Translate this page into:
Intrauterine devices & infection: Review of the literature
Reprint requests: Dr David Hubacher, Senior Epidemiologist, FHI 360, 359 Blackwell Street, Suite 200, Durham, NC 27701, USA e-mail: dhubacher@fhi360.org
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The relationship between use of an intrauterine device (IUD) and pelvic inflammatory disease (PID) has been studied extensively over the past 50 years. Previous research has led to considerable controversy and debate. Numerous limitations in the studies make it difficult to draw any firm conclusions from the past research or to design new approaches to study the topic. The main research barriers include uncertainty of infection/diagnoses, and inappropriate comparison groups for IUD users. Natural history studies of the aetiology of disease and observational research among IUD users suggest that the risk of PID is very low. Research linking previous IUD use to the more distant endpoint of tubal infertility reveals that the risks may be even lower than the risks of PID.
Keywords
Aetiology
infection
intrauterine device
pelvic inflammatory disease
research limitations
Introduction
The concern that intrauterine devices (IUDs) might cause or facilitate gynaecologic infection has a long and controversial history, dating to the 1940s1. The introduction and rapid adoption of modern IUDs in the 1960s, followed by increased popularity in the 1970s, gave scientists many opportunities to conduct research on IUD-related infections. Despite nearly 50 years of research, we still lack a clear understanding that is accepted by all. Even with modern research methods that employ more sophisticated approaches and strategies that can help eliminate the shortcomings of prior research, we still do not have perfect information.
This article reviews the available information about the relationship between IUD use and gynaecologic infection. Because the majority of pelvic inflammatory disease (PID) is thought to be caused by unbridled exposure to sexually transmitted bacteria2, this review draws from the medical literature on contraceptive use and sexually transmitted infections. It is important to emphasize that this article provides evidence for non-hormonal IUDs only. The levonorgestrel intrauterine system, for example, may have a different effect on the aetiology of infection, compared to copper IUDs.
Barriers to understanding
Four main barriers prevent complete understanding of the role of the IUD in gynaecologic infections: the asymptomatic nature of many infections, the unknown timing of bacterial exposure in relation to insertion and use of an IUD, lack of an appropriate comparison group for IUD users, and imprecise PID diagnoses. These and other limitations make it difficult to conduct research on this topic3.
(i) Asymptomatic infections: Because an infection may produce mild or no symptoms, women may be inaccurately characterized as free of disease, flawing research, especially when searching for associations between previous infection and IUD use. This problem is particularly true for Chlamydia trachomatis, which can cause a significant number of asymptomatic cervical and upper genital tract infections. The damage may be discovered only years later through diagnostic work-up for infertility or chronic pelvic pain.
(ii) Timing of bacterial exposure in relation to insertion and use of an IUD: Documenting the presence of sexually transmitted bacteria in the genital tract, with or without accompanying infection (again possibly asymptomatic) is a necessary first step in understanding how or if the IUD increases risk. Presence of bacteria before the IUD is inserted can have a completely different aetiology compared to bacteria acquired after the IUD is in situ. Treatment of detected bacteria removes both the risk of infection and the ability to fully understand the aetiology.
(iii) Lack of appropriate comparison group: To obtain valid results, researchers must compare IUD users with women who have the same levels of infection risk. This requirement is difficult to fulfill, for several reasons. First, self-perceived differences in risk of infection often dictate the decision on which birth control method to use. For example, a woman who is unsure of her partner's sexual behaviour might opt for condoms. Or high coital frequency might prompt a woman to seek intrauterine contraception because it is highly effective, but high coital frequency itself increases the risk of infection in general populations.
A woman who chooses sterilization may be at the lowest risk of infection, because often her sexual relationship is likely to be stable and monogamous. Researchers have attempted to control for such confounding factors by collecting information on the number of sexual partners, use of condoms, and other infection risk factors456. These measures, which are often ascertained by the subject report, are notoriously unreliable and cannot fully account for the underlying differences in risk among study groups789.
