg/ml menadione. Each plate was immediately put inside the modified candle jar, and before closing the jar lid, anaerobiosis was initiated by lighting a small white wax candle and putting 5 g of acidified copper-coated steel wool on an open plate kept inside. This simple in-house developed method was standardized earlier5 and was found suitable for the initiation of anaerobiosis at bedside. Simultaneously, a separate inoculated plate was placed in a jar with GasPak system (Anaerogas Pack- Hi-Media, India) and another inoculated plate for aerobic incubation.
After 48 h of incubation at 37°C, the anaerobic plates were examined for growth and used for aero-tolerance study by aerobic incubation on blood agar plate after subculture. Colony morphology was noted and bacterial morphology was observed from Gram-stained smears. Aerobic bacteria were identified based on the results of standard biochemical tests6. The sensitivity tests were performed by modified Kirby–Bauer disk diffusion method following the Clinical and Laboratory Standards Institute guideline, 20137. Suspected anaerobic isolates, verified by aero-tolerance study, were put into a fresh set of modified candle jars to perform biochemical tests8. The biochemical tests included fermentation, indole, nitrate disk reduction, catalase and urease tests. Special-potency disk test (vancomycin, 5 μg; kanamycin, 1000 μg; and colistin, 10 μg)8, sodium polyanethol sulphonate disk test8, bile esculin hydrolysis test, lipase and lecithinase test, pigment production test and colony observation of fluorescence study were also included for presumptive identification of anaerobes up to the genus level8. Isolated anaerobes were tested for antibiotic susceptibility by the E-test9 (BioMérieux, France) in the same modified candle jar system. Antibiotics tested were metronidazole, clindamycin, cefoxitin, imipenem and penicillin.
Results
Thirty five of the 43 patients had poor glycaemic control and 11 had osteomyelitic features. All were culture positive. A total of 80 isolates were detected from 43 patients. In 27 (62.78%) cases, more than one microorganism was isolated and single microorganism from the remaining 16 (37.2%) cases. Of the 43 patients, 22 (51.16%) were infected by aerobes only and 20 (46.51%) patients were infected by both aerobic and anaerobic organisms. Anaerobe was isolated as pure growth from only one patient. Both in the Gas-pack and modified candle jar methods, growth of anaerobic organisms was comparable. Of the 11 cases with features of osteomyelitis, anaerobes were isolated from nine cases.
Of the 80 isolates, 59 (73.75%) were aerobic organisms and 21 (26.25%) were anaerobic organisms. Among anaerobes, the most common isolates were Peptostreptococcus spp., (n=9, 42.85%); followed by Bacteroides spp., (n=6, 28.57%); Veillonella spp., (n=3, 14.28%), Porphyromonas spp., (n=2, 9.52%) and Clostridium perfringens from one sample (Table I).
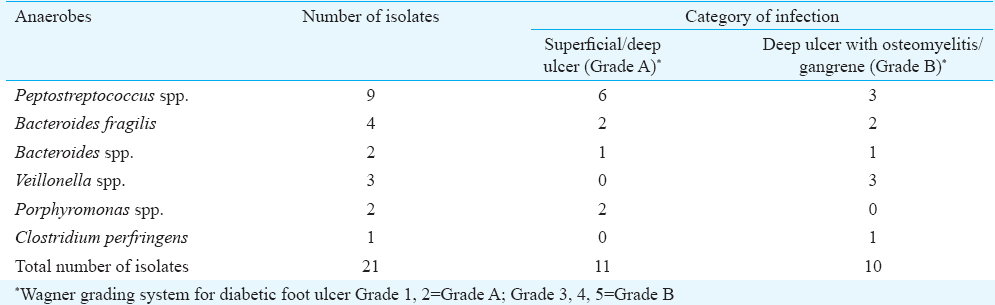
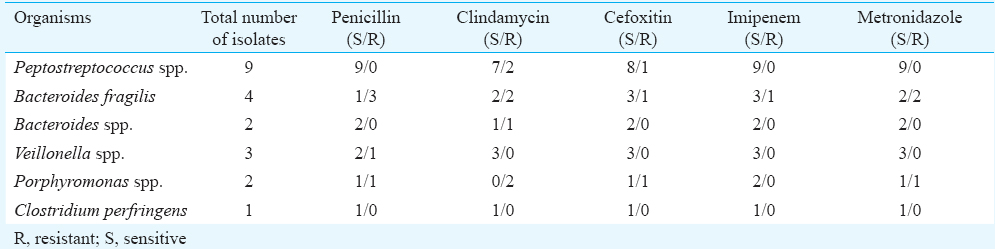
Of the 21 anaerobic isolates, 8 (38.09%) were resistant to clindamycin, followed by 5 (23.81%) to penicillin and 4 (19.05%) to cefoxitin. Imipenem and metronidazole had lowest resistance rates at 4.76 and 14.29 per cent, respectively. All Gram-positive anaerobes were sensitive to penicillin, metronidazole and imipenem. All Gram-negative anaerobic Cocci (n=3) isolated were sensitive to all antibiotics except one isolate which showed resistance to penicillin. Gram-negative bacilli showed higher resistance to clindamycin (62.5%) and penicillin (50%) followed by cefoxitin (25%). Only one isolate of Bacteroides group showed resistance to all antibiotics (Table II).
