Translate this page into:
Immunologic response among HIV-infected patients enrolled in a graduated cost-recovery programme of antiretroviral therapy delivery in Chennai, India
Reprint requests: Dr Suniti Solomon, Director, YR Gaitonde Centre for AIDS Research & Education, VHS Adyar, Taramani, Chennai 600 113, India e-mail: suniti@yrgcare.org
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Sustainability of free antiretroviral therapy (ART) roll out programmes in resource-limited settings is challenging given the need for lifelong therapy and lack of effective vaccine. This study was undertaken to compare treatment outcomes among HIV-infected patients enrolled in a graduated cost-recovery programme of ART delivery in Chennai, India.
Methods:
Financial status of patients accessing care at a tertiary care centre, YRGCARE, Chennai, was assessed using an economic survey; patients were distributed into tiers 1- 4 requiring them to pay 0, 50, 75 or 100 per cent of their medication costs, respectively. A total of 1754 participants (ART naïve = 244) were enrolled from February 2005-January 2008 with the following distribution: tier 1=371; tier 2=338; tier 3=693; tier 4=352. Linear regression models with generalized estimating equations were used to examine immunological response among patients across the four tiers.
Results:
Median age was 34; 73 per cent were male, and the majority were on nevirapine-based regimens. Median follow up was 11.1 months. The mean increase in CD4 cell count within the 1st three months of HAART was 50.3 cells/μl per month in tier 1. Compared to those in tier 1, persons in tiers 2, 3 and 4 had comparable increases (49.7, 57.0, and 50.9 cells/μl per month, respectively). Increases in subsequent periods (3-18 and >18 months) were also comparable across tiers. No differential CD4 gains across tiers were observed when the analysis was restricted to patients initiating ART under the GCR programme.
Interpretation & conclusions:
This ART delivery model was associated with significant CD4 gains with no observable difference by how much patients paid. Importantly, gains were comparable to those in other free rollout programmes. Additional cost-effectiveness analyses and mathematical modelling would be needed to determine whether such a delivery programme is a sustainable alternative to free ART programmes.
Keywords
ART
cost-recovery programme
HIV
India
treatment outcomes
Highly active antiretroviral therapy (HAART) has revolutionized the management of HIV by suppressing viral replication and prolonging survival12345. Data from the US and Europe suggest that current survival of treated HIV-infected persons is comparable to the general population at least in the initial five years after the initiation of ART6. Initially HAART was expensive and inaccessible to about 85 per cent of HIV-infected persons living in the developing world7. The introduction of free antiretroviral therapy (ART) rollout programmes by national governments and programmes such as the President Emergency Plan for AIDS Relief (PEPFAR) have greatly improved access to HAART in the developing world. At the end of 2011, 8 million people were estimated to be receiving ART in low- and middle-income countries7.
Despite the success of these programmes, several factors ensure that the number of people requiring HAART will continue to grow. First, there is a continued lack of effective prevention measures; in 2007, for every two patients initiated on HAART, five new infections occurred8. Additionally, recent data from randomized clinical trials, observational and simulated studies suggest that patients should be initiated on HAART at higher than currently recommended CD4 counts to maximize survival and secondary prevention benefits of HAART91011121314. As more people require HAART, sustainability of programmes to provide lifetime ART to a patient free-of-charge is questionable especially with agencies such as the Global Fund to fight AIDS, TB and Malaria (GFATM) reporting a deficit in funds15.
India is home to approximately 2.3 million HIV-infected persons, and HAART has been available free-of-charge via the Government of India's (GOIs) programme since 20041617. As of December 2010, about 385,000 persons have been initiated on HAART via ART programmes in the government and the private sector combined17. In 2008, there were about 50,000 patients on ART in the private sector18. Assuming an annual cost of USD 200 (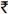 11,000) for a first-line regimen19 and approximately 300,000 persons receiving ART in the government ART sector, the GOI currently spends about USD 60 million (
11,000) for a first-line regimen19 and approximately 300,000 persons receiving ART in the government ART sector, the GOI currently spends about USD 60 million (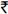 328 crores) per year on ART.
328 crores) per year on ART.
The YR Gaitonde Centre for AIDS Research and Education (YRGCARE) medical center is located in Chennai, India, and has initiated over 4,500 patients on HAART. In 2005, as part of a grant from the GFATM, YRGCARE initiated a graduated cost recovery (GCR) programme for HAART delivery in which HAART costs are distributed across economic strata such that high-income groups subsidize HAART expenses for low-income groups. The objective of this analyses was to describe the immunological outcomes of 1,754 HIV-infected patients who received HAART through this programme between 2005 and 2008 at YRGCARE, Chennai.
Material & Methods
Study setting: YRGCARE is a not-for-profit, non-governmental organization established in 1993 and is among the largest providers of HIV care in the Indian private sector. YRGCARE is not a government recognized free ART rollout center; therefore, patients must pay for their medications out-of-pocket. In 2004, YRGCARE was awarded a grant by the GFATM (Round II Objective III) to implement a graduated cost recovery (GCR) model of HAART delivery, which was initiated in February 2005.