A second difficulty in comparing risk among women using different contraceptive methods is that some methods reduce risk: condoms may protect women from bacterial exposure, and oral contraceptives may protect women from upper genital tract infection by thickening the cervical mucus barrier10. Copper IUDs do neither. Thus, when compared to some contraceptives, the IUD may appear riskier even though these do not add to the intrinsic risk of infection.
(iv) Imprecise diagnoses: PID is difficult to diagnose with high sensitivity and specificity, even in a research setting with predefined criteria. Laparoscopic evaluation, the gold standard for diagnosis, is highly invasive for general evaluation of possible acute PID.
Natural history of gynaecologic infection
Sexually transmitted bacteria that ascend through the genital tract may not produce discrete signs of disease at different anatomic sites. A single bacterial exposure causes chronic infection and the disease begins with the acquisition of bacteria. If host defenses fail, acquisition may lead to cervical infection, followed by PID, and finally tubal infertility.
Although published research provides some clues as to how often one stage of infection develops into the next, the evidence is insufficient to allow definitive conclusions. At each step, the quality of the information varies, making it difficult to understand with confidence the complete aetiology of disease. The evidence as we have it from cervical infection to PID and from PID to tubal infertility is outlined below (Fig. 1).
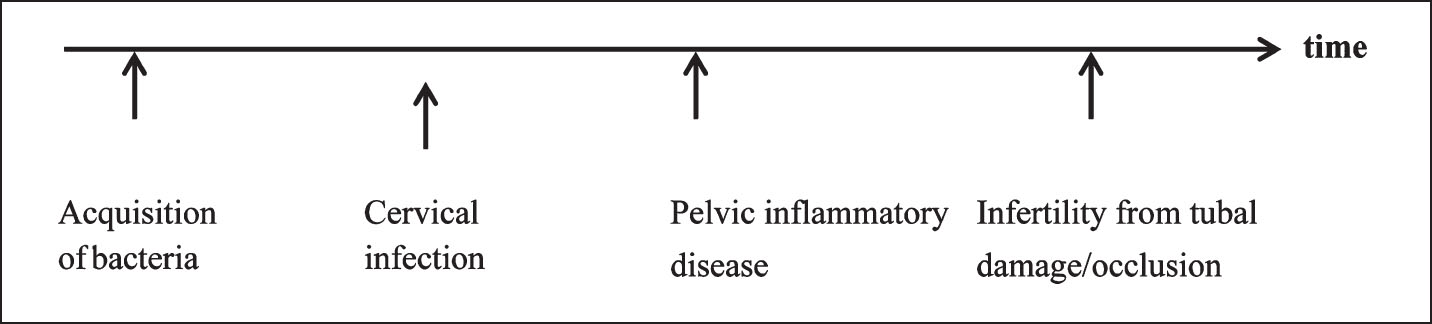
- Aetiology of infection.
Cervical infection to PID: How often does cervical infection lead to PID? Some evidence comes from studies conducted in the United States that discovered that penicillin was an ineffective treatment for chlamydial infection. In two separate studies, 16 and 30 per cent of women with chlamydial infection developed PID1112. In research from The Netherlands, none of the 30 women who had chlamydial infections developed PID13. Rahm14 estimated that either 2 or 5 per cent of participants in a prospective study developed PID secondary to chlamydial cervical infection, depending on whether some PID diagnoses were missed. In a small study of 20 women with chlamydial infection, Paavonen and coworkers15 found that 20 per cent women developed PID within four weeks. A study of gonococcal exposure that had time to progress during contact tracing and before treatment found that nine of 16 women (47%) developed symptoms consistent with PID (median time of 11 days after exposure)16. As noted in two review articles1718, small study sizes, varying quality of the research, and other factors, make it difficult to use existing research to summarize the risks.
PID to tubal infertility: How often does PID damage the lumen of the fallopian tubes, resulting in tubal infertility? The best evidence comes from a seminal Swedish study19 in which women who had laparoscopically confirmed PID were followed up for 6 to 14 yr in the national health system. Among women who had one episode of PID, 13 per cent were diagnosed with tubal infertility. With two episodes of PID, 35 per cent developed PID. With three or more episodes of PID, 75 per cent of women developed tubal infertility. None of the 100 control subjects (negative for PID based on laparoscopy) developed tubal infertility over the same follow up period.
