Translate this page into:
Intricacies of using temperature of different niches for assessing impact on malaria transmission
Reprint requests: Dr Ramesh C. Dhiman, National Institute of Malaria Research (ICMR), Sector 8, Dwarka, New Delhi 110 077, India e-mail: r.c.dhiman@gmail.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
The influence of temperature on the life cycle of mosquitoes as well as on development of malaria parasite in mosquitoes is well studied. Most of the studies use outdoor temperature for understanding the transmission dynamics and providing projections of malaria. As the mosquitoes breed in water and rest usually indoors, it is logical to relate the transmission dynamics with temperature of micro-niche. The present study was, therefore, undertaken to understand the influence of different formats of temperature of different micro-niches on transmission of malaria for providing more realistic projections.
Methods:
The study was conducted in one village each of Assam and Uttarakhand States of India. Temperatures recorded from outdoor (air) as well as indoor habitats (resting place of mosquito) were averaged into daily, fortnightly and monthly and were used for determination of transmission windows (TWs) for Plasmodium vivax (Pv) and P. falciparum (Pf) based on minimum temperature threshold required for transmission.
Results:
The daily temperature was found more useful for calculation of sporogony than fortnightly and monthly temperatures. Monthly TWs were further refined using fortnightly temperature, keeping in view the completion of more than one life cycle of malaria vectors and sporogony of malaria parasite in a month. A linear regression equation was generated to find out the relationship between outdoor and indoor temperatures and R2 to predict the percentage of variation in indoor temperature as a function of outdoor temperature at both localities.
Interpretation & conclusions:
The study revealed that the indoor temperature was more than outdoors in stable malarious area (Assam) but fluctuating in low endemic area like Uttarakhand. Transmission windows of malaria should be determined by transforming outdoor data to indoor and preferably at fortnightly interval. With daily recorded temperature, sporogonic and gonotrophic cycles can also be calculated which is otherwise not possible with monthly data. The study highlights that the projections made for malaria in view of climate change need to be seen with limitation of difference in outdoor and indoor temperatures at different locations, highlighting the need for local data generation at least at sub-district level.
Keywords
Indoor
malaria
outdoor
Plasmodium falciparum
Plasmodium vivax
temperature
transmission windows
It is well known that transmission dynamics of malaria is climate sensitive and temperature affects the development of immature stages of mosquito vectors in water bodies, extrinsic incubation period of malaria parasite in adult mosquitoes and their survival12345. With the projected threat of climate change on vector borne diseases, various studies have been undertaken in the last two decades using climatic factors for modelling the projection of malaria transmission67891011. In almost all the studies modelling has been done using monthly mean temperature of outdoors recorded by meteorological stations. Similarly, for studying the capacity of mosquitoes to transmit parasites, the researchers have considered constant temperatures412 and, therefore, the daily/fortnightly temperature variations and consequent impact on vector-parasite interactions have been overlooked. Studies on climate and malaria transmission have indicated the significance of microclimate of adult mosquitoes and diurnal temperature range on malaria transmission1314. As the immature stages of mosquitoes complete their life cycle in water and adults usually rest indoors in human dwellings1518, cattle sheds or sometimes outdoors, the air temperature recorded does not seem logical to apply uniformly to various habitats19. A study undertaken in urban area of Chennai (India) observed varying temperature in different micro-niches20. Though malaria transmission is influenced by temperature, rainfall, relative humidity (RH) and wind speed, etc., temperature is the most important limiting factor for absence/presence of vectors/malaria at a given place in spite of most suitable rainfall or RH. The present study was planned to assess the implication of outdoor versus indoor temperatures in different ecological settings using different formats of temperatures (4 hourly, daily, fortnightly and monthly) on transmission windows of malaria.
Material & Methods
Two geographically different villages, one from a malaria endemic plain area (Lahorijaan Tea Estate), under primary health centre (PHC) Bokajan, Karbi Anglong, Assam and the other from a malaria vulnerable hilly area Bhorsa, under PHC Bhimtal, Nainital, Uttarakhand, India, were selected for the study. The study villages are located >1800 km apart having varying climatic conditions, altitude, malaria endemicity and vectors. Lahorijaan is situated at 138 m altitude having Anophales minimus, An fluviatilis and increased density of An culicifacies as malaria vectors with high malaria endemicity. On the other hand, Bhorsa is a malaria vulnerable area with recent occurrence of malaria cases since 2009 (unpublished observation), located in hilly area at 609 m altitude where An. culicifacies and An. fluviatilis are the major malaria vectors. Resting An. fluviatilis, and An. culicifacies vectors were reported from indoors in district Nainital and Karbi Anglong while An. minimus was reported to be outdoor resting mosquito2122. Houses in Lahorijaan were made of usually bamboo sheet plastered with mud having tin roof while in Bhorsa, the dwellings were stony/brick made and plastered with cement. Temperature was recorded from indoors (from human dwellings as usual resting habitats of mosquitoes) and outdoors in open space (where usually the meteorological data were recorded by meteorological station). For recording the temperature, HOBO data logger (Onset, USA) were installed on walls in corner of human dwellings where mosquitoes usually rest and in open space were fixed in Stevenson screen above 2 m from the ground (i.e. two HOBOs per village) as per standard method for recording air temperature23. The study was conducted from June, 2012 to May, 2013 and October, 2012 to September, 2013 at Lahorijaan and Bhorsa, respectively. The study was launched simultaneously at both the sites but due to malfunctioning of data-loggers at Bhorsa, the date of recording temperature differed. Data on monthly incidence of malaria in the concerned PHC for the study period were collected so as to compare with determined transmission window (TW) of malaria. In Uttrakhand, Bhimtal PHC did not report any malaria case during the study period; however, stray malaria cases were recorded in 2009, 2011 and 2012 from Bhorsa (unpublished observation).
