Translate this page into:
HIV-malaria interactions in North-East India: A prospective cohort study
Reprint requests: Dr Sarala K. Subbarao, Division of Epidemiology & Communicable Diseases, Indian Council of Medical Research, V. Ramalingaswamy Bhavan, Ansari Nagar, New Delhi 110 029, India e-mail: subbaraosk@gmail.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
The interactions between HIV and malaria co-infection have been shown to influence each other in their clinical outcomes in Sub-Saharan Africa. This study was carried out in the two States of north east India endemic for both HIV and malaria infections, to study the interactions between the two diseases in the HIV-infected population.
Methods:
In this prospective study, a total of 333 HIV-infected individuals were followed up for a period of 6-18 months in Mizoram and Manipur during 2010-2011. The study assessed the changes in viral load and also the therapeutic efficacy of artesunate plus sulphadoxine-pyrimethamine (AS+SP) combination therapy in HIV-infected and HIV-uninfected individuals with Plasmodium falciparum malaria.
Results:
Viral load in HIV-infected malaria patients on day zero (D0) ranged from 1110 to 147,000 copies/ml. The log transformation of the geometric means of HIV viral loads revealed no significant difference on different days of follow up. There was 100 per cent adequate clinical and parasitological response (ACPR) after treating with artemisinin based combination therapy (ACT) both in HIV-infected and HIV-uninfected P. falciparum-positive individuals. Similarly, chloroquine showed 100 per cent ACPR in P. vivax HIV-infected individuals.
Interpretation & conclusion:
The study showed no significant increase in HIV viral load in malaria cases. All HIV-infected and HIV-uninfected P. falciparum malaria-positive cases responded to the treatment with 100 per cent ACPR.
Keywords
Anti-malaria treatment
CD4 counts
HIV
malaria
north-east States
Plasmodium falciparum
viral load
The impact of interactions of malaria and HIV/AIDS was observed in sub-Saharan countries in Africa where HIV/AIDS epidemics and sTable malaria are endemic12. HIV-infected adults are also known to have more febrile episodes than HIV-uninfected adults due to malarial parasites and other pathogens such as Mycobacterium, Streptococcus and non-typhi Salmonella species3. The effect of HIV-1 viral infection on clinical outcome of malaria in HIV-malaria co-infections in rural Uganda was found to be associated with an increase in frequency of clinical episodes of malaria and also on parasitaemia and the effect was more pronounced in persons with CD4+ counts <20045. Antimalarial treatment failure may be more common in HIV-infected adults with low CD4 cell counts compared to those not infected with HIV678.
Malaria episodes have been found to be associated with at least a transient increase in HIV viral load. In Malawi, HIV-1 plasma viral loads were significantly higher in patients with malaria infection than in those without, and these levels remained higher for up to 10 wk after malaria treatment910. A mathematical model based on data from Kenya, which has a high prevalence of HIV-malaria co-infections suggested that HIV-malaria co-infection could also result in increased HIV transmission due to recurrent malaria episodes2. These studies have shown that in areas with sTable malaria, HIV increases the risk of malaria infection and influences clinical malaria in adults, and malaria may accelerate HIV transmission and possibly disease progression, especially in those with advanced immunosuppression. Hence, one can assume that in settings with unsTable malaria, HIV-infected adults would also be at increased risk of complicated and severe malaria and death. As malaria epidemiology varies in different geographical regions, there is a need to carry out such studies in India. In 2010-2011, India had the third largest number (2.39 million) of people living with HIV/AIDS in the world and an adult prevalence of 0.31 per cent [National AIDS Control Organization (NACO) Report, 2010-2011]11. Malaria has been a major public health problem in the country, and in 2010 and 2011, 1.60 and 1.31 million confirmed malaria cases, respectively, were reported by the National Vector Borne Disease Control Program (NVBDCP). Of the reported cases, 50-52 per cent were due to Plasmodium falciparum12.
In Mumbai, Maharashtra, in one of the studies where HIV infection seropositivity was 1.8 per cent, significantly higher seropositivity of HIV infection was observed in severe P. falciparum cases13. In another study in Mumbai, HIV seropositivity in malaria-positive patients was significantly higher than in healthy blood donors14. In a study reported from Chennai, Tamil Nadu, in stored plasma samples of HIV-cohort, considerable number of malaria co-infections was found. Plasmodium vivax was reported to be the predominant species in Chennai, but the proportion of P. falciparum in co-infections were several folds higher in HIV-malaria co-infected patients than in the general population15. The present study was, therefore, aimed to find out the impact of episodes of malaria on HIV viral loads in Indian populations, and whether the treatment being offered to malaria cases under the National Malaria Control Programme was effective in HIV-infected individuals.
