Translate this page into:
Impaired systemic vascular reactivity & raised high-sensitivity C reactive protein levels in chronic obstructive pulmonary disease
Reprint requests: Dr Anjana Talwar, Department of Physiology, All India Institute of Medical Sciences New Delhi 110 029, India e-mail: anjanatalwar@gmail.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Chronic obstructive pulmonary disease (COPD) is characterized by slowly progressive airflow limitaion, chronic lung inflammation and associated systemic manifestations. The objective of this preliminary study was to investigate the levels of high sensitivity C reactive protein (hs CRP) and tumour necrosis factor-α (TNF-α) as markers of systemic inflammation and assessment of systemic vascular reactivity that may play an important role in development of cardiovascular disease in COPD patients.
Methods:
Systemic vascular reactivity was assessed non-invasively by measuring peripheral pulse waveform changes during reactive hyperemia (RH) in 16 COPD patients and 14 controls by photoplethysmography technique (PPG). Parameters measured were pulse wave amplitude (PWA), slope and pulse transit time (PTT). Tumour necrosis factor-α (TNF-α) and hs CRP were measured as markers of inflammation.
Results:
PWA during the 1st, 2nd and 3rd minutes post release of occlusion were significantly higher than the baseline means in controls, whereas in the patient group there was no significant change in the PWA during any of the observed time periods following release of occlusion, in comparison to the baseline means. Similar results were observed in slope values for patients and controls. Maximum percentage change in PWA during RH with reference to baseline was significantly lower in patients as compared to controls (26.78±20.19 vs 57.20±19.80%, P<0.001). Maximum percentage change in slope during RH with reference to baseline was significantly lower in patients as compared to controls (19.77±10.73 vs 39.25±13.49%, P<0.001). A vascular tone response as represented by PTT was also impaired in the 3rd minute of RH as compared to baseline mean values in COPD patients only.
Interpretation & conclusions:
Our findings showed raised hs CRP levels and impaired systemic vascular reactivity in COPD patients. Whether these may increase the risk of cardiovascular disease in COPD patients need to be confirmed in future studies with large sample size and appropriate study design.
Keywords
CRP
endothelial dysfunction
nitric-oxide
reactive hyperemia
systemic inflammation
Chronic obstructive pulmonary disease (COPD) is associated with an inflammatory response of lungs to noxious particles or gases, particularly cigarette smoke1. However, the pathological mechanisms and manifestations of COPD are not restricted to lung2. Systemic inflammation is one of the key mechanisms that may be responsible for the extrapulmonary manifestations, including cardiovascular complications in COPD3. There are only a few reports showing a decrease in endothelium mediated vasodilatation in systemic vasculature in stable COPD45. The dysfunction of endothelium has been linked to systemic inflammation in many studies67. It is suggested that a rise in the levels of inflammatory markers causes downregulation of endothelial nitric oxide synthase8.
Various techniques have been used for assessment of endothelial function. Venous occlusion plethysmography has been used to assess vasomotor responses of forearm resistance vessels by infusing acetylcholine in the brachial artery9. However, this technique requires arterial catheterization and is thus considered invasive. By examining flow mediated dilatation (FMD) of conduit artery by ultrasound is a non-invasive assessment of endothelial function10. Assessment of endothelial dysfunction based on photoplethysmographic (PPG) digital volume pulse offers a simple, objective and operator independent method of assessment of endothelial dysfunction as compared to FMD measured by ultrasound11. Additionally, PPG allows estimation of changes in the vascular tone by measurement of pulse transit time (PTT). Monitoring of peripheral pulse waveform changes during reactive hyperemia (RH) has also been used as a validated technique to assess vascular reactivity and endothelial function1112.
This preliminary study was undertaken to investigate endothelial dysfunction by the model of reactive hyperemia using photoplethysmography (PPG) in COPD patients; to evaluate vascular function by peripheral pulse waveform changes during post-occlusion reactive hyperemia in stable COPD patients, and to assess markers of systemic inflammation.