Study population: The study population comprised a subset of patients on HAART at YRGCARE. The criteria for inclusion in the GCR programme were modified once during the course of the programme. Between February 2005 and March 2007, patients were eligible for inclusion in the GCR programme if they were (i) ART naïve and being initiated on a first-line regimen; or (ii) had initiated HAART after May 2004 (when the GFATM award was granted); or (iii) were substituting a medication in their ART regimen (e.g. efavirenz for nevirapine, or zidovudine for stavudine) irrespective of date of initiation. From April 1, 2007, all patients on HAART irrespective of initiation date or regimen type were considered eligible for the GCR programme. A total of 2095 patients were enrolled into the GCR programme between February 2005 and January 2008 (1227 were enrolled prior to April 1, 2007 and 868 were enrolled on or after April 1, 2007). At the time of analysis, 1754 had at least one visit after enrolling in the programme and constituted the analytic sample.
Graduated cost-recovery (GCR) programme: A survey instrument was developed in collaboration with the Department of Economics, University of Madras, Chennai. This survey captured income/expense information on six main domains which were weighted as follows: (i) income (personal and household) – 25 per cent; (ii) occupation -15 per cent; (iii) current value of household assets (e.g. property, possession of luxury products such as televisions, refrigerators, air-conditioners -10 per cent; (iv) household medical expenses (e.g. HIV-related as well as other chronic diseases such as diabetes and hypertension) relative to household income -25 per cent; (v) recurring monthly expenses (e.g. utility bills, loans) relative to household income -10 per cent; and (vi) number of dependents -15 per cent20. For the six economic indicators, the weighted score ranged from 100-400.
This survey was administered to 100 consecutive patients who visited the YRGCARE HIV clinics. Based on their responses, cut-off values were established for the four pre-determined categories: tier 1- free HAART: scores 100-150; tier 2 -patient pays 50 per cent of maximum retail price (MRP): scores 151-190; tier 3- patient pays 75 per cent of MRP: scores 191-280; and tier 4 -patient pays 100 percent of the MRP: scores 281-400. Patients were also asked about their willingness to pay for their medications and were given four options: 0, 50, 75 or 100 per cent of their medication costs. If the patients’ willingness to pay was higher than what was determined by the survey, the patients’ tier was modified to the higher payment category. After these cut-offs were established, the instrument was administered to another 200 consecutive patients; validity was assessed by comparing the proportions in each tier among the first 100 to the next 200.
YRGCARE established an arrangement with the pharmaceutical companies in which antiretrovirals (ARVs) were provided to the YRGCARE pharmacy at 60 per cent of the MRP for the GCR programme. Based on this price reduction, it was estimated that to provide a particular ART regimen (e.g. stavudine plus lamivudine plus nevirapine or zidovudine plus lamivudine plus efavirenz) free-of-charge to 20 per cent of patients and recover 100 per cent of the cost price for that particular regimen, 20 per cent of the patients had to pay 50 per cent of the MRP, 40 per cent of patients had to pay 75 per cent of the MRP, and 20 per cent had to pay 100 per cent of the MRP for the same regimen. Accordingly, patients initiating each regimen were distributed at a ratio of 1:1:2:1 across tiers 1, 2, 3 and 4 (depending on the results of their GCR survey), respectively. The block size for recruitment for each regimen was 100, permitting 20 patients to get medications for free, 20 for 50 per cent of MRP, 40 for 75 per cent of MRP and 20 for 100 per cent of MRP. If certain tiers within a block were filled up, patients who qualified for the same tier had to wait until that block was completely filled and a new block for the same regimen became available. The survey instrument was re-administered annually to assess changes in patients’ ability/willingness-to-pay, with appropriate modification of tiers.
All patients registered in the GCR programme were monitored with complete blood counts, liver function tests, and CD4+ cell counts, which were performed free-of-charge every three months for all enrollees regardless of tier. Physician consultation costs were also waived. The costs of the monitoring tests and physician costs were covered under the GFATM grant. Prior to the implementation of the GCR programme, nearly all patients had to pay MRP for antiretrovirals at the YRGCARE pharmacy as well as monitoring tests and physician consultations. A small number of patients below the poverty line were provided ART free-of-charge by means of independent donations from private donors; these patients were not included in the analysis. Thus, no patients experienced an increase in the cost of their medications as a result of their participation in the programme. The provision of free monitoring tests and waiver of consultation of fees were included as incentives for the enrollment of tier 4 patients (100% MRP), the only tier for which the cost of ART did not decrease after implementation of the GCR programme.
Statistical analysis: Data were extracted from a comprehensive electronic health management system database available at YRGCARE. This database contains information related to GFATM enrollment, demographic and risk behaviour information from the time of diagnosis of HIV infection, HIV natural history data [e.g. CD4+ cell counts, body weight, opportunistic infections (OIs)] and additional laboratory information (e.g. haemoglobin). Differences across tiers at baseline were compared using the Kruskal-Wallis test for continuous variables and the Chi-squared test for categorical variables.