Aetiology of infection with an IUD
Event rates observed in natural history data outlined above may be altered by IUD use. The risks may also vary, depending on when the IUD is inserted in relation to the different disease steps (Fig. 2). For example, if the IUD is in situ prior to bacterial exposure, does the IUD facilitate infection of the upper genital tract? If the cervix is infected and an IUD is inserted, the chances of upper genital tract infection may be different. If months pass between acquisition of bacteria and IUD insertion (without developing cervical infection), does the insertion procedure increase the risk of lower and upper genital tract infection? We have no evidence to answer most of these questions.
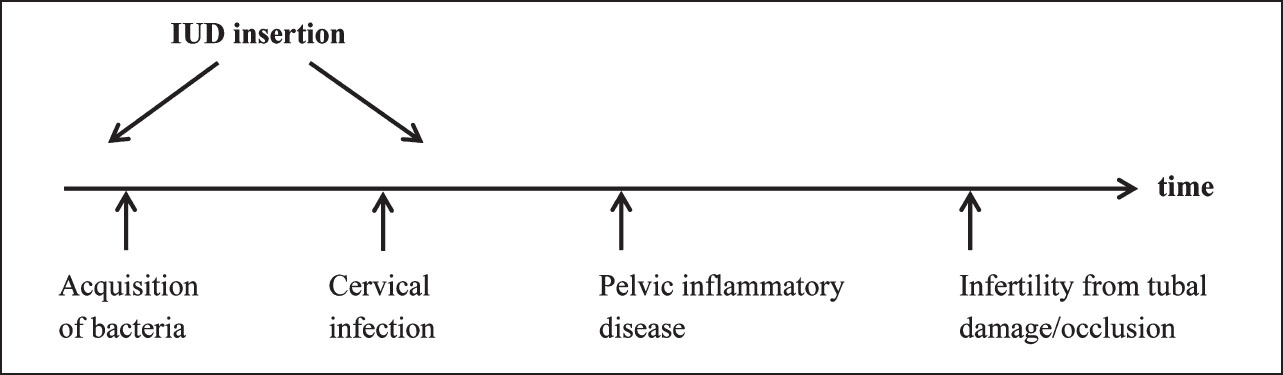
- Aetiology of infection with intrauterine device (IUD).
Background rates of PID among IUD users: How common is PID in a general population of IUD users? The best evidence comes from a compilation of IUD studies conducted by the World Health Organization20. Key points from the study are: (i) 22,908 women received an IUD (75% of the devices were copper-containing), some were followed up to 10 years (Greater than 51,000 person-years of IUD use). (ii) PID defined as oral temperature > 38° C, and abdominal tenderness with guarding, and positive pelvic exam (adnexal or cervical motion tenderness, or palpable adnexal mass), (a) 81 cases of PID were followed up; (b) average incidence was 1.6 events per 1000 person-years; (c) Highest rate in the first month: 4 times higher than the average rate.
This evidence shows that the risk of PID among IUD users is low and similar to the rate in a general population of sexually active women. However, the higher rate during the first month suggests that the insertion procedure may cause additional cases of PID.
Antibiotic prophylaxis at IUD insertion: The concern that the insertion procedure might increase PID risk led several groups of researchers to investigate whether prophylactic use of antibiotics could reduce PID incidence. A systematic review and meta-analysis of four randomized trials on this topic21 found low and equal PID rates between the antibiotic and placebo groups. Thus, with today's focus on careful selection of IUD users (i.e., low risk of acquiring sexually transmitted infection), PID rates can remain low, without the need for prophylactic medications.