Material & Methods
The study was conducted in the department of Physiology, All India Institute of Medical Sciences (AIIMS), New Delhi, India. The study included 16 consecutively selected ex-smoker male patients with stable COPD from Medicine outpatient department during June 2011-July 2012, and 14 non smoking healthy controls. Patients diagnosed to have COPD, as defined by the GOLD guidelines13 were included in the study.
Patient inclusion and exclusion criteria: The inclusion criteria for patients with stable COPD included an age of 40-65 yr, smoking history of at least 10 pack years or more, evidence of airflow obstruction on spirometry (FEV1/FVC < 0.7). The patient group included moderate and severe COPD patients. The mild patients were not excluded by design. Patients receiving long-term oxygen therapy or oral corticosteroids, hypertension, heart disease, diabetes mellitus, chronic renal diseases, collagen vascular diseases, pulmonary hypertension, history of COPD exacerbation in the last four weeks and obstructive sleep apnoea (OSA) were excluded from the study. Overall, 20 patients were excluded. Those COPD patients having apnoea hypopnoea index >5 were excluded from the study. The age matched controls were chosen from the staff of the Institute after advertisement. The subjects were explained about the study protocol and a written informed consent was obtained from all before enrolment. The study protocol was approved by the Institutional Ethics Committee.
Spirometry was performed using a dry rolling spirometer (SPIROAIR, MEDISOFT, PK Morgan, Kent, UK) according to European respiratory guidelines14. COPD staging was done according to GOLD guidelines, 200913.
Assessment of systemic vascular reactivity: Systemic vascular reactivity was assessed by measuring pulse wave form (PWF) and pulse transit time (PTT) responses during reactive hyperemia using simultaneously acquired lead II ECG and finger PPG signals15. Both ECG and PPG signals were acquired using digital data acquisition system Powerlab 8/30 (AD Instruments, Australia).
Patients and controls were asked to report in the vascular function laboratory after overnight fasting. After 15 min of supine rest baseline blood pressure was recorded. Sphygmomanometer cuff was kept fastened to the right arm for producing arterial occlusion after baseline recording. Disposable Ag-AgCl electrodes were applied for recording standard bipolar limb lead II ECG. PPG probe was fixed to the middle finger of right hand using the Velcro strap without creating undue pressure. After appropriate placement of electrodes and PPG probe, signal acquisition was done using data acquisition and analysis software Lab Chart7 (AD Instruments, Australia). The entire recording period included five min of baseline recording, five min of arterial occlusion and five min of reactive hyperemia. All subjects were asked to lie supine with eyes closed during the entire recording period. After acquiring baseline ECG and PPG signals, arterial occlusion was produced by raising the cuff pressure 50 mmHg above the baseline systolic blood pressure1011. The maintenance of arterial occlusion was verified real time by looking for the absence of PPG signal from the monitor. Arterial occlusion was released after five min by deflating cuff pressure completely and recording was continued during the period of reactive hyperemia. All signals recorded were analyzed offline using LabChart Pro 7® software (AD Instruments, Australia) for extraction and calculation of the following parameters with appropriate peak detection algorithms for ECG and PPG signals: (i) amplitude of PPG pulse waveform: defined as the magnitude of difference between maximum signal voltage and baseline signal voltage for each PPG pulse waveform; (ii) maximum slope of upstroke: maximum positive value of the first derivative of PPG signal for each PPG pulse waveform; and (iii) pulse transit time (PTT): time interval between each R wave peak and foot (defined as point at which signal voltage is 10 per cent above the preceding baseline value) of the corresponding PPG pulse waveform (Figure).
