Translate this page into:
In vitro comparison of the cytotoxic effects of statins on U266 myeloma cell line
For correspondence: Dr Hatice Terzi, Department of Hematology, Faculty of Medicine, Cumhuriyet University, Sivas 58140, Turkey e-mail: dr.terzi@hotmail.com
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Statins are one of the most widely used drugs and have antilipidemic effects as well as antioxidant, anti-inflammatory, anti-angiogenic and anti-tumorigenic effects. It has been shown that the synergistic combinations of statins which can provide better clinical benefit in the treatment of cancer and if administered with other anticancer agents, may be an alternative treatment modality. The aim of this study was to assess the efficacy of administrating statin in multiple myeloma (MM) cell line on cell proliferation.
Methods:
U266 myeloma cells were cultured in 25 or 75 cm2 flasks by using cell culture medium mixtures obtained with the supplementation of 10 per cent foetal bovine serum and one per cent of penicillin-streptomycin into RPMI 1640 medium. When the cells reached confluence (reached to the density of 70%), they were reproduced by passaging. Cytotoxicity was evaluated by using the XTT test.
Results:
Statins (atorvastatin and simvastatin), were administered to the U266 myeloma cell line at 100, 50, 25, 12.5, 6.25 and 3.12 μM concentrations. Inhibitor concentration 50 (IC50) values calculated for atorvastatin and simvastatin were determined as 94 and 38 μM, respectively. While 100, 50, 25, 12.5, 6.25 and 3.12 μM concentrations were used for bortezomib, the IC50 value calculated for this agent was 18.2 nM. When six concentrations of bortezomib used in the study were combined with 12.5 μM inactive concentrations of statins that did not cause inhibition in cell proliferation, both atorvastatin and simvastatin increased the effect of bortezomib at all the concentrations used, and simvastatin showed a stronger efficacy than atorvastatin.
Interpretation & conclusions:
Our in vitro results indicated that atorvastatin and simvastatin when used along with the conventional treatment in myeloma patients, may improve the effectiveness of the standard therapy and prevent the bortezomib-induced cytotoxic and neurotoxic side effects when used at a low dose. Further studies need to be done in MM patints to confirm these findings.
Keywords
Bortezomib
cancer
cytotoxic effect
multiple myeloma
standard therapy
statin
Multiple myeloma (MM) is a malignant plasma cell disease characterized by uncontrolled proliferation of monoclonal plasma cells in the bone marrow12. The risk of MM increases with increasing age1. The treatment of MM has progressed considerably within the last 15 yr with the use of immunomodulatory drugs such as thalidomide, lenalidomide and pomalidomide and the use of proteasome inhibitors such as bortezomib, carfilzomib and ixazomib34. Although the combination regimens of these drugs and autologous stem cell transplantation prolong the mean survival time of MM patients, but it is an incurable progressive disease characterized by multiple relapses due to resistance of the residual disease, and therefore, multiple treatments are needed5678. Bortezomib was the first proteasome inhibitor that was approved for recurrent/refractory MM patients by the U.S. Food and Drug Administration in 2003 and was subsequently approved for the treatment of newly diagnosed MM patients9. Despite the fact that bortezomib revolutionized the treatment of MM, there were certain limitations in the treatment. The majority of patients initially responding to bortezomib may later develop resistance to the drug and may show a relapse. Most importantly, peripheral neuropathy (PN) is a dose-limiting toxicity of bortezomib, which can potentially cause permanent nerve injury in the extremities10. The other common side effects of bortezomib include fatigue, gastrointestinal effects and mild cytopaenia. Bortezomib is administered intravenously and via subcutaneous route, and this administration has demonstrated similar efficacy and bioavailability and showed a significantly lower incidence of PN compared to iv administration11121314. More effective combinations in which bortezomib is used at lower doses are needed so that the side effects will reduce and compliance to treatment will increase. It has been revealed that statins also have different effects together with the lipid-lowering effect. These effects, which are independent of the reduction of cholesterol, are called pleiotropic effects15. It has been suggested that statins prevent tumour recurrence by showing an oncoprotective effect16. This study was undertaken to evaluate the effect of administrating statins in MM cell line on cell proliferation when administered with bortezomib in vitro.