Linear regression was used to characterize predictors of changes in the CD4+ cell count per month on HAART (slope) after enrollment in the GCR programme. The regression model incorporated generalized estimating equations (GEE) to account for repeated correlated measurements within individuals over time; the exchangeable correlation structure was specified. The primary exposure of interest is expressed as an interaction between the GCR programme tier and time on HAART. This interaction term was included to account for the fact that patients were enrolled into the GCR program at varying points after initiation of therapy. Time on HAART was stratified according to the biphasic CD4+ response that was observed when HIV-infected patients were initiated on HAART: <3 months, during which a rapid increase in CD4+ cell count is observed due to increases in the number of memory and naïve CD4+ T-lymphocytes; 3-18 months, in which a less dramatic increase in CD4+ cell count is expected that can be attributed to increases only in the naïve CD4+ T-lymphocytes; and >18 months2122. Thus differences across the programme tiers were expressed separately for the three different strata of time since HAART initiation. For ease of comparison, the reference was changed such that within each time on HAART stratum, immunological response could be compared among tiers 2, 3 and 4 to a single reference. A sub-analysis restricted to those individuals who initiated ART after the GCR programme (n=244) was also conducted. Two sensitivity analyses that included proportion of patients with CD4 cell count >200 cells/μl and >350 cells/μl as the outcome were performed and logistic regression was used with generalized estimating equations. Results did not differ by payment tier (data not shown).
Because there were only a few individuals in the <3 month stratum, the minimum detectable difference in CD4 slope was calculated across tiers given several parameters. We assumed the actual observed sample sizes within each stratum, a two-sided alpha=0.05, 80 per cent power, a standard deviation of 20 cells/μl and a correlation within individuals of 0.4-0.7. All statistical analyses were performed using Intercooled STATA Version 10.1 (College Station, TX, USA).
Results
The distribution of the 1754 patients was as follows: tier 1: n=371; tier 2: n=338; tier 3: n=693; tier 4: n=352. The median age was 34 yr [interquartile range (IQR): 30-40]. 73 per cent were male, and the predominant mode of HIV transmission was heterosexual. Persons were registered at YRGCARE for a median 16 months prior to enrollment in the programme. Of these, 1505 (86%) were on HAART prior to enrolling in the programme, and the remaining initiated HAART through the programme. The median CD4+ cell count at the time of HAART initiation was 188 cells/μl (IQR: 99-352), and 606 (60%) patients were classified as WHO stage III/ IV. The median follow up time after enrollment into the GCR programme was 11.1 months (IQR: 5.2-21.5).