Among the 59 aerobic organisms, 38 (49.32%) were Gram-negative and 21 (27.27%) were Gram-positive. The most commonly isolated aerobic organisms were Proteus spp., (n=19, 32.20%); followed by Staphylococcus aureus, (n=12, 20.33%); Klebsiella spp., (n=11, 18.64%); Enterobacter spp., (n=3, 5.08%); Pseudomonas spp., (n=2, 3.38%); Escherichia coli, (n=2, 3.38%); Enterococcus spp., (n=5, 10.20%); diphtheroids, (n=4, 8.16%) and Citrobacter including imipenem, co-trimoxazole, tetracyclin espp., (n=1, 1.6%).
The aerobic Gram-negative organisms were found to be highly drug resistant. The first-line antibiotics tested were amikacin, amoxicillin, amoxicillin-clavulanic acid, cefotaxime, cefpodoxime, piperacillin-tazobactam and levofloxacin. Of these, piperacillin-tazobactam was found to be most sensitive (84%), followed by levofloxacin (72%) and amikacin (56%). Maximum resistance was observed with amoxycillin (92%), followed by amoxycillin-clavulanic acid (60%) and cephalosporins (72%).
Eight isolates of aerobic Gram-negative bacilli were found to be resistant to almost all the first-line drugs and were tested for the second-line antibiotics, including imipenem, co-trimoxazole, tetracycline and polymyxin B (not for Proteus spp.). Of these, seven were found to be sensitive to imipenem. All organisms tested for polymyxin B were sensitive.
The Staphylococcus isolates were tested against amoxycillin, cefoxitin, vancomycin, linezolid, oxacillin and piperacillin-tazobactam. All except one were found to be sensitive to all these drugs. One organism showed reduced sensitivity to amoxicillin and identified as methicillin-resistant S. aureus (MRSA) by cefoxitin resistance.
Enterococcus isolates were tested against amoxycillin, vancomycin, linezolid, gentamicin and piperacillin-tazobactam. All were sensitive to all the tested drugs. All four diphtheroid isolates were sensitive to amoxycillin, vancomycin, linezolid, cefoxitin, cefotaxime and piperacillin-tazobactam.
Discussion
By using modified, simple, cost-effective method4 for anaerobic culture, 26.25 per cent of obligate anaerobes were isolated from diabetic foot ulcer cases. There was a large proportion of polymicrobial infections with both aerobes and anaerobes. Gram-negative aerobic bacilli were found to be predominant and most of them were resistant to antibiotics. Piperacillin-tazobactam combination was found to be the most effective antibiotic. However, our study was conducted mainly in patients with advanced stage of ulcers; many of them had been previously treated. This could explain the high degree of antibiotic resistance amongst the isolates as well as low anaerobe isolation, as most of such pathogens are amenable to treatment with common antibiotics.
In a study by Colayco et al10, of the 126 ulcers examined, 24 per cent of isolates were strict anaerobes. Chopped meat broth in anaerobic GasPak jars was used in their study for primary isolation of anaerobes. The most commonly isolated anaerobes were Peptostreptococcus spp. in 27 per cent of patients, followed by Actinomyces israelii in 13 per cent of cases. Of the 29 anaerobes tested, 48 per cent were resistant to metronidazole and 24 per cent to clindamycin. Imipenem and ampicillin-sulbactam had the lowest resistant rates at 3.4 per cent each. Banoo et al11 reported 11.77 per cent anaerobic isolates from diabetic foot ulcer cases. Robertson's cooked meat broth was used for primary inoculation and anaerobic jar for anaerobe isolation and identification. The predominant anaerobic organisms were Peptostreptococcus spp. (45.5%), whereas Pseudomonas spp. (21.9%) was the most common aerobic organism followed by Klebsiella spp. (19.4%). All the aerobic Gram-negative organisms were sensitive to imipenem (100%). Gram-positive organisms were 100 per cent sensitive to vancomycin. MRSA was detected in 66.7 per cent of cases. All the anaerobes were sensitive to metronidazole, clindamycin, cefoxitin and penicillin G11.
In a study from Singapore12, 102 strains (79%) of strict anaerobic bacteria were isolated in an anaerobic chamber from 30 specimens of diabetic foot ulcers. The predominant anaerobic isolates were Peptostreptococcus spp. (46%) and Bacteroides fragilis group (19%). Antibiotic resistance was detected in 18 per cent for clindamycin, one per cent for metronidazole and two per cent for imipenem.