Four-hourly generated temperature data from indoor and outdoor habitats were plotted to show the daily variations within a day as well as daily (31 days) as a representative of peak malaria month i.e., July and October at Lahorijaan and Bhorsa, respectively based on data in previous years collected from concerned PHCs. Four hourly data of temperature were then averaged to daily mean temperature, fortnightly and monthly mean temperature. Transmission windows for malaria parasite development were determined following the lower cut-off of temperature ranging from 14.5 and 16°C for Plasmodium vivax (Pv) and P. falciparum (Pf) while 32°C as upper cut-off for both parasites89.
F-test one tail with 95% confidence interval was applied for comparing the variance in four hourly and daily temperatures in both habitats at both localities. To find out the correlation between these two variables (viz. outdoor temperature as independent variable and indoor temperature as dependent variable) a linear regression equation (on fortnightly and monthly basis) was generated. A coefficient of determination (R2) was derived to find out as to how much variation in indoor temperature could be explained by the relationship to outdoor temperature.
Results
Figure 1 depicts four-hourly and daily temperatures recorded indoor and outdoor, during peak malaria month of July at Lahorijaan location. The four-hourly indoor temperature remained in the range of 27- 35.5°C as minimum and maximum (mean temperature = 30.44± 2.02, variance= 4.08) while the outdoor temperature ranged from 24.4-35.5°C (mean temperature =28.83±2.75, variance= 7.61) (Fig. 1a). Within a day the four-hourly indoor temperature was more than outdoor temperature and the difference being a maximum of 4.49°C but more variation was observed in outdoor temperature (P<0.05). In outdoor, the fluctuation within a day ranged from 0.83 to 10.1°C while in indoors it was 1.16-8.2°C indicating stability of temperature in indoors. When the temperature was averaged on daily basis, the mean indoor temperature was found always more than the outdoor (Fig. 1b) and the difference ranged from 0.97-3.08°C, the maximum being in the month of November.
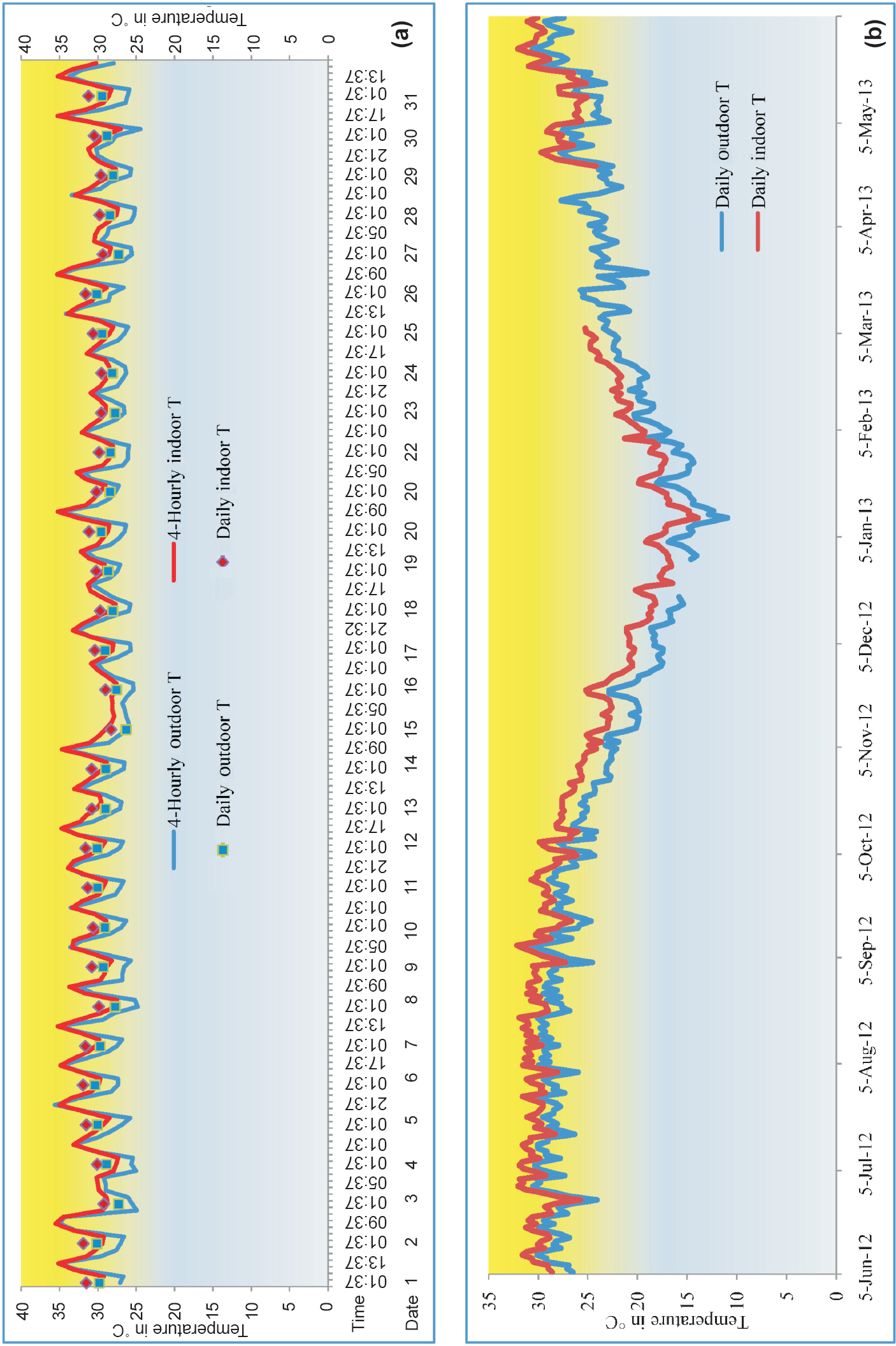
-
(a). Four-hourly and daily temperatures from outdoor and indoor habitats for peak malaria month (July 1-31, 2012), (b) Daily mean temperature from outdoor and indoor for the period of June 2012 - May-2013 at Lahorijaan Tea Estate (TE), Assam.