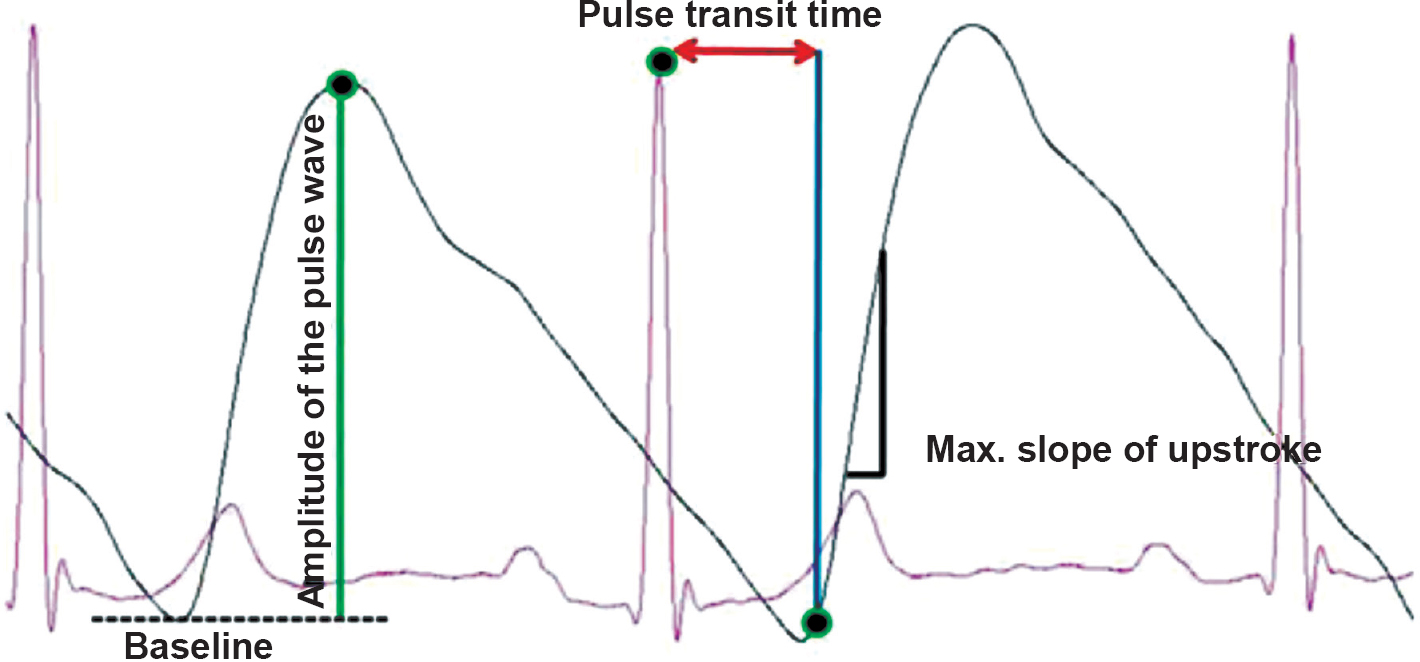
-
Figure shows overlapped and magnified image of simultaneously acquired lead II ECG and photoplethysmography (PPG) signals labelled with various function parameters.
Identification of R wave peaks of ECG and peaks and foots of PPG pulse waveform signal was done using the peak detection module of Lab chart software. Beat to beat values were extracted for the baseline and the reactive hyperemia data for all the above mentioned parameters. Mean values of each parameter were computed for the entire baseline recording and for every one min period of RH16. The time course of responses during RH within each group in comparison to their respective baseline means was analyzed. To compare the responses between groups, standardization with respect to respective baseline means was done for the RH data of each subject. Standardization was done by dividing averaged every minute data during reactive hyperemia (1st to 5th minute values) by averaged baseline values for each subject. The standardized baseline value was considered as 1(one) for each patient. To assess the peak response, maximum percentage change in PWA and slope response during RH was calculated and compared between groups.
Estimation of inflammatory markers: Venous blood sample (5 ml) was collected under aseptic precautions on the day of vascular function assessment. Serum was separated and stored in two aliquots at -80°C. The levels of TNF-α (tumour necrosis factor-α) and hs CRP (high sensitivity C reactive protein) were estimated by ELISA using commercially available ELISA kits (Biocheck, Inc. UK). Samples were estimated in duplicates and average values were used for calculations.
Statistical analyses: Each parameter was tested for normality in the distribution of data using Shapiro-Wilk test17, PWA and slope of arterial pulse waveform and pulse transit time were normally distributed, subsequently data were expressed as mean±SD. Intragroup comparison for absolute as well as standardized values for amplitude and slope of pulse waveform and pulse transit time during reactive hyperemia was assessed by using repeated measures Two way ANOVA, followed by post-hoc comparison18 by least square deviation method to see the changes over the period of time and intergroup comparison at specific time points. As there was a lack of sphericity after applying Mauchly's test19, Greenhouse-Geisser correction was used. Intergroup comparisons of maximum percentage change in PWA and slope and that of hs CRP and TNF-α levels were done using Mann-Whitney test.