Gadepalli et al13 from New Delhi demonstrated that Gram-negative aerobes were most frequent isolates (51.4%), followed by Gram-positive aerobes and anaerobes, 33.3 and 15.3 per cent, respectively. Seventy two per cent of patients were positive for multidrug resistant (MDR) organisms. Extended-spectrum β-lactamase production and methicillin resistance were noted in 44.7 and 56.0 per cent of bacterial isolates, respectively. They concluded that infections with MDR organisms were common in diabetic foot ulcers and were associated with inadequate glycaemic control demanding more surgical interventions. Anandi et al14 from Tamil Nadu reported 20.27 per cent of anaerobic isolates and 79.72 per cent of aerobic isolates from diabetic foot ulcer.
Isolation of anaerobes requires special measures during collection, transportation, inoculation of specimens and handling growth, to avoid toxic oxygen (O2) exposure as much as possible. It also requires a pre-reduced enriched medium for inoculation and incubation at strict anaerobic condition. Commercially used methods for anaerobic culture15 are often costly, time-consuming as well as cumbersome and not available in most of the centres in developing countries. With the anaerobic jar technique, the anaerobe isolation rate from diabetic foot ulcers was low16. The isolation rate of anaerobic bacteria using automated anaerobic system (Anoxomat) was also low, only in 19 per cent of cases reported by Garg et al17. The impact of sampling methods and transport medium is also important for successful isolation, as a poor correlation has been obtained for culture results from superficial wound swabs compared with deep tissue or bone samples18.
An in-house developed modified candle jar technique was a cheaper and simpler alternative to a conventional GasPak system which can be applied for isolation of clinically significant anaerobes by the quick reduction of major bulk of O2 using a lighted candle in a screw-capped air-tight jar, along with slow reduction of residual 1-2 per cent O2 by acidified copper-coated steel wool. Candle combustion in a sealed jar produces 15-16 per cent of vacuum due to consumption of O2 in spite of addition of 4-5 per cent of carbon-dioxide leaving 1-2 per cent of O2 from the total 21 per cent present in the air, which makes possible to purge out a substantial part of residual O2 by the second step reaction. This minimized the risk of toxic O2 exposure to the anaerobes by averting the need for a transport medium and delay of processing. Pre-reduced medium can also be obtained ready at hand applying the same candle jar method at minimal cost and efforts.
The study had some limitations. The sample size was small. Bacterial isolation was not attempted before any antimicrobial therapy in diabetes patients. Future studies could be planned to observe relation with an incidence of microbial colonization and factors precipitating pressure ulcers in such patients.
Conflicts of Interest: Patent entitled “Anaerobic culture on modified blood agar plate kept in a two steps combustion candle jar system” applied. Application no. 191/KOL/2012 A.
References
- Detection of anaerobic infection in diabetic foot ulcer using PCR technique and the status of metronidazole therapy on treatment outcome. Wounds. 2012;24:283-8.
- [Google Scholar]
- The diabetic foot. Soft tissue and bone infection. Infect Dis Clin North Am. 1990;4:409-32.
- [Google Scholar]
- Anaerobic culture on growth efficient bi-layered culture plate in a modified candle jar using a rapid and slow combustion system. Indian J Med Microbiol. 2013;31:173-6.
- [Google Scholar]
- Mackie and McCartney Practical Medical Microbiology 1988
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; with 23rd informational supplement. CLSI document M100-S23. Wayne, PA: CLSI; 2013.
- [Google Scholar]
- Wadsworth anaerobic bacteriology manual. Belmont, California: Star Publishing Company; 1986.
- Evaluation of the E test for susceptibility testing of anaerobic bacteria. J Clin Microbiol. 1991;29:2197-203.
- [Google Scholar]
- Microbiologic and clinical profile of anaerobic diabetic foot infections. Philipp J Microbiol Infect Dis. 2002;31:151-60.
- [Google Scholar]
- Bacterial and clinical profile of diabetic foot patients. Ann Trop Med Public Health. 2012;5:69-73.
- [Google Scholar]
- Anaerobic culture of diabetic foot infections: Organisms and antimicrobial susceptibilities. Ann Acad Med Singapore. 2008;37:936-9.
- [Google Scholar]
- A clinico-microbiological study of diabetic foot ulcers in an Indian tertiary care hospital. Diabetes Care. 2006;29:1727-32.
- [Google Scholar]
- Improved isolation of anaerobic bacteria from the mouse cecum by maintaining continuous strict anaerobiosis. Proc Soc Exp Biol Med. 1967;124:903-9.
- [Google Scholar]
- Isolation, identification and antimicrobial susceptibility of anaerobic bacteria: A study re-emphasizing its role. J Clin Diagn Res. 2014;8:DL01-2.
- [Google Scholar]
- Culture of percutaneous bone biopsy specimens for diagnosis of diabetic foot osteomyelitis: Concordance with ulcer swab cultures. Clin Infect Dis. 2006;42:57-62.
- [Google Scholar]