Persons in higher tiers (who paid more for medications) tended to be older and were more often male (Table I). Persons in higher tiers were also more likely to have initiated HAART prior to the programme, but this difference was not significant. Those in higher tiers also tended to have higher CD4 cell counts, lower WHO disease stage, higher haemoglobin levels and body weight (P<0.0001) compared to those in lower tiers. Persons who paid more for their medication were also more likely to be on a protease inhibitor (PI)-based regimen.
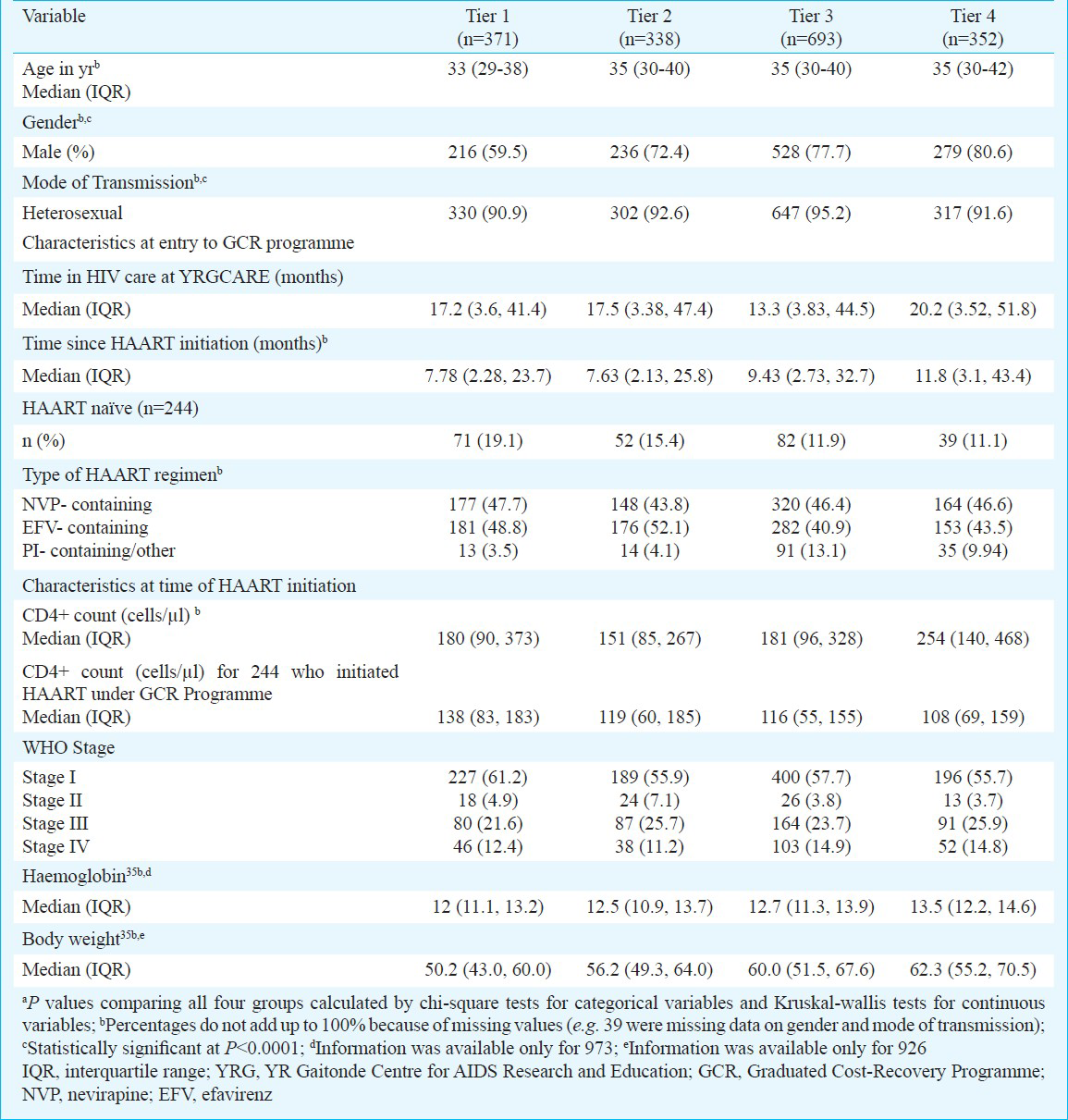
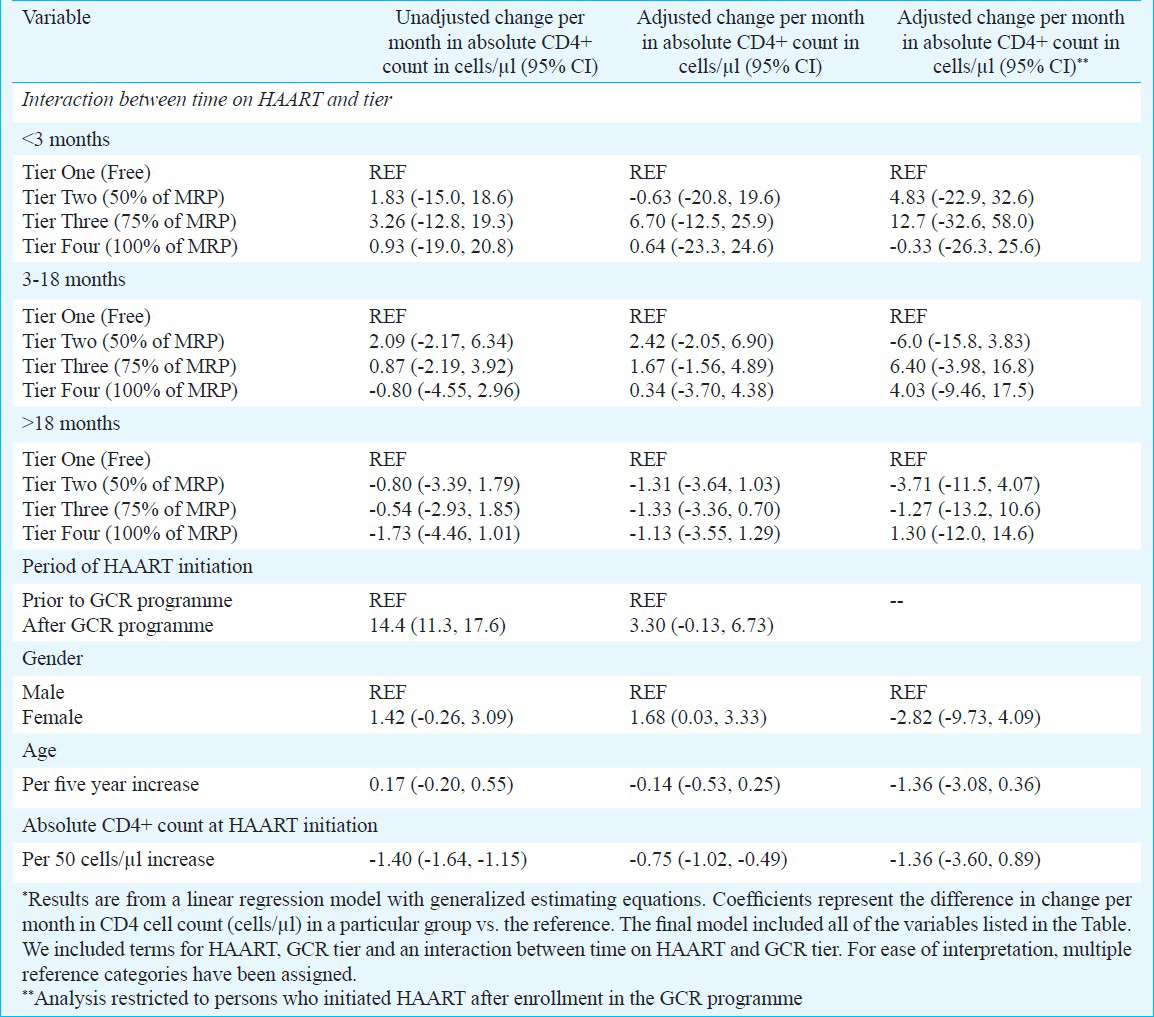
Changes in CD4 cell count after enrollment in the cost-recovery programme: The mean increase in CD4+ cell count within the 1st three months of HAART was 50.3 cells/μl per month in tier 1 (95% CI: 35.9, 64.7). Compared to those in tier 1, persons in tiers 2, 3 and 4 had comparable increases (49.7, 57.0, and 50.9 cells/μl per month, respectively). After this initial period, increases in all groups were comparable for both 3-18 months and >18 months (Figure). When analysis was restricted to those who initiated HAART after being enrolled in the GCR programme, results were similar (data not shown).
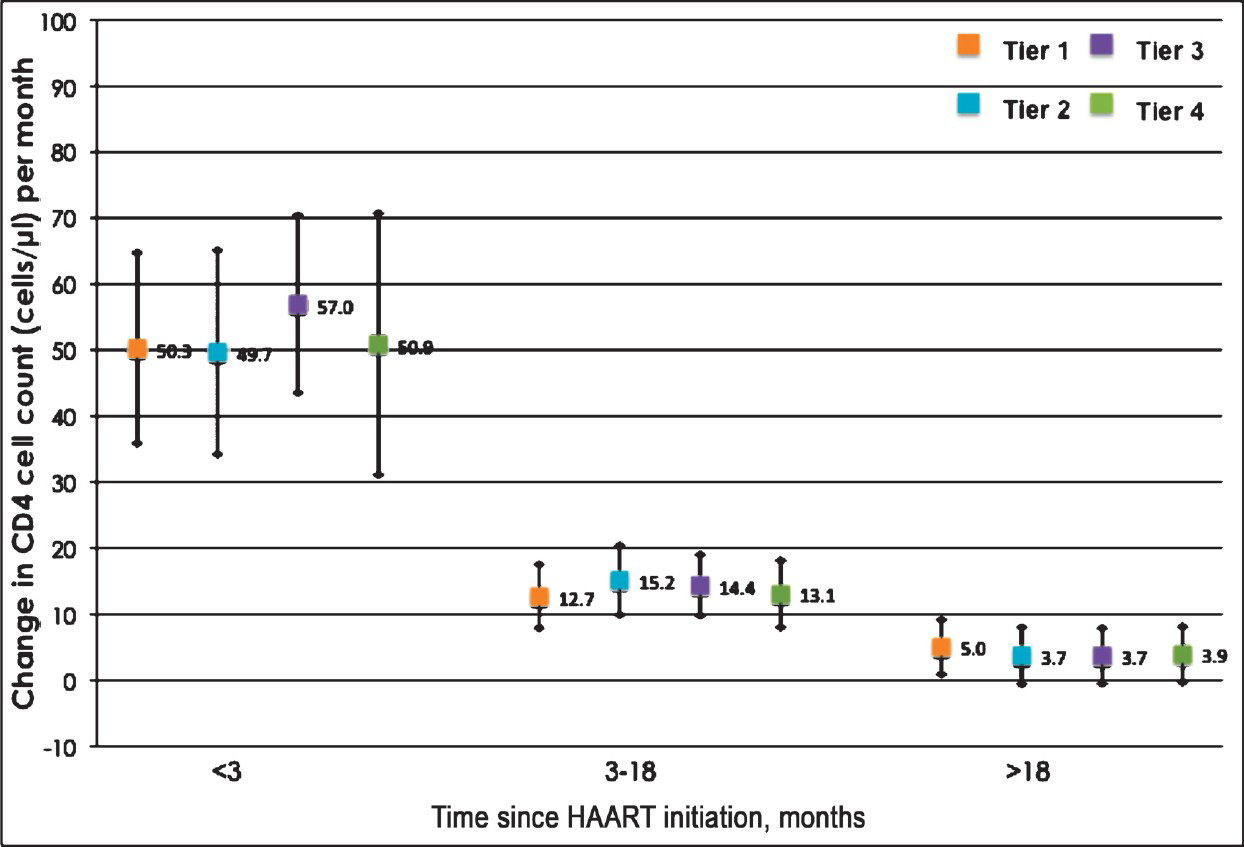
- Mean gain and 95% confidence interval in CD4+ T-lymphocyte count in cells/μl per month stratified by tier and time since HAART initiation among patients enrolled in the GFATM-funded graduated cost recovery (GCR) programme in Chennai, India. Tier 1: Free; Tier 2: 50% of maximum retail price (MRP); Tier 3: 75% of MRP; Tier 4: MRP.