At Bhorsa, four-hourly indoor temperature ranged from 13.9-28.2°C (indoor mean temperature = 19.75 ± 3.46, variance = 12.0) while outdoor temperature from 12.9-30.2°C (outdoor temperature mean = 19.1 ± 4.12, variance= 16.99) which showed lower range of indoor temperature more than outdoor temperature while the upper range of temperature was more in outdoors (Fig. 2a). Within a day, the four-hourly indoor temperature was most of the time more than outdoor except day time and the maximum difference between outdoor and indoor temperatures was 8°C. Significantly more variation was observed in outdoor temperature (P<0.05). In outdoor situation, temperature within a day ranged from 2-12°C while it was 2-4°C indoors indicating less fluctuation in indoor as compared to outdoor temperature. There was fluctuation in daily indoor and outdoor temperatures from October to March (up to ±2°C). After March 2013, the daily indoor temperature remained mostly more than outdoor up to the difference of 7.3°C in the month of August 2013 (Fig. 2b).
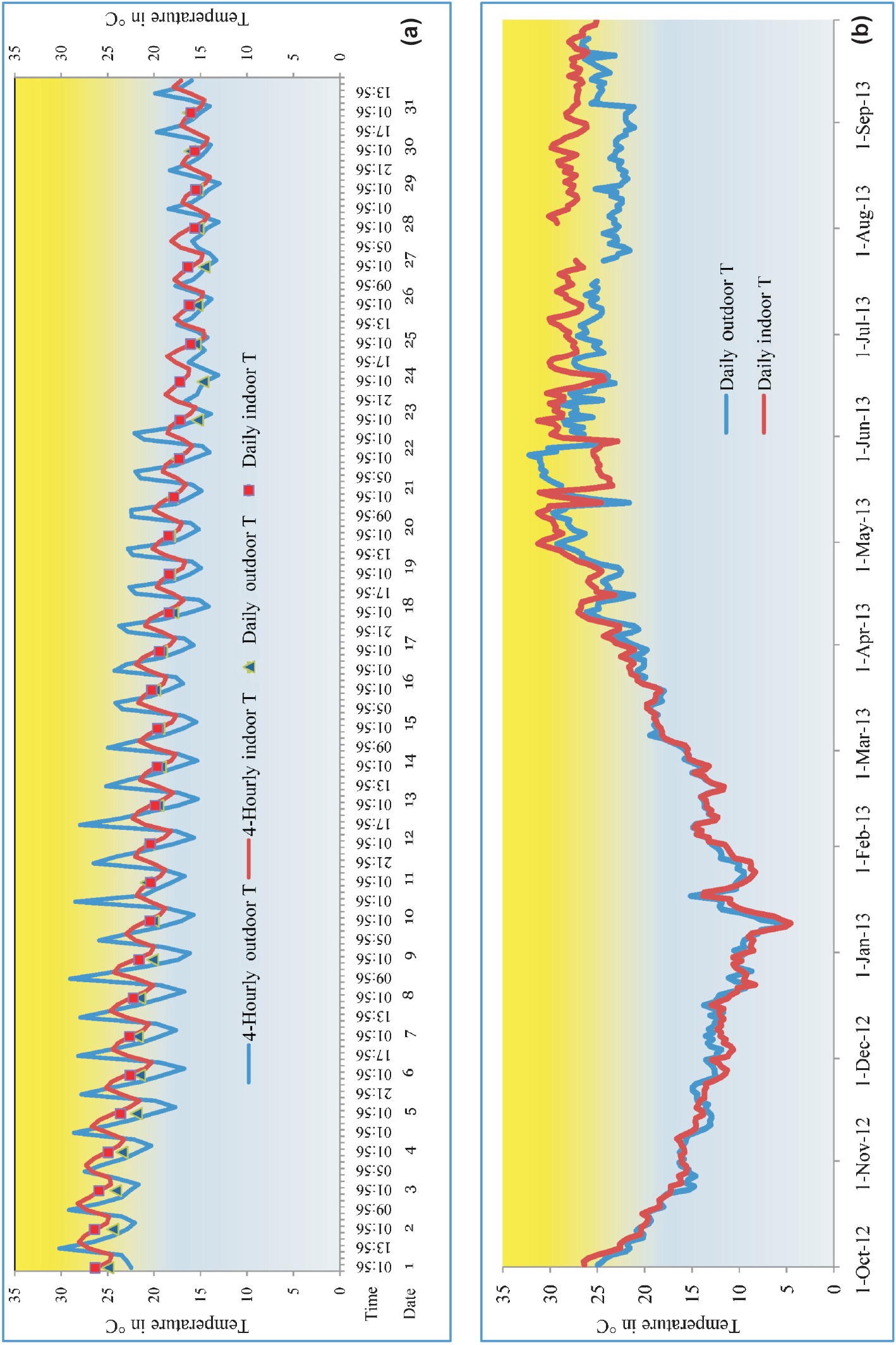
-
(a). Four-hourly and daily tempeatures from outdoor and indoor for peak malaria month (October) showing fluctuation in outdoor temperature, (b) Daily mean temperature from outdoor and indoor for the period of October-2012 - September-2013 at Bhorsa.