Material & Methods
This study was conducted in the laboratory of the department of Pharmacology, Medical Faculty of Cumhuriyet University Sivas, Turkey. The multiple myeloma (MM) cancer cell lines (U266 myeloma) were obtained from American Type Cell Collection (ATCC) cell collection. The study was approved by the Ethics Committee of the Cumhuriyet University.
The cells, which were adherent cell lines and grew as monolayers, were routinely cultured in RPMI-1640 medium (Roswell Park Memorial Institute-1640; Gibco, Invitrogen, Carlsbad, CA, USA) supplemented with 10 per cent heat-inactivated foetal bovine serum, one per cent L-glutamine and one per cent penicillin-streptomycin in 75 cm2 polystyrene flasks (Corning Life Sciences, Tewksbury, MA, USA) and maintained at 37°C in a humidified atmosphere with five per cent CO2. Growth and morphology were monitored, and the cells were passaged when they reached 90 per cent confluence. Cell culture supplies were obtained from Life Technologies (Darmstadt, Germany).
XTT viability assay: After verifying cell viability using trypan blue dye exclusion test17 by cellometer automatic cell counter (Nexcelom Inc., Lawrence, MA, USA), the cells were seeded at approximately 1×104 cells/well in a final volume of 100 μl in 96 well flat-bottomed microtitre plates with or without various concentrations of bortezomib (100, 50, 25, 12.5 and 6.25 nM), atorvastatin (100, 50, 25, 12.5, 6.25 and 3.12 μM) and simvastatin (100, 50, 25, 12.5, 6.25 and 3.12 μM). After single administration of the test drugs, bortezomib was combined with non-toxic concentrations of both atorvastatin and simvastatin. The plates were incubated at 37°C in a five per cent CO2 incubator for 48 h. The medium was not refreshed during this time. At the end of incubation, 100 μl of XTT {2,3-bis(2-methoxy-4-nitro-5-sulphophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide} (Roche Applied Science, Mannheim, Germany) was added to each well, and the plates were incubated at 37°C for another 4 h. Absorbance was measured at 450 nM against a reference wavelength at 650 nM using a microplate reader (DTX 880 Multimode Reader, Beckman Coulter, USA). The mean of triplicate experiments for each dose was used to calculate the half maximal inhibitory concentration (IC50) values.
Statistical analysis: Data were analyzed by using SPSS software v22 (SPSS Inc., Chicago, IL, USA). The results were expressed as mean±standard deviation, and the data were analyzed by one-way analysis of variance test followed by Dunnett's t test for multiple comparisons. The effect size (ES) was calculated to assess the responsiveness by comparing the results. ES was calculated at 0.812.
Results & Discussion
Statins used in this study, atorvastatin and simvastatin, were applied on U266 myeloma cell line at 100, 50, 25, 12.5, 6.25 and 3.12 μM concentrations (Fig. 1). In the administration of statin alone, both statins caused a concentration-dependent cytotoxic effect. Inhibitor concentration 50 (IC50) values calculated for atorvastatin and simvastatin were found as 94 and 38 μM, respectively. While 100, 50, 25, 12.5, 6.25 and 3.12 μM concentrations were used for bortezomib, the IC50 value calculated for this agent was 18.2 nM. When six concentrations of bortezomib used in the study were combined with 12.5 μM inactive concentrations of statins that did not cause inhibition in cell proliferation, it was determined that both atorvastatin and simvastatin increased the effect of bortezomib in all the concentrations and in this regard, simvastatin showed a stronger efficacy than atorvastatin (Figs 2 and 3). The contribution of this combination to the anticancer effect was limited not only to the concentrations of bortezomib that displayed activity on its own, but also the combination of low and inactive concentrations of statins, where bortezomib alone was not active, and caused a significant cytotoxic effect.