In univariate analysis comparing the rate of change in CD4+ cell count over time (cells/μl per month), no significant difference was observed across tiers regardless of the time since HAART initiation (Table II). Other factors significantly associated in univariate analysis with change in CD4 count included initiating HAART through the GCR programme and CD4 count at the time of initiation. In a multivariate analysis that adjusted for these factors and other factors deemed important a priori, there was still no significant difference in the rate of CD4 increase by tier, after accounting for the fact that persons were at different stages in their treatment trajectory. For example, between 3-18 months after HAART initiation, compared to those in tier 1, persons in tier 2 gained 2.42 more cells/μl per month (95% CI: -2.05, 6.90), persons in tier 3 gained 1.67 more cells/μl per month (95% CI: -1.56, 4.89) and those in tier 4 gained 0.34 more cells/μl per month (95% CI: -3.70, 4.38). After adjustment, only higher CD4+ cell counts at initiation and female gender remained significantly associated with CD4 change. Further, when the analysis was limited to individuals who initiated HAART after enrollment into the GCR programme, no significant differences were observed in CD4 gains across tiers (Table II).
Discussion
The GCR programme at YRGCARE stratified patients into tiers according to their ability to pay. These preliminary data of comparable immunological outcomes across tiers suggest that out-of-pocket payment programmes tailored to address disparities in income may be a feasible way to deliver HAART to HIV-infected persons in resource constrained settings.
The majority of ART programmes worldwide are free rollouts through the local government and/or via organizations such as PEPFAR in collaboration with the local government and/or other organizations such as Médecins sans Frontières (MSF). The gains experienced by patients across all tiers in our programme were comparable to those observed in those programmes, which provide medication free-of-charge5232425262728. For example, a study from Malawi observed that among 1266 patients receiving HAART, the median increase in CD4+ cell count over 8 months was 165 cells/μl23. A study of the national programme in Nigeria involving 50,000 patients observed increases in CD4+ cell count from 260 cells/μl at initiation of HAART to 370 cells/μl 6 months after initiation24. An analysis of patients receiving HAART through the MSF programme in Cambodia reported gains in CD4+ cell count of 101 and 154 cells/μl at 6 and 12 months post HAART initiation25. The gains in CD4+ cell count in this programme were also comparable to the government of India's free ART rollout programme: 6 months- 142 cells/μl and 12 months- 184 cells/μl5.
The primary difference between these programmes and our programme was cost of the programme. Costs associated with the implementation of our programme essentially included salaries for the physicians, nurses, counselors and interviewers, the laboratory costs associated with monitoring of therapy and operational costs. The cost of HAART itself was cross-subsidized across the various tiers; therefore, there were no costs associated with the medications. Operational costs (utilities, accounting, administration, site-maintenance, etc.) were covered by the GFATM grant. Free ART rollout programmes also incur the costs described above including operational costs - additionally, these would also require the full cost of purchasing antiretrovirals. Assuming that a first-line regimen costs approximately 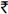 11000 (USD 200) per patient per year19, delivering HAART free-of-charge to the 1,754 patients included in this analysis would have cost an additional
11000 (USD 200) per patient per year19, delivering HAART free-of-charge to the 1,754 patients included in this analysis would have cost an additional 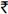 1.9 crores (USD 350,800) per year. Assuming this model was expanded to include all patients enrolled in the GOI's free HAART roll out programme (n~300,000), the cost-savings associated with cross-subsidizing the medications would be
1.9 crores (USD 350,800) per year. Assuming this model was expanded to include all patients enrolled in the GOI's free HAART roll out programme (n~300,000), the cost-savings associated with cross-subsidizing the medications would be 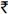 328 crores (USD 60 million) per year. Costs savings could be several orders of magnitude higher if early initiation of treatment becomes the norm (e.g. all persons with HIV are started on treatment regardless of CD4 cell count) as suggested by the HPTN 052 trial14. Future analyses should more formally evaluate the cost-effectiveness of such programmes.
328 crores (USD 60 million) per year. Costs savings could be several orders of magnitude higher if early initiation of treatment becomes the norm (e.g. all persons with HIV are started on treatment regardless of CD4 cell count) as suggested by the HPTN 052 trial14. Future analyses should more formally evaluate the cost-effectiveness of such programmes.