At Lahorijaan, determination of TWs based on monthly indoor temperature showed opening for 12 months for Pv, as compared to 11 months using outdoor temperature (Fig. 3a). On the other hand, the TWs of Pf showed opening for 12 and 10 months with indoor and outdoor temperatures, respectively (Fig. 3b). The data of temperature computed at fortnightly basis showed opening of TWs for all the 24 fortnights (12 months) using indoor temperature for Pv and Pf while with outdoor temperature, TWs were open for 23 fortnights for Pv and 21 fortnights for Pf (Fig. 3c, d). In the fortnights commencing from December 1 to January 2, indoor temperatures were 20.0, 17.8, 16.7 and 18.0°C while outdoor temperatures were 17.5, 15.1, 14.2 and 15.6°C showing that indoor temperature was more in critical colder months making all the fortnights open for malaria transmission.
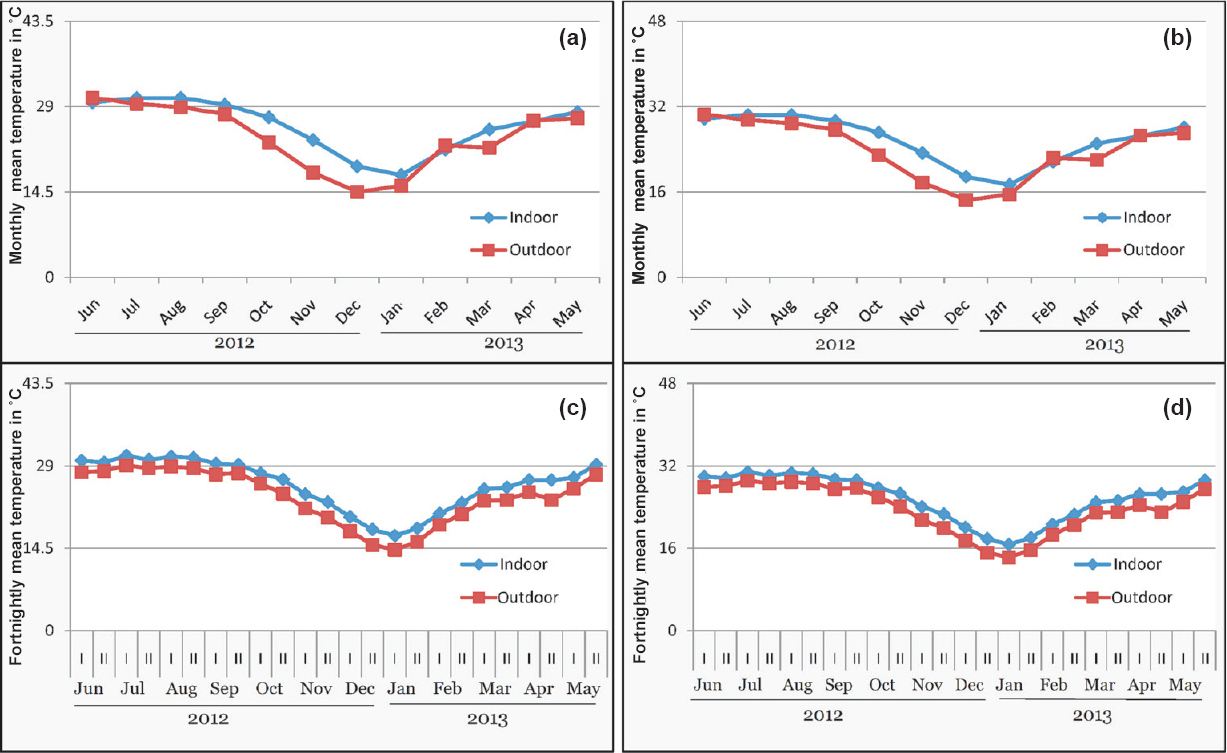
- Transmission windows (TW) of malaria based on monthly temperatures (a) Pv (b) Pf, and fortnightly temperature (c) Pv (d) Pf at Lahorijaan TE. With indoor temperature, TWs are open for 12 months / 24 fortnights while with outdoor temperature, TWs are closed for 0-2 months /1-3 fortnights for Pv & Pf, respectively. I and II represent two fortnights of a month.