Material & Methods
The study was carried out from December 2009 to November 2011 in West Aizawl and East Aizawl districts of Mizoram and Moreh area of Chandel district bordering Myanmar in Manipur. Both the regions are endemic for malaria and HIV infections. In 2010, malaria annual parasite incidence (API) values were 1.11, 1.24 and 0.01, and in 2011, the API values were 0.32, 0.40 and 0.29, respectively in Aizawl East, Aizawl West and Chandel districts (NVBDCP reports 2010 and 2011)12. During 2010-2011, adult HIV prevalence was 0.78 per cent in Manipur and 0.42 per cent in Mizoram states (NACO report, 2010-2011)11. The study was planned as a prospective study, which included a total of 333 HIV-infected individuals. In Mizoram, the study was conducted in the departments of Pathology and Medicine, Civil Hospital, Aizawl, whereas in Manipur, the study was conducted in the department of Community Medicine, Regional Institute of Medical Sciences and in community health centre (CHC) hospital in Moreh area of Chandel district. The study protocol was approved by the Ethical Committee of the Regional Medical Research Centre (RMRC) Dibrugarh, Assam. Informed written consent was obtained from all participants before enrolment in the study. Participants were free to withdraw from the study at any time.
Selection of HIV-infected individuals: The study was restricted to individuals aged 15-45 yr who were confirmed to be HIV seropositive by standard diagnostic tests. Recruitment of HIV-infected individuals started in January 2010, and a total of 333 consecutive HIV patients (202 from Aizawl district in Mizoram +131 from CHC Hospital in Moreh area of Chandel district in Manipur) were selected for the study following inclusion and exclusion criteria. Of these, in Mizoram males and females were 101 each, whereas in Manipur females were 65 and males 66. Majority of the individuals (279) were enrolled at the initiation of the study and these were followed up for the entire period of the study. The remaining 54 (16.2%) cases (30 from Mizoram and 24 from Manipur) were enrolled 6-7 months before the completion of the study. These 54 persons were followed up for six months period only. Pregnant women and HIV-infected individuals with AIDS-defining illness, with tuberculosis, with filariasis or on malaria prophylaxis were excluded. HIV-infected individuals with CD4 counts ≥250 cells/µl were included in the study, and they were not on ART as per the NACO guidelines that were in practice at the time this study was initiated16. HIV-infected individuals who fulfilled the inclusion criteria were selected from ART Centres of NACO located in the sites selected. Efforts were also made to enrol HIV-infected individuals who visited the Civil Hospital in Aizawl, Mizoram, and Moreh Hospital, Chandel district, Manipur, for any treatment.
Personal data collection and clinical examinations at the time of selection and at follow up visits: Personal information (name, age, marital status, educational qualifications, family structure and smoking habit), body temperature, blood pressure and weight were noted at the time of enrolment and followed up at eight weekly intervals. Finger prick blood was collected for malaria parasite examination. Monitoring for current signs and symptoms of tuberculosis and of history was also done in the beginning. The same procedure was followed at eight weekly follow up visits. Similarly, examinations were done to rule out filariasis and AIDS-defining illness. None of the 333 patients were on co-trimoxazole.
For CD4 count and viral load estimation: Two samples of blood (2-3 ml) were collected from each study participant in ethylenediaminetetraacetic acid (EDTA) vials. Samples collected for CD4 counts were stored at room temperature (20-25°C) and counts were done within 48 h at the study sites. For viral load estimation, plasma was separated within 6 h of collection by centrifugation at 800-1600×g for 10 min at room temperature. Plasma was stored at -20°C and transported maintaining the cold chain to RMRC, Dibrugarh, for HIV-1 viral load estimation.
CD4 absolute counts were performed in BD FACSCount system using BD FACSCount CD4 reagents (BD Biosciences, San Jose, CA, USA) following manufacturer's instructions. HIV-1 viral load was performed in a Cobas TaqMan 48 analyzer (Roche Diagnostics, Rotkreuz, Switzerland) using CE-IVD/FDA-IVD approved Cobas TaqMan HIV-1 test, v2.0., and high Pure System kits (Roche Diagnostics, Mannheim, Germany) following manufacturer's instructions. This test involved real-time TaqMan probe polymerase chain reaction (PCR) technology for the detection of HIV-1 RNA by targeting two highly conserved regions of the HIV-1 genome to compensate for the possibility of mutations or mismatches. Blood samples were collected from all individuals, but only the initial 163 samples were processed to estimate the viral load to get the trend, with an idea that depending on the need stored samples will be used.
Selection of malaria-positive cases for therapeutic efficacy study: At the time of inclusion of HIV-infected cases and during their follow up visits, they were examined for malaria positivity. Those who were found positive for malaria were included in malaria therapeutic efficacy study. For the selection of HIV-uninfected malaria-positive cases, those who visited the Civil Hospital, Aizawl, with fever for treatment and found positive for malaria were requested to undergo HIV test. Those who agreed, as part of the requirement of NACO guidelines17 were counselled by the certified counsellors at HIV-testing centres. Malaria-positive patients found negative for HIV infection and agreed to participate in the study were included as controls.