Results
Amongst COPD patients, 12 had moderate COPD and four had severe COPD. The mean age of patients was 57.11±2.81 yr. Patients had body mass index (BMI) of 22.66 ± 4.52 kg/m2, blood pressure of 128 ± 16/83 ± 8 mm Hg, mean smoking history of 16 ± 6.67 pack years, fasting plasma glucose of 88 ± 11.23 mg/dl, and total cholesterol of 193 ± 23 mg/dl. Controls aged 56.14 ± 2.38 yr, had body mass index of 23.17 ± 4.12 kg/m2 and blood pressure of 126 ± 15/84 ± 8 mm Hg. There was a significant difference in forced expiratory volume in 1st second (FEV1%) and ratio of forced expiraotry volume in 1st second and forced vital capacity (FEV1/FVC) ratio between the patient group (45.63 ± 15.74%, 0.43 ± 0.09) and control group (85.04 ± 13.04%, 0.78 ± 0.08) (P<0.001 and P<0.001), respectively.
Intragroup mean values at different time points during reactive hyperemia are shown in Table 1a, b. In the control group, PWAs during the1st, 2nd and 3rd minutes post release of occlusion were significantly higher than the baseline means. In the patient group, there was no significant change in the PWA during any of the observed time periods following release of occlusion, in comparison to the baseline means. Following the release of arterial occlusion in the control group, pulse wave slopes during the 2nd and 3rd minutes were significantly higher than the baseline values. In the patient group, there was no significant change in the pulse wave slope during any of the observed time periods following release of occlusion, in comparison to the baseline means.
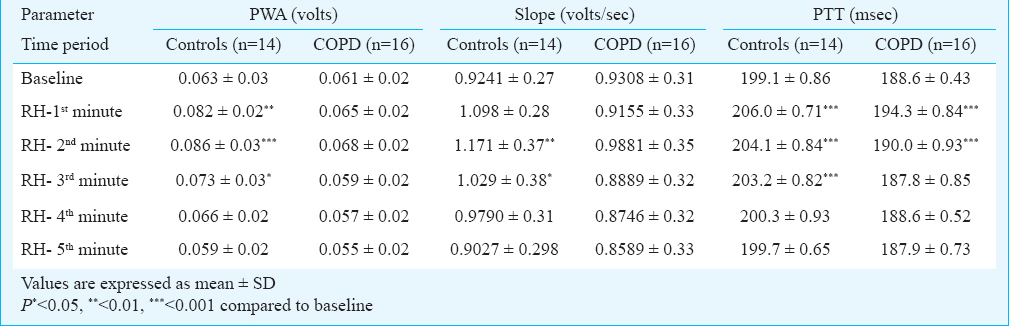
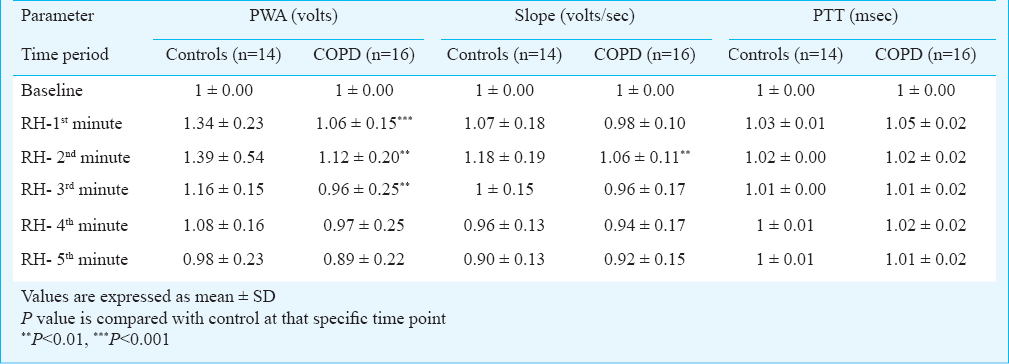
On intergroup comparison after standardization, it was observed that the rise in pulse wave amplitude was significantly lower in the patient group during 1st, 2nd and 3rd minute of RH when compared to controls (1.06 ± 0.15 vs 1.34 ± 0.23 volts, P<0.001) for 1st minute, (1.12 ± 0.20 vs 1.56 ± 0.54 volts, P<0.01) for the 2nd minute and (0.96 ± 0.25 vs 1.16 ± 0.15 volts, P<0.01) for the 3rd minute, respectively. On intergroup comparison after standardization, it was observed that the rise in standardized pulse wave slope was significantly lower in the patient group during the 2nd minute of RH when compared to controls (1.06 ± 0.11 vs 1.18 ± 0.19 volts, P<0.01). On intergroup comparison for PTT after standardization, no significant difference was observed between controls and patient group at any time point post occlusion.