At Bhorsa, with indoor and outdoor monthly temperatures, months of opening of TWs were same for Pv and Pf, i.e. closed from November to February (Fig. 4a, b). However, based on fortnightly mean indoor temperature, TWs showed opening for 17 and 16 fortnights for Pv and Pf, respectively (Fig. 4c, d). At Lahorijaan the indoor temperature was consistently more during nine months while at Bhorsa it was highly fluctuating between outdoor and indoor. When the monthly malaria data were compared with TWs based on indoor temperature it matched with the closing of TWs at Lahorijaan (Fig. 5).
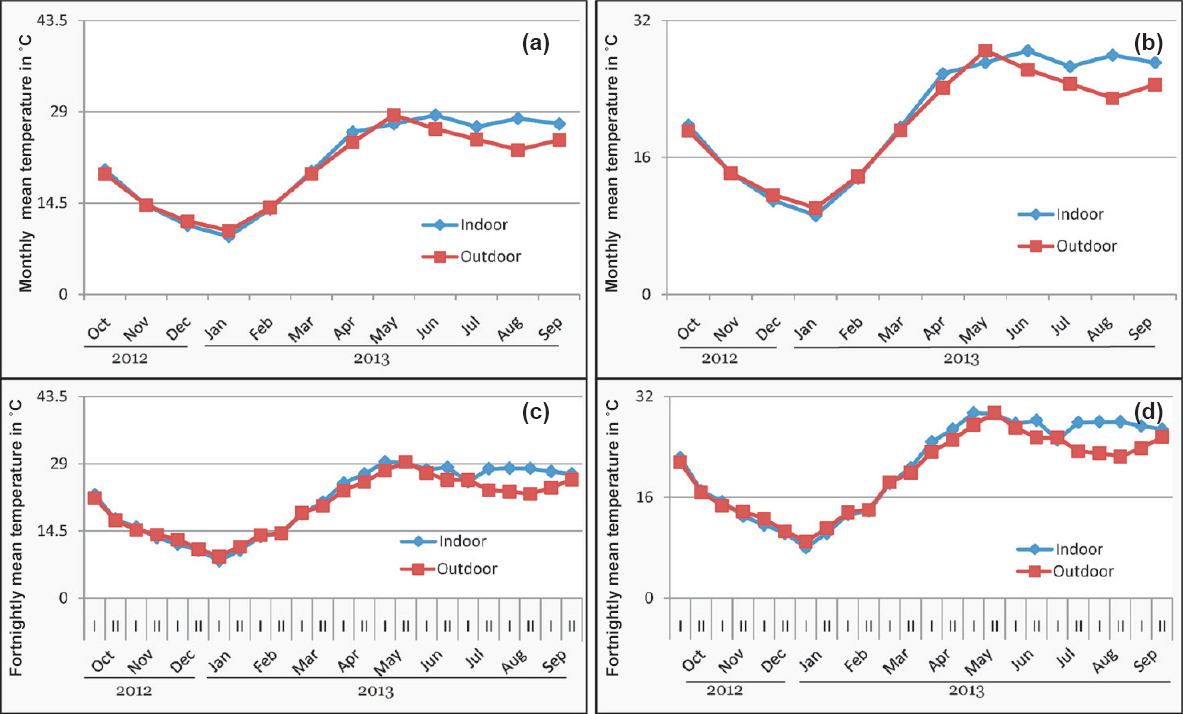
- Transmission windows (TWs) of malaria based on monthly temperature (a) Pv (b) Pf showing opening of windows for all months and based on fortnightly temperature (c) Pv (d) Pf, showing opening of window for 17 and 16 fortnights for Pv and Pf transmission, respectively at Bhorsa. I and II represent two fortnight of a month.