It is important to note that there are certain limitations to this model of ART delivery. First, the ratio of 1:1:2:1 must exist among patients paying 0, 50, 75 and 100 per cent, respectively, of the MRP for each particular regimen. Given that the large majority of the patients accessing care in the free ART programmes and government ART dispensaries fall below poverty line, this model may be difficult to implement. A possible strategy in India would require collaboration between the public (free government roll out where lower-income and impoverished populations access care) and private sectors (wealthier patients who pay out-of-pocket). Currently, different pharmacies serve the private and public sectors, but if common clinics/pharmacies could be established to provide ART to both groups, more high-income patients could be reached, and such a ratio may be attainable. This would require establishment of public-private partnerships for ART delivery.
Second, patients across all tiers should purchase, procure and take their medications in a similar fashion. If there were differences in regular access or adherence to therapy across tiers, the cost recovery mechanism would fail. In depth counselling on the importance of adherence and avoidance of treatment interruption is critical. Third, long-term outcomes of this programme need to be evaluated, especially as patients begin to fail first-line regimens. The costs of second-line regimens are several orders of magnitude higher than those of first-line regimens. Patients who are able to pay the entire cost of a first-line regimen may not be able to pay the cost of a second-line regimen. However, the cost of second-line regimens remains a major barrier in free HAART programs as well2930 further highlighting the importance of adherence counselling and treatment literacy among HIV-infected persons.
The major limitation in this analysis was the lack of HIV RNA data, the gold standard for measurement of treatment response31. However, at YRGCARE, as is the case in most resource-constrained settings, the cost of viral load testing [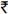 5000 (USD 100) per test] puts it out of reach for most patients on HAART. It is important to note, however, that the primary objective of this analysis was not to compare the efficacy of the GCR model of ART delivery against other free roll out programmes but rather to compare treatment outcomes across four different tiers of patients within the GCR programme. As patients across tiers were of the same ethnicity, were all likely infected with HIV subtype C32 and were exposed to similar ART regimens, one would expect that immunologic-virologic discordance would be comparable across tiers. Thus, it is possible that our finding of comparable immunologic responses is further reflective of similar virologic response33. But CD4 count still remains a strong predictor of risk of AIDS-related conditions and mortality among patients on HAART34. The lack of any formal measure of adherence in this programme was an additional limitation. One further limitation was not having a substantial number of patients who initiated HAART after enrolling in the programme - thus, power to detect differences across tiers for the first three months after HAART initiation was limited. While we had 80 per cent power to detect differences in gains of ±5 cells/month in the other strata (3-18 months and >18 months), we had 80 per cent power only to detect differences of ±20 cells/month in the <3 month strata both because of the small time window and the limited number of patients who initiated HAART after the programme. However, most of the differences observed did not appear to be clinically significant. Finally, a limitation of the GCR instrument itself was the possibility of under-reporting of income, as documentation was not required. However, under-reporting of income would have been similar across all four tiers.
5000 (USD 100) per test] puts it out of reach for most patients on HAART. It is important to note, however, that the primary objective of this analysis was not to compare the efficacy of the GCR model of ART delivery against other free roll out programmes but rather to compare treatment outcomes across four different tiers of patients within the GCR programme. As patients across tiers were of the same ethnicity, were all likely infected with HIV subtype C32 and were exposed to similar ART regimens, one would expect that immunologic-virologic discordance would be comparable across tiers. Thus, it is possible that our finding of comparable immunologic responses is further reflective of similar virologic response33. But CD4 count still remains a strong predictor of risk of AIDS-related conditions and mortality among patients on HAART34. The lack of any formal measure of adherence in this programme was an additional limitation. One further limitation was not having a substantial number of patients who initiated HAART after enrolling in the programme - thus, power to detect differences across tiers for the first three months after HAART initiation was limited. While we had 80 per cent power to detect differences in gains of ±5 cells/month in the other strata (3-18 months and >18 months), we had 80 per cent power only to detect differences of ±20 cells/month in the <3 month strata both because of the small time window and the limited number of patients who initiated HAART after the programme. However, most of the differences observed did not appear to be clinically significant. Finally, a limitation of the GCR instrument itself was the possibility of under-reporting of income, as documentation was not required. However, under-reporting of income would have been similar across all four tiers.
In conclusion, this GCR model of HAART delivery was associated with significant CD4 increases with no observable differences based on how much patients were able to pay. Given the lack of effective prevention measures and the disproportionate rate of new infections compared with persons being initiated on HAART, the long-term sustainability of free HAART programmes will be challenging. Our data suggest that a cost-recovery model may be a feasible alternative; more observational and modelling exercises are needed to evaluate the role of such a GCR programme in the delivery of ART in the developing world.
Acknowledgment
The authors thank the clinicians and the staff at YRGCARE who were responsible for the data extraction and data entry processes and acknowledge Ms. Sangeetha P., Ms. Pearl Paice, Ms. Sripriya P., Shri Suresh James, and Prof. Duraisamy P. who helped with the development and implementation of the GFATM instrument.
References
- Changing patterns of mortality across Europe in patients infected with HIV-1. EuroSIDA Study Group. Lancet. 1998;352:1725-30.