Vascular tone responses during reactive hyperemia were assessed by changes in pulse transit time. Beat to beat pulse transit times were obtained from simultaneously acquired ECG and PPG signals. Following the release of occlusion, in control subjects pulse transit time was significantly higher during the 1st, 2nd and 3rd minutes, post occlusion (RH) in comparison to the baseline values. In COPD patients, the PTT was higher only in the 1st minute after release of occlsuion and in 2nd minute in comparison to the baseline values. This initial rise was followed by a recovery to baseline values by 4th minute after release of occlusion in the control group, whereas in COPD group this initial rise was followed by a recovery to near baseline values by 3rd minute after release of occlusion.
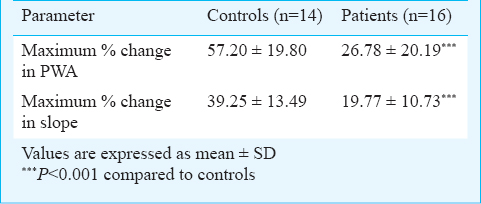
Magnitudes of peak responses in pulse wave amplitude and slope were assessed by calculating maximum percentage change during reactive hyperemia with reference to baseline means in both the groups. Maximum percentage changes in pulse wave amplitude and pulse wave slope were significantly (P<0.001) lower in the patient group as compared to controls (Table II). No significant difference in TNF-α levels was observed between COPD and control groups [3.59 (2.52-4.96) vs 3.35 (2.24-4.54) pg/ml], while a significant difference in hs CRP levels was observed between the two groups [0.58 (0.58-1.06) vs 10.45 (1.89-15.00) mg/l].
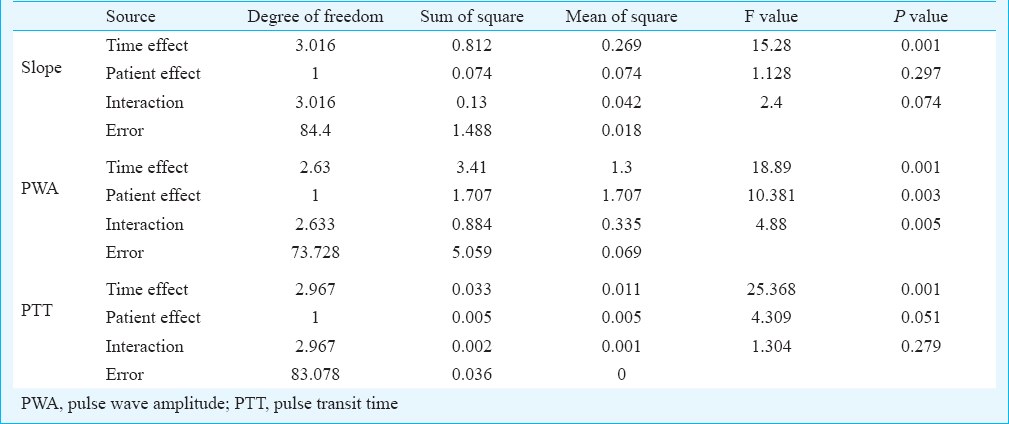
Regarding interaction effect, slope changes over a period of time and interaction between the groups were not significant. Pulse wave amplitude changes over a period of time and interaction between the groups were significant (P=0.005). Pulse transit time changes over a period of time and interaction between the groups were not significant (Table III). Thus, controls were found to have significantly different progression compared to patients in PWA with time following occlusion, appeared to be significantly different in slope and not different in PTT with time.