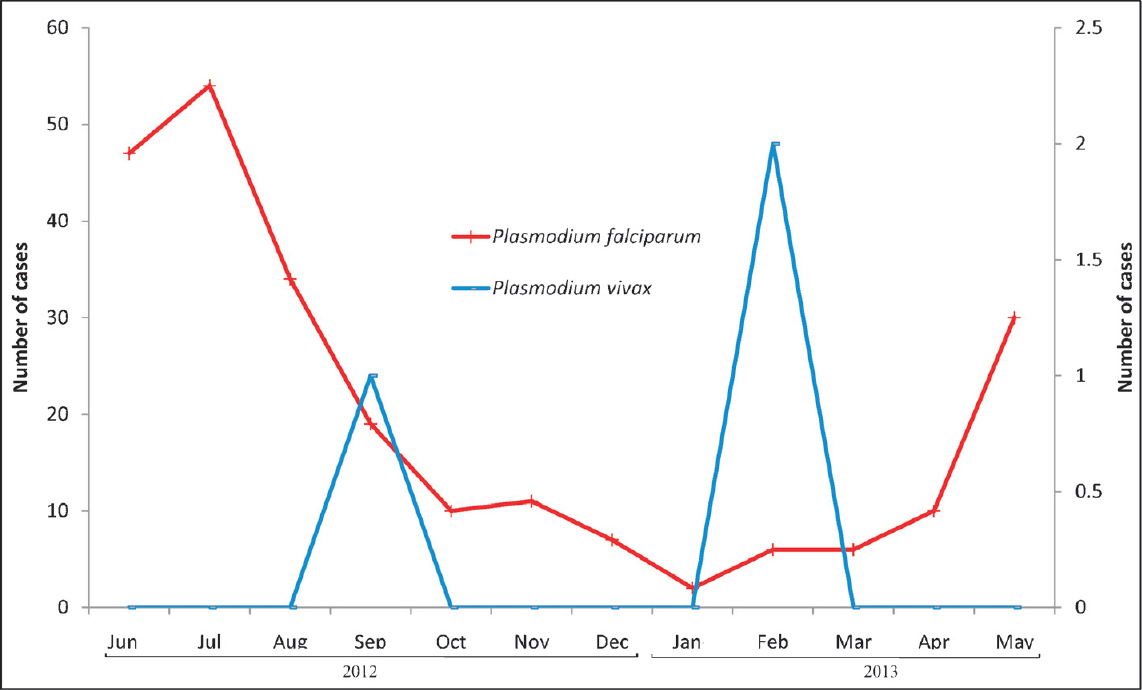
- Monthly malaria cases collected from Bokajaan PHC for Lahorijaan study site, showing occurrence of malaria cases throughout the year.
The correlation between monthly recorded indoor and outdoor temperatures was r = 0.974 (P<0.001) at Lahorijaan and r = 0.937 (P<0.001) at Bhorsa, respectively (Fig. 6a, c). In simple linear regression equation the first derivative (dy/dx) showed the rate of change in indoor temperature over the outdoor as an increasing function of dy/dx as 0.762 and 1.144 in Lahorijaan and Bhorsa, respectively. The variation in indoor temperature as a function of outdoor temperature could be predicted up to 87 per cent (R2 = 0.878) and 94 per cent (R2= 0.949) for high and low endemic areas with standard error 1.642 and 1.712, respectively. With fortnightly recorded temperature, similar results were also obtained with more strong correlation and the coefficient of determination at both localities [Lahorijaan (R2= 0.993, r = 0.996, P<0.001), Bhorsa (R2 = 0.951, r = 0.975, P<0.001)] (Fig. 6b, d).
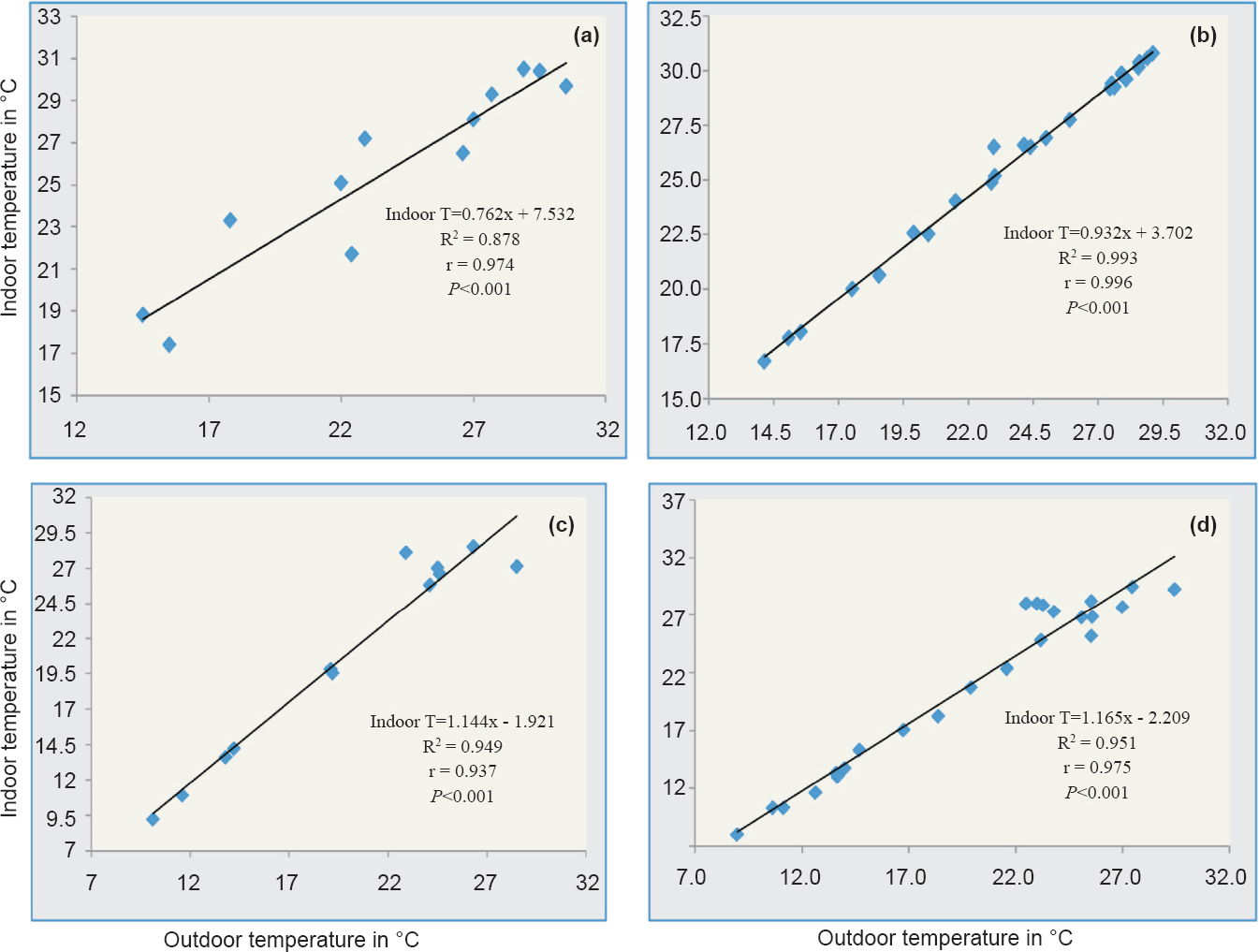
- Scatter plot showing indoor temperature as a function of outdoor temperature and linear regression equation generated. (a) R2 measuring the proportion of variation in monthly indoor temperature from outdoor temperature up to 87 per cent (P<0.001) and (b) up to 99.3 per cent (P<0.001) with fortnightly indoor temperature, at Lahorijaan, whereas, (c) up to 94.9 per cent (P<0.001) of variability in monthly indoor temperature, and (d) explained up to 95 per cent (P<0.001) with fortnightly indoor temperature at Bhorsa.
Discussion
The study revealed that temperatures of the indoor habitats were always more than the outdoor in Lahorijaan, indicating that the indoor resting habitats provided better conditions for resting of adult mosquitoes and development of parasite in the mosquito vectors in high endemic areas of malaria. On the other hand, fluctuations in indoor and outdoor temperatures at Bhorsa site were quite frequent affecting the transmission dynamics typical of unstable malarious area. The importance of temperature of external air and of resting habitats of mosquitoes was recognized decades ago2425, for calculation of sporogony of malaria parasites. Although indoor biting and resting behaviour of anophelines has been studied in India262728 as well as globally2930, but studies assessing the impact of temperature of micro-niches on survival of vectors species and resultant malaria transmission are rare.
Four-hourly data of temperature showed different outcome in terms of daily, fortnightly and monthly temperature and corroborated the postulation that small differences between temperatures could result in large differences31 in transmission of malaria. Based on the hourly temperature, diurnal, nocturnal and the temperature prevalent at the time of mosquitoes coming out of indoor habitats for foraging and egg laying can be determined. In addition to