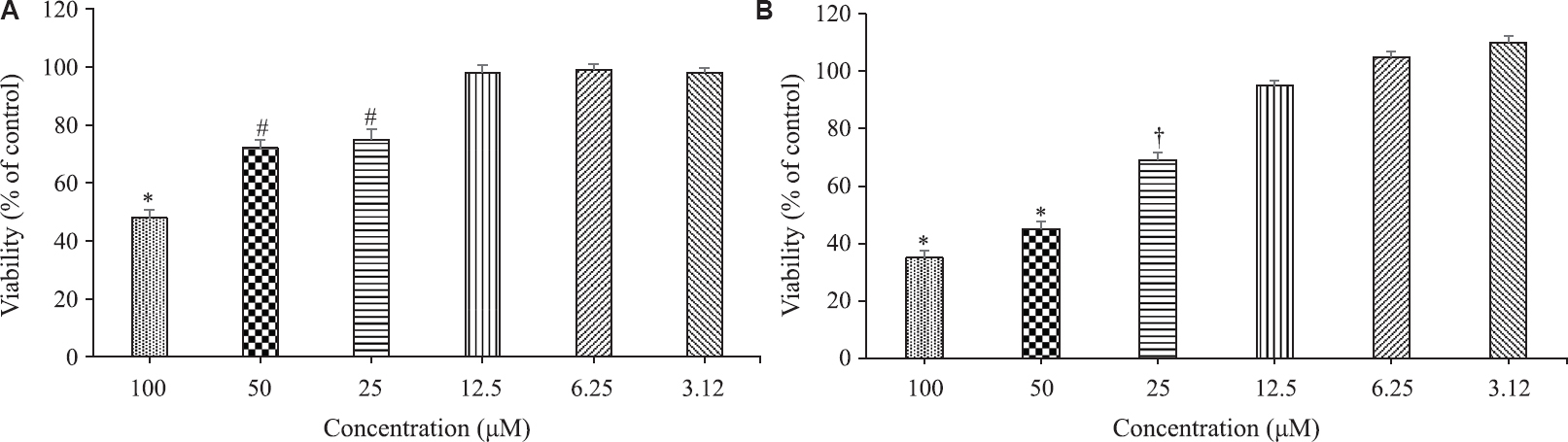
- Evaluation of cytotoxic effects of atorvastatin (A) and simvastatin (B) on U266 myeloma cell line. Values are mean±SEM of triplicate experiments. *P<0.05 compared to all the other groups; #P<0.05 compared to atorvastatin 100, 12.5, 6.25 and 3.12 μM groups; †P<0.05 compared to simvastatin 100, 50, 12.5, 6.25 and 3.12 μM groups.
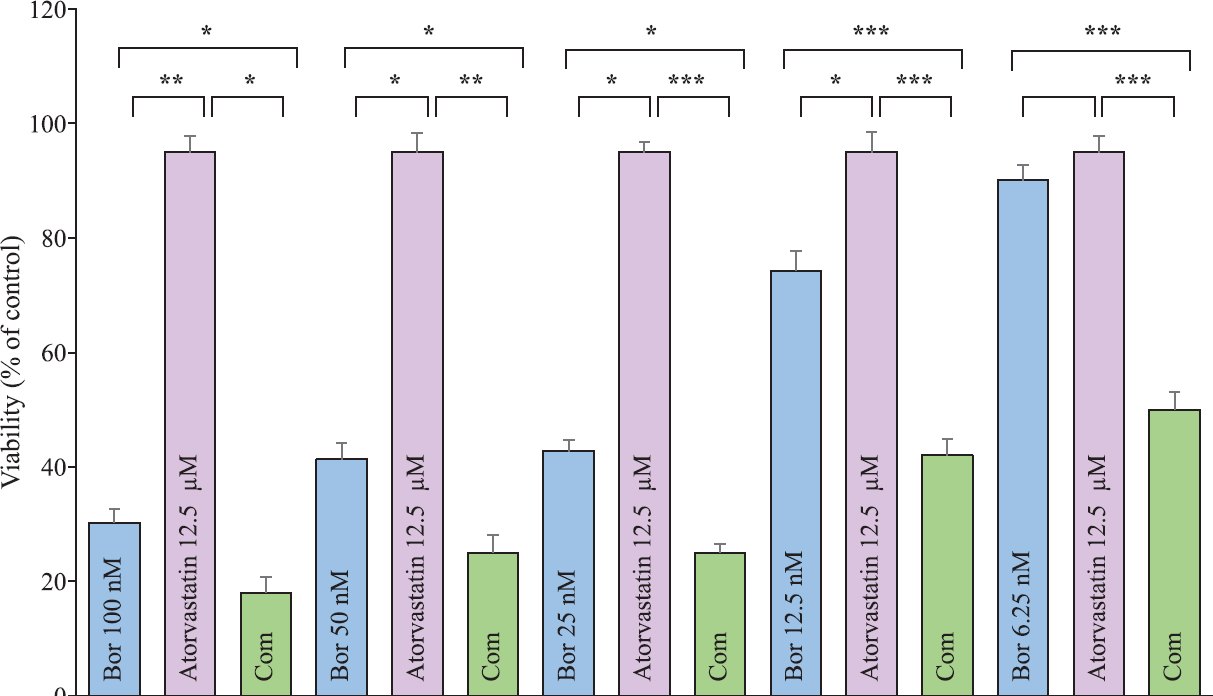
- Evaluation of the cytotoxic effects of 12.5 μM concentration of atorvastatin and 100, 50, 25, 12.5 and 6.25 μM concentrations of bortezomib on U266 myeloma cell line along with the combination of 12.5 μM concentration of atorvastatin with each concentration of bortezomib (Bor stands for bortezomib and Com stands for the combination of bortezomib with atorvastatin). Values are mean±SEM of triplicate experiments (P*<0.05, **<0.01, ***<0.001).

- Evaluation of the cytotoxic effects of 12.5 μM concentration of simvastatin and 100, 50, 25, 12.5 and 6.25 μM concentrations of bortezomib on U266 myeloma cell line along with the combination of 12.5 μM concentration of simvastatin with each concentration of bortezomib (Bor stands for bortezomib and Com stands for the combination of bortezomib with simvastatin). Values are mean±SEM of triplicate experiments (P*<0.05, **<0.01, ***<0.001).
Ahmed et al18 investigated the effectiveness of simvastatin in relapsed refractory chronic lymphocytic leukaemia, and the combination of standard therapy and administration of simvastatin was shown to be an alternative approach. Statins inhibit the HMG-CoA (3-hydroxy-3-methylglutaryl coenzyme A) reductase enzyme and inhibit the synthesis of farnesyl pyrophosphate and geranylgeranyl