Discussion
In this study, reactive hyperemia was chosen as a physiological model to assess the vascular reactivity to temporary arrest of circulation. The temporary arterial occlusion leads to vasodilatation of resistance vessels and decrease in tone of both resistance and conduit arteries due to myogenic vasorelaxation and metabolic mechanisms2021. The release of arterial occlusion to restore the circulation leads to transient augmentation of blood flow to the ischaemic tissues via resistance vessels and flow mediated vasodilatory response in the proximal conduit vessel10. The maximum percentage changes in pulse wave amplitude and slope responses during reactive hyperemia have been shown to coincide with peak responses of flow mediated dilatation of brachial artery done by B mode ultrasound technique111215. The PWA response during reactive hyperemia was dependent on endothelial nitric oxide synthesis as assessed by blocking nitric oxide (NO) synthesis with nitro L-NG nitroarginine methyl ester (L-NAME)12. The vascular tone changes in the conduit and resistance arteries occur primarily due to myogenic vasorelaxation during arterial occlusion and also partly dependent on flow mediated release of nitric oxide during reactive hyperemia152223.
In this study, higher PWA and slope responses during the first, second and third minutes of reactive hyperemia in the control subjects as compared to baseline indicated normal flow mediated vasodilatation of the proximal conduit vessels. The PWA changes were in agreement with the earlier study by Selvaraj et al15 in healthy subjects. In COPD patients, significantly lower PWA and slope responses during RH as well as maximum percentage changes in pulse wave amplitude and slope indicated a significant impairment of endothelial dysfunction in conduit arteries. Similar impairment in endothelial dysfunction has been shown in earlier studies using different techniques4524.
The vascular tone significantly decreased (increase in pulse transit time) from the baseline subsequent to release of occlusion followed by recovery towards baseline values in both control and patient groups. However, PTT values remained significantly higher than baseline values in 3rd min of RH in controls but not in patients with COPD. This could be due to loss of vasodilatory response in COPD that might have contributed to non-sustainence of decrease in tone as compared to controls.
On release of occlusion, the shear stress due to flowing blood causes endothelium to cause release of NO that causes vasodilation in conduit vessels. The response starts immediately on release of occlusion and peaks between 60-90 sec10. In the present study, significant interaction between group and time suggested that the endothelial response to shear stress was different amongst the two groups not only in terms of quantum but also in its temporal profile during the phase of RH indicating a lower systemic reactivity following releasae of occlusion at each time point in COPD patients and thus an impaired endothelial function. Impairment of vasodilatory function is one of the earliest manifestations of atherosclerotic changes in a vessel and may contribute to increased risk of cardiovascular morbidity in these patients.
Raised hs CRP levels in COPD patients as compared to controls have been reported earlier24; hs CRP decreases nitric oxide availability leading to endothelial dysfunction25. It has been shown to be an important determinant of endothelium mediated vascular function in coronary artery disease patients26 as well as healthy subjects27.
In our study, levels of TNF-α were not significantly different in the two groups. Karadag et al28 have reported similar results in stable COPD patients. Another group reported raised TNF-α only in lean patietns with COPD as compared to patient with normal BMI29. This may explain the lack of higher TNF-α in the present study as patients with COPD had BMI in the normal range.
In conclusion, the endothelium dependent vascular dilatation was found to be impaired in the stable patients with COPD. hs CRP, as a marker of inflammation was also raised in COPD patients. Thus, systemic inflammation and dysfunction of the endothelium together may contribute to the occurrence of cardiovascular complications in patients with COPD. However, our study being preliminary in nature had a limitation of small sample size. Therefore, studies with appropriate design and large sample size need to be done to confirm these findings.
Acknowledgment
The authors acknowledge the research grant received from All India Institute of Medical Sciences, New Delhi.
Conflicts of Interest: None.