daily temperature, it is possible to determine sporogonic and gonotrophic cycles and degree days as well32. With monthly temperature, sporogonic and gonotrophic cycles cannot be calculated and most of the finer details of fluctuation within 30 days are masked and do not seem appropriate33 for analyzing the relationship between temperature and transmission dynamics and distinctively for the completion of life of mosquito and extrinsic incubation period, which is usually less than one month. Blanford et al34 have also mentioned that the use of only mean monthly temperature cannot define the transmission environment for parasite development. The applicability of using temperature at less than monthly resolution would affect the projected scenarios of malaria transmission in view of climate change as these have been done on the basis of monthly mean temperatures. Paaijmans et al14 have demonstrated that there is remarkable difference in mosquito infection rate at a difference of 2°C and also reduction in vectorial capacity at higher temperature, which further highlights the importance of difference between indoor and outdoor temperatures as crucial factor in host parasite interaction. Based on the difference in outdoor and indoor temperatures, the unstability/ stability of malaria transmission could also be explained by high fluctuation versus consistent temperature.
Most of the models of climate change have projected changes in climate or disease at global or regional level at monthly interval. Therefore, the projections made for malaria in view of climate change based on air temperature should be viewed with limitation of not using temperature data of indoors. This study highlights the importance of local data generation at least at sub-district level for understanding/ projecting the impact of climate change in view of variations in outdoor and indoor temperatures at different locations. The transmission windows of malaria should be determined based on mean temperature of indoor (for indoor resting vectors, which can be derived from outdoor temperature also using the regression model in similar geographic areas) preferably with fortnightly temperature. Further studies are required on resting behaviour of malaria vectors, implications of different types of houses in varying temperature and relative humidity versus outdoor temperature in different eco-epidemiological settings.
Acknowledgment
Authors thank the Indian Council of Medical Research, New Delhi, for financial assistance. The technical assistance provided by Ms. Teena Pathak and Shri. Benudhar Mukhi, Research Assistants and Shri Neechu Keshava Rao and Shri Sarthe Tisso, Senior Field Attendants and Shri Mrinmoy Barua and Shri Rohit Kumar, Field workers at respective field sites is also acknowledged.
Conflicts of Interest: None.
References
- The influence of temperature on the development of malaria plasmodia in the mosquito. Leningrad Pasteur Instt Epidemiol Bacteriol. 1935;2:108-9.