Insertion through an infected cervix: effect on PID incidence: If natural history studies show that between 0 and 47 per cent of untreated cervical infections will progress naturally to PID, how does IUD insertion through an infected cervix affect PID risk? In other words, does IUD insertion contaminate the uterus with a clinically significant amount of bacteria? Evidence to answer these questions does not exist. A systematic review by Mohllajee and colleagues17 at the Centers for Disease Control and Prevention failed to identify any properly designed published research. Because ethical considerations preclude implementation of a study with such a design, it is impossible to know how PID incidence might be altered by inserting an IUD through an infected cervix, compared to simply leaving the cervical infection untreated and providing an alternative method of birth control. The review by Mohllajee and colleagues, however, found six published studies that tallied events after inserting an IUD through an infected cervix222324252627. These prospective studies lack a comparison group since these involved only IUD users, some of whom had a cervical infection at the time of insertion. Among women who had an IUD inserted through an infected cervix, 0 to 5 per cent developed PID. IUD users who did not have an infection at the time of insertion appeared to have a lower incidence of PID. Still, it is difficult to draw firm conclusions from these studies, for two main reasons. First, because the number of women with infection was small and there were only a few PID events, the confidence intervals around the point estimates were very wide. Second, an unknown proportion of women who had an infection were contacted to return to the clinic for treatment, thus possibly preventing development of PID. In addition, timing of initiation of antibiotic treatments probably varied considerably in the studies.
Tubal infertility and IUD use
The latest research to examine the relationship between IUD use and tubal infertility was conducted in Mexico City28. Three key features of this effort distinguish it from past work: a non-litigious society void of IUD controversy; collection of serum samples from participants to detect past exposure to C. trachomatis; and any past use of an IUD was limited to copper devices. The case-control study found that women with tubal infertility had used the IUD previously with same frequency as pregnant primigravid controls. Thus the research found that past use of a copper IUD did not increase the risk of tubal infertility; however, previous exposure to C. trachomatis (as determined by presence of chlamydial antibodies) increased the risk more than two-fold. This effort lends support to the theory that sexually transmitted bacteria, and not the IUD, are to blame for tubal infertility. Because the research was conducted among only nulligravid women, the results should be reassuring to women who want to use an IUD before having children.
Attributable risk
The concept of attributable risk, as applied to IUD use and resulting PID, was developed because clinicians often do not know whether a patient has an active cervical infection when inserting a device29. Thus, to prevent complete paralysis of IUD services in the face of these unknowns, the attributable risk model gives clinicians a better sense of the magnitude of the risks. Using a hypothetical model and assumptions about risk under worst-case scenarios (10% prevalence of cervical infection in the clinic population; relative risk of PID from IUD use is 2.5 times higher than for the general population; and 5 per cent of cervical infections will progress to PID after an IUD insertion), approximately 1 in 333 insertions would result in PID that was directly attributable to the IUD (less than one-third of 1%). Also estimated in the model was that the attributable risk could be halved (1 in 667 insertions or about 0.15%) if clinicians used simple questions to screen out high-risk women.
Conclusions
We do not know for certain whether the IUD is a cause, facilitator, or innocent bystander in the aetiology of gynaecologic infection. The best evidence suggests that the risk of PID among IUD users is very low. Although research has shown that the insertion procedure may increase the risk of PID, prophylactic use of antibiotics appears unnecessary because PID rates, even in the first month, are low. Recent evidence suggests that any link between IUD use and subsequent infertility is less certain.
Conflict of interest
David Hubacher has served on Scientific Advisory Boards for Bayer HealthCare and Teva Pharmaceutical. He has received product donations from Bayer, Teva, and Merck for his independent and externally-funded research ideas. Bayer has funded an additional year of follow up on participants in one of Dr Hubacher's NIH-funded research projects (investigator-led R01).
References
- Intra-uterine contraceptive devices. Proceedings of the Conference. New York City. April 30 - May 1 1962. Amsterdam: Excerpta Medica International Congress Series No 54; 1962.
- [Google Scholar]
- Pitfalls of research linking the intrauterine device to pelvic inflammatory disease. Obstet Gynecol. 2013;121:1091-8.
- [Google Scholar]
- Hormonal contraceptive use, cervical ectopy, and the acquisition of cervical infections. Sex Transm Dis. 2004;31:561-7.
- [Google Scholar]
- Hormonal contraception and risk of sexually transmitted disease acquisition: results from a prospective study. Am J Obstet Gynecol. 2001;185:380-5.