References
- Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532-55.
- [Google Scholar]
- Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases?. The potential role of systemic inflammation in chronic obstructive pulmonary disease. Circulation. 2003;107:1514-9.
- [Google Scholar]
- Impaired flow-mediated dilation is associated with low pulmonary function and emphysema in ex-smokers: the Emphysema and Cancer Action Project (EMCAP) Study. Am J Respir Crit Care Med. 2007;176:1200-7.
- [Google Scholar]
- Reduced six-minute walking distance, high fat-free-mass index and hypercapnia are associated with endothelial dysfunction in COPD. Respir Physiol Neurobiol. 2012;183:128-34.
- [Google Scholar]
- Endothelial dysfunction in peripheral arterial disease is related to increase in plasma markers of inflammation and severity of peripheral circulatory impairment but not to classic risk factors and atherosclerotic burden. J Vasc Surg. 2003;38:374-9.
- [Google Scholar]
- Atorvastatin lowers C-reactive protein and improves endothelium-dependent vasodilation in type 2 diabetes mellitus. J Clin Endocrinol Metab. 2002;87:563-8.
- [Google Scholar]
- A self-fulfilling prophecy: C-reactive protein attenuates nitric oxide production and inhibits angiogenesis. Circulation. 2002;106:913-9.
- [Google Scholar]
- Impaired vasodilation of forearm resistance vessels in hypercholesterolemic humans. J Clin Invest. 1990;86:228-34.
- [Google Scholar]
- Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257-65.
- [Google Scholar]
- Finger photoplethysmogram pulse amplitude changes induced by flow-mediated dilation. Physiol Meas. 2008;29:625-37.
- [Google Scholar]
- Role of nitric oxide in the regulation of digital pulse volume amplitude in humans. J Appl Physiol. 2006;101:545-8.
- [Google Scholar]
- GOLD Scientific Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disese. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163:1256-76.
- [Google Scholar]
- Monitoring of reactive hyperemia using photoplethysmographic pulse amplitude and transit time. J Clin Monit Comput. 2009;23:315-22.
- [Google Scholar]
- Peripheral vascular endothelial function testing as a noninvasive indicator of coronary artery disease. J Am Coll Cardiol. 2001;38:1843-9.
- [Google Scholar]
- An analysis of variance test for normality (Complete sample) Biometrica. 1965;52:591-611.
- [Google Scholar]
- Significance test for sphericity of a normal n-variate distribution. Ann Math Stat. 1940;11:204-9.
- [Google Scholar]
- The role of myogenic relaxation, adenosine and prostaglandins in human forearm reactive hyperaemia. J Physiol (Lond). 1987;389:147-61.
- [Google Scholar]
- On the role of mechanosensitive mechanisms eliciting reactive hyperemia. Am J Physiol Heart Circ Physiol. 2002;283:H2250-9.
- [Google Scholar]
- Effect of reactive hyperemia on carotid-radial pulse wave velocity in hypertensive participants and direct comparison with flow-mediated dilation: a pilot study. Angiology. 2010;61:100-6.
- [Google Scholar]
- Flow-mediated changes in pulse wave velocity: a new clinical measure of endothelial function. Eur Heart J. 2006;27:302-9.
- [Google Scholar]
- Determinants of systemic vascular function in patients with stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;178:1211-8.
- [Google Scholar]
- Cross-sectional evaluation of brachial artery flow-mediated vasodilation and C-reactive protein in healthy individuals. Eur Heart J. 2004;25:1754-60.
- [Google Scholar]
- Relation of inflammation to vascular function in patients with coronary heart disease. Atherosclerosis. 2000;149:403-11.
- [Google Scholar]
- Endothelial dysfunction as a possible link between C-reactive protein levels and cardiovascular disease. Clin Sci. 2000;98:531-5.
- [Google Scholar]
- The value of C-reactive protein as a marker of systemic inflammation in stable chronic obstructive pulmonary disease. Eur J Intern Med. 2008;19:104-8.
- [Google Scholar]
- Tumor necrosis factor-alpha levels and weight loss in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1994;150:1453-5. J
- [Google Scholar]