pyrophosphate, which are the products of mevalonate pathway other than cholesterol. The effects of statins can be regarded as apoptotic, immunosuppressive, anti-thrombotic, anti-angiogenic and oncoprotective1920. It has also been shown that high-dose statin triggers the apoptosis of tumour cells21.
It is also known that statins deteriorate the oxidative stress/inflammation cycle by reducing the release of inflammatory mediators and lipid peroxidation20. Statins also inhibit the peroxisome proliferator-activated receptor (PPAR)-alpha and PPAR-gamma activated by peroxisome proliferators that are known as the inflammatory mediators. Statins can also be accepted as having immunosuppressive nature due to these characteristics22. Although statins are a quite large family, the pleotropic effects shown by the agents in this family can be different from each other23. In this study, it was revealed that simvastatin and atorvastatin showed strong anticancer activities. van der Weide et al24 investigated the efficacy of high-dose simvastatin on acute myeloid leukaemia (AML) blast cells in newly diagnosed or relapsed AML patient groups. It was shown that simvastatin treatment increased chemosensitization by the geranylgeranylation inhibition. In another study, the apoptotic activity of statins was investigated in MM cells25. One group of myeloma cells was administered only with statin, only thalidomide was administered to the second group of myeloma cells and thalidomide + statin combination was administered to the third group of myeloma cells. The apoptotic activity in the group to which the combination was administered was significantly higher than that of other groups.