- [Google Scholar]
- Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853-60.
- [Google Scholar]
- The safety, tolerability and effectiveness of generic antiretroviral drug regimens for HIV-infected patients in south India. AIDS. 2003;17:2267-9.
- [Google Scholar]
- The changing natural history of HIV disease: before and after the introduction of generic antiretroviral therapy in southern India. Clin Infect Dis. 2005;41:1525-8.
- [Google Scholar]
- Two-year treatment outcomes of patients enrolled in India's national first-line antiretroviral therapy programme. Natl Med J India. 2010;23:7-12.
- [Google Scholar]
- Changes in the risk of death after HIV seroconversion compared with mortality in the general population. JAMA. 2008;300:51-9.
- [Google Scholar]
- Global report: UNAIDS report on the global AIDS epidemic. Geneva, Switzerland: UNAIDS; 2012.
- [Google Scholar]
- Early versus standard antiretroviral therapy for HIV-infected adults in Haiti. N Engl J Med. 2010;363:257-65.
- [Google Scholar]
- Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009;360:1815-26.
- [Google Scholar]
- Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373:48-57.
- [Google Scholar]
- Effectiveness of highly active antiretroviral therapy in reducing heterosexual transmission of HIV. J Acquir Immune Defic Syndr. 2005;40:96-101.
- [Google Scholar]
- Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet. 2009;373:1352-63.
- [Google Scholar]
- Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493-505.
- [Google Scholar]
- Crisis looms as Global Fund forced to cut back on Aids, malaria and TB grants. 2011. The Guardian 2011, UK on November 23. Available at: http://www.guardian.co.uk/society/sarah-boseley-global-health/2011/nov/23/aids-tuberculosis
- [Google Scholar]
- Scaling up India's Respons to HIV and AIDS: An Overview. In: Oral presentation at XVII International AIDS Conference. August 3-8, 2008. 2008. Abstract #WESAT/30/
- [Google Scholar]
- The cost effectiveness of antiretroviral treatment strategies in resource-limited settings. AIDS. 2007;21:1333-40.
- [Google Scholar]
- Enhancing HAART through graduated cost recovery in GFATM - round II objective III [Abstract no. MOPE0706] In: XVI International AIDS Conference. 2006. Abstract #MOPE0706
- [Google Scholar]
- Biphasic kinetics of peripheral blood T cells after triple combination therapy in HIV-1 infection: a composite of redistribution and proliferation. Nat Med. 1998;4:208-14.
- [Google Scholar]
- T cell changes after combined nucleoside analogue therapy in HIV primary infection. AIDS. 1999;13:1077-81.
- [Google Scholar]
- Scaling up of highly active antiretroviral therapy in a rural district of Malawi: an effectiveness assessment. Lancet. 2006;367:1335-42.
- [Google Scholar]
- Management of HIV-1 infection with a combination of nevirapine, stavudine, and lamivudine: a preliminary report on the Nigerian antiretroviral program. J Acquir Immune Defic Syndr. 2005;40:65-9.
- [Google Scholar]
- Positive outcomes of HAART at 24 months in HIV-infected patients in Cambodia. AIDS. 2007;21:2293-301.
- [Google Scholar]
- Rapid scaling-up of antiretroviral therapy in 10,000 adults in Cote d’Ivoire: 2-year outcomes and determinants. AIDS. 2008;22:873-82.
- [Google Scholar]
- Effectiveness of antiretroviral treatment in a South African program: a cohort study. Arch Intern Med. 2008;168:86-93.
- [Google Scholar]
- Expansion of HIV-1 screening and anti-retroviral treatment programs in a resource-poor setting: results from a faith-based organization in Jos, Plateau State, Nigeria. Afr Health Sci. 2007;7:93-100.
- [Google Scholar]
- Second-line combination antiretroviral therapy in resource-limited settings: facing the challenges through clinical research. AIDS. 2007;21(Suppl 4):S55-63.
- [Google Scholar]
- Antiretroviral roll-out: the problem of second-line therapy. Lancet. 2009;374:185-6.
- [Google Scholar]
- Treatment for adult HIV infection: 2006 recommendations of the International AIDS Society-USA panel. JAMA. 2006;296:827-43.
- [Google Scholar]
- HIV type 1 genotypic variation in an antiretroviral treatment-naive population in southern India. AIDS Res Hum Retroviruses. 2005;21:301-5.
- [Google Scholar]
- Discordant immunological and virological responses to antiretroviral therapy. J Antimicrob Chemother. 2006;58:506-10.
- [Google Scholar]
- Treatment and prognosis of AIDS-related lymphoma in the era of highly active antiretroviral therapy: findings from the Swiss HIV Cohort Study. Antivir Ther. 2007;12:931-9.
- [Google Scholar]
- Tattooing and risk of transmitting HIV in Quthing prison, Lesotho. Int J STD AIDS. 2007;18:363-4.
- [Google Scholar]