- [Google Scholar]
- Climate change and vector-borne diseases: a regional analysis. Bull World Health Organ. 2000;78:1136-47.
- [Google Scholar]
- McCarthy JJ, Canziani OF, Leary NA, Dokken DJ, White KS, eds. Intergovernmental Panel on Climate changes. Climate change 2001. Impacts, adaptation and vulnerability, Contribution of Working Group II to the Third Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK: Cambridge University Press; 2001. p. :1032.
- Tropical temperatures can inhibit development of the human malaria parasite Plasmodium falciparum in the mosquito. Proc Exper Appl Entomol. 2011;12:151-6.
- [Google Scholar]
- Estimating air temperature and its influence on malaria transmission across Africa. PLoS One. 2013;8:56487.
- [Google Scholar]
- Potential effect of climate change on malaria transmission in Africa. Lancet. 2003;362:1792-8.
- [Google Scholar]
- Modelling the global constraints of temperature on transmission of Plasmodium falciparum and P. vivax. Parasit Vectors. 2011;4:92.
- [Google Scholar]
- Potential impact of global climate change on malaria risk. Environ Health Perspect. 1995;103:458-64.
- [Google Scholar]
- A climate based distribution model of malaria transmission in Africa. Parasitol Today. 1999;15:105-11.
- [Google Scholar]
- National and regional impacts of climate change on malaria by 2030. Curr Sci. 2011;101:372-83.
- [Google Scholar]
- How malaria models relate temperature to malaria transmission. Parasit Vectors. 2013;6:20.
- [Google Scholar]
- Influence of climate on malaria transmission depends on daily temperature variation. Proc Natl Acad Sci USA. 2010;107:15135-9.
- [Google Scholar]
- The influence of mosquito resting behaviour and associated microclimate for malaria risk. Malar J. 2011;10:183.
- [Google Scholar]
- Warmer temperatures reduce the vectorial capacity of malaria mosquitoes. Biol Lett. 2012;8:465-8.
- [Google Scholar]
- Anopheles parensis: the main member of the Anopheles funestus species group found resting inside human dwellings in Mwea area of central Kenya toward the end of the rainy season. J Am Mosq Control Assoc. 2003;19:130-3.
- [Google Scholar]
- Mosquito Ecology: Field sampling methods, springer science & business media (3rd ed). Netherlands: Springer; 2007.
- Anopheline fauna and malaria incidence in Changlang district (Arunachal Pradesh) Indian J Malariol. 1993;30:135-43.
- [Google Scholar]
- Identification of Anopheles fauna in a hyperendemic falciparum area of Orissa State, India. Indian J Med Res. 2008;127:178-82.
- [Google Scholar]
- Characterizing microclimate in urban malaria transmission settings: a case study from Chennai, India. Malar J. 2013;12:84.
- [Google Scholar]
- Anopheles minimus: its bionomics and role in the transmission of malaria in Assam, India. Bull World Health Organ. 1996;74:61-6.
- [Google Scholar]
- Seasonal prevalence of malaria vectors and its relationship with malaria transmission in three physiographic zones in Uttaranchal state, India. J Vect Borne Dis. 2007;44:75-7.
- [Google Scholar]
- Draft report/guidelines for setting up automatic weather stations (AWSs) and automated rain gauge (ARGs), Ministry of Agriculture, Government of India. Available from: http://agricoop.nic.in/imagedefault/credit/GuidlinesforAWSandWeather%20Data-15.04.pdf
- [Google Scholar]
- The dependence upon temperature of the speed of development of malaria plasmodia in the mosquito. Med Parazitol (Mosk). 1946;15:19-28.
- [Google Scholar]
- World Health Organization (WHO). Manual on practical entomology in malaria. Part II. Geneva: WHO; 1975. p. :1-191.
- An outbreak of Plasmodium falciparum malaria due to Anopheles minimus in central Assam, India. Indian J Malariol. 2001;38:32-8.
- [Google Scholar]
- Vector incrimination in Tamulpur primary health centre, district Nalbari, lower Assam during malaria outbreak 1995. Indian J Med Res. 1996;103:146-9.
- [Google Scholar]
- Reappraisal on anopheline mosquitoes of Garhwal region, Uttarakhand, India. J Vector Borne Dis. 2008;45:112-23.
- [Google Scholar]
- Host choice by indoor-resting Anopheles arabiensis in Ethiopia. Trans R Soc Trop Med Hyg. 1997;91:376-8.
- [Google Scholar]
- Indoor resting by outdoor biting females of Anopheles gambiae complex (Diptera: Culicidae) in the Sahel of northern Senegal. J Med Entomol. 1997;34:285-9.
- [Google Scholar]
- Malaria resurgence in the East African highlands: Temperature trends revisited. Proc Natl Acad Sci USA. 2006;103:5829-34.
- [Google Scholar]
- Sporogonic cycles based on degree-days for malaria parasite development in different eco-epidemiological settings. Jpn J Infect Dis. 2016;69:87-90.
- [Google Scholar]
- Temperature variation makes ectotherms more sensitive to climate change. Glob Chang Biol. 2013;19:2373-80.
- [Google Scholar]
- Implications of temperature variation for malaria parasite development across Africa. Sci Rep. 2013;3:1300.
- [Google Scholar]