- [Google Scholar]
- Incidence of uncomplicated genital infections in women using oral contraception or an intrauterine device: a prospective study. Sex Transm Dis. 1990;17:23-9.
- [Google Scholar]
- Validity of self-reported ‘safe sex’ among female sex workers in Mombasa, Kenya - PSA analysis. Int J STD AIDS. 2007;18:33-8.
- [Google Scholar]
- Concerns regarding design, analysis, and interpretation of the morrison study on hormonal contraceptive use and acquisition of cervical infections. Sex Transm Dis. 2005;2:644.
- [Google Scholar]
- Condom use as a dependent variable: measurement issues relevant to HIV prevention programs. AIDS Educ Prev. 1998;10:548-57.
- [Google Scholar]
- Decreased risk of symptomatic chlamydial pelvic inflammatory disease associated with oral contraceptive use. JAMA. 1990;263:54-9.
- [Google Scholar]
- The treatment of pelvic inflammatory disease. Am J Obstet Gynecol. 1980;138:1042-7.
- [Google Scholar]
- Effect of treatment regimens for Neisseria gonorrhoeae on simultaneous infection with Chlamydia trachomatis. N Engl J Med. 1984;310:545-9.
- [Google Scholar]
- The natural course of asymptomatic Chlamydia trachomatis infections: 45% clearance and no development of clinical PID after one-year follow-up. Int J STD AIDS. 2002;13(Suppl 2):12-8.
- [Google Scholar]
- Asymptomatic carriage of Chlamydia trachomatis - a study of 109 teenage girls. Eur J Sex Transm Dis. 1986;3:91-4.
- [Google Scholar]
- Treatment of nongonococcal urethritis with trimethoprim-sulphadiazine and with placebo. A double-blind partner-controlled study. Br J Vener Dis. 1980;56:101-4.
- [Google Scholar]
- Risk of acquiring gonorrhea and prevalence of abnormal adnexal findings among women recently exposed to gonorrhea. JAMA. 1983;250:3205-9.
- [Google Scholar]
- Does insertion and use of an intrauterine device increase the risk of pelvic inflammatory disease among women with sexually transmitted infection? A systematic review. Contraception. 2006;73:145-53.
- [Google Scholar]
- The incidence of pelvic inflammatory disease in untreated women infected with Chlamydia trachomatis: a structured review. Int JSTD AIDS. 2007;18:727-31.
- [Google Scholar]
- Effect of acute pelvic inflammatory disease on fertility. Am J Obstet Gynecol. 1975;121:707-13.
- [Google Scholar]
- Intrauterine devices and pelvic inflammatory disease: an international perspective. Lancet. 1992;339:785-8.
- [Google Scholar]
- Antibiotic prophylaxis for intrauterine contraceptive device insertion. Cochrane Database Syst Rev. 2001;2:CD001327.
- [Google Scholar]
- Preventing IUCD-related pelvic infection: the efficacy of prophylactic doxycycline at insertion. Br J Obstet Gynaecol. 1990;97:412-9.
- [Google Scholar]
- Genital tract infections associated with the intrauterine contraceptive device can be reduced by inserting the threads into the uterine cavity. Br J Obstet Gynaecol. 1992;99:676-9.
- [Google Scholar]
- Effect of prophylactic antibiotics on morbidity associated with IUD insertion: results of a pilot randomized controlled trial. IUD Study Group. Contraception. 1994;50:319-27.
- [Google Scholar]
- IUD users in Norway are at low risk for genital C. trachomatis infection. Contraception. 1996;54:209-12.
- [Google Scholar]
- The risk of inadvertent intrauterine device insertion in women carriers of endocervical Chlamydia trachomatis. Contraception. 1998;58:105-9.
- [Google Scholar]
- Use of sexually transmitted disease risk assessment algorithms for selection of intrauterine device candidates. Contraception. 1999;59:97-106.
- [Google Scholar]
- Use of copper intrauterine devices and the risk of tubal infertility among nulligravid women. N Engl J Med. 2001;345:561-7.
- [Google Scholar]
- Risk of clinical pelvic inflammatory disease attributable to an intrauterine device. Lancet. 2001;357:443.
- [Google Scholar]