In this in vitro study, simvastatin and atorvastatin showed strong anticancer activities. Furthermore, the combination of statins with bortezomib even at very low concentrations increased the efficacy of the latter. The data presented in the present study suggest that if atorvastatin and simvastatin are used as a supplementary treatment in the conventional treatment of myeloma patients, both atorvastatin and simvastatin may improve the efficacy of the standard therapy. Bortezomib was used in much lower doses which would prevent potential side effects including neurotoxicity and bone marrow suppression. However, these in vitro results need to be confirmed in in vivo system.
Acknowledgment
Authors thank Cumhuriyet University Research Center, Sivas, Turkey, for their technical support.
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- Oncogenomics to target myeloma in the bone marrow microenvironment. Clin Cancer Res. 2011;17:1225-33.
- [Google Scholar]
- Oral ixazomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;374:1621-34.
- [Google Scholar]
- Clinical course of patients with relapsed multiple myeloma. Mayo Clin Proc. 2004;79:867-74.
- [Google Scholar]
- Prognostic value of deep sequencing method for minimal residual disease detection in multiple myeloma. Blood. 2014;123:3073-9.
- [Google Scholar]
- Emerging biological insights and novel treatment strategies in multiple myeloma. Expert Opin Emerg Drugs. 2012;17:407-38.
- [Google Scholar]
- Phase 2 trial of ixazomib in patients with relapsed multiple myeloma not refractory to bortezomib. Blood Cancer J. 2015;5:e338.
- [Google Scholar]
- Managing treatment-related peripheral neuropathy in patients with multiple myeloma. Blood Lymphat Cancer. 2016;6:37-47.
- [Google Scholar]
- Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: A randomised, phase 3, non-inferiority study. Lancet Oncol. 2011;12:431-40.
- [Google Scholar]
- Updated survival analysis of a randomized phase III study of subcutaneous versus intravenous bortezomib in patients with relapsed multiple myeloma. Haematologica. 2012;97:1925-8.
- [Google Scholar]
- Subcutaneous bortezomib incorporated into the bortezomib-thalidomide-dexamethasone regimen as part of front-line therapy in the context of autologous stem cell transplantation for multiple myeloma. Haematologica. 2014;99:e33-4.
- [Google Scholar]
- Subcutaneous versus intravenous bortezomib in patients with relapsed multiple myeloma: Subanalysis of patients with renal impairment in the phase III MMY-3021 study. Haematologica. 2015;100:e207-10.
- [Google Scholar]
- HMG-CoA reductase inhibitors and the malignant cell: The statin family of drugs as triggers of tumor-specific apoptosis. Leukemia. 2002;16:508-19.
- [Google Scholar]
- The compound cis-(dichloro)tetrammineruthenium(III) chloride induces caspase-mediated apoptosis in K562 cells. Toxicol In Vitro. 2010;24:1562-8.
- [Google Scholar]
- Pharmacokinetics of high-dose simvastatin in refractory and relapsed chronic lymphocytic leukemia patients. Cancer Chemother Pharmacol. 2013;72:1369-74.
- [Google Scholar]
- Apoptosis induction and cyclooxygenase-2 regulation in human colorectal adenoma and carcinoma cell lines by the cyclooxygenase-2-selective non-steroidal anti-inflammatory drug NS-398. Int J Cancer. 2000;86:553-60.
- [Google Scholar]
- Specific COX-2 inhibitor, meloxicam, suppresses proliferation and induces apoptosis in human HepG2 hepatocellular carcinoma cells. J Gastroenterol Hepatol. 2006;21:1814-20.
- [Google Scholar]
- Effects of selective cyclooxygenase-2 inhibitor NS-398 on 5-fluorouracil chemotherapy and progression of colon cells: An experimental study. Zhonghua Yi Xue Za Zhi. 2004;84:583-6.
- [Google Scholar]
- Treatment with high-dose simvastatin inhibits geranylgeranylation in AML blast cells in a subset of AML patients. Exp Hematol. 2012;40:177-186e6.
- [Google Scholar]
- Indomethacin promotes apoptosis in gastric cancer cells through concomitant degradation of survivin and aurora B kinase proteins. Apoptosis. 2014;19:1378-88.
- [Google Scholar]