Diagnosis for malaria: Finger prick blood was used for rapid diagnostic test (RDT) and to prepare thick and thin smears. Blood slides were examined after staining with three per cent Giemsa for 30 min. A bivalent RDT kit (FalciVax, Zephyr Biomedicals) was used to identify P. falciparum and P. vivax parasite antigens. RDT kit was used initially to assess malaria positivity and identify species, which was later confirmed by examining Giemsa-stained blood smears under a microscope. Asexual parasites were counted against 300 leucocytes. Parasite density was expressed as the number of asexual parasites/µl blood by assuming a mean normal leucocyte count of 8000/µl. Of the total blood smears examined, 10 per cent were cross-checked at the RMRC, Dibrugarh, Assam, for quality assurance.
Therapeutic efficacy of antimalarial drugs and HIV estimations: To examine the response to malaria treatment, HIV-infected individuals with mono-infection of P. falciparum were followed up for 42 days and of P. vivax cases for 28 days. Malaria-positive HIV-uninfected individuals were included as controls to study the treatment efficacy. All P. falciparum malaria cases found amongst HIV-infected and also HIV-uninfected individuals were treated with artesunate (4 mg/kg of body weight) for three days (on day 0, 1 and 2), and sulphadoxine (25 mg/kg body weight) plus pyrimethamine (1.25 mg/kg of body weight) as a single dose on day 0 and primaquine (0.75 mg/kg body weight) on day 2. Both the drugs were given orally under the direct supervision of the investigators, and the patients were not admitted in the hospital. P. vivax cases found amongst HIV-infected individuals were treated with chloroquine (10 mg/kg body weight on day 0 and day 1 and 5 mg/kg body weight on day 2) and primaquine (0.25 mg/kg body weight daily) starting on day 1 for 14 days. Monitoring of therapeutic efficacy was done by following up the patients on days 1, 2, 3, 7, 14, 28 and 42 following WHO protocol18. On days 1, 2 and 3, finger prick blood while on days 7, 14, 28 and 42, intravenous 2-3 ml blood were collected from each participant to conduct the required tests. On day 1, temperature was measured; on days 2 and 3, temperature was measured and thick smear was examined for the presence of malaria parasite and on days 7, 14, 28 and 42 in addition to the above viral load estimations were made.
Statistical analysis: Chi-square test was used to calculate the differences in proportions, whereas parasite densities as well as viral load were expressed as geometric mean with range showing maximum and minimum.
Results
A total of 333 HIV-infected individuals (202 cases from Mizoram and 131 cases from Manipur) were included into the study. Male female ratio in both the study areas was 1:1. The age range of participants from Mizoram was between 17 and 50 yr and the mean age was 28.9±6.3 yr, and the age range of the participants from Manipur was 18 and 45 yr and the mean age was 34.5±6.5 yr. Blood pressure was found to be within normal limits and there was no change in their weight during the study. None was suspected to have developed tuberculosis during the study period.
Viral load estimation in HIV-infected individuals: Of the 333 samples collected, 163 samples were used for the estimation of viral load. Of these 163 samples, 134 were validated, and in the remaining 29 samples, viral RNA could not be amplified by quantitative PCR. Variation was observed in the viral loads in both the States ranging from 2680 to 1,790,000 copies/ml blood in Manipur and 257-4,660,000 copies/ml blood in Mizoram.
CD4 counts: Average CD4 counts (geometric mean) of the participants were 443 cells/µl (range 257-1259) in Mizoram and 453 cells/µl (range 251-1422) in Manipur.
Detection of malaria cases among the HIV infected individuals: A total of 22 malaria positive cases were found among the 333 HIV-infected participants with a slide positivity rate (SPR) of 6.6 per cent. Of these, 18 were identified as P. falciparum, three as P. vivax and one as mixed infection of P. falciparum and P. vivax in RDT and the same was confirmed by microscopic examination. These were found in HIV-infected individuals at the time of their enrolment into this study. Among the 202 HIV-infected participants from Mizoram, 10 P. falciparum, three P. vivax and one mixed infection malaria-positive cases with SPR of 5.4 per cent were found. In Manipur, all eight malaria positive cases were of P. falciparum among the 131 HIV-infected persons with a SPR of 6.1 per cent. Parasite density on day 0 of P. falciparum cases ranged from 5560 to 165,000/µl blood in HIV-infected and 12,840-26,120/µl blood in HIV-uninfected. Parasite densities were significantly (P<0.05) higher in HIV-uninfected than in HIV-infected malaria-positive cases. During the 18-month follow up, of the 83.8 per cent of the HIV-infected patients who came for all eight weekly visits, and of the 16.2 per cent who visited three to four times during the study, none were found positive for malaria parasites.
Therapeutic efficacy study in Mizoram and Manipur: Of the 19 cases of P. falciparum detected among HIV-positive cases, four [one with mixed infection with P. vivax, two with low parasitaemia as well as low CD4 counts/µl (<200) and one with low parasitaemia] were excluded. Of the remaining 15 P. falciparum/HIV co-infected cases, 10 from Mizoram and five from Manipur and six HIV-uninfected P. falciparum cases from Mizoram were followed to study the efficacy of artesunate plus sulphadoxine-pyrimethamine (AS+SP) combination treatment. One of the cases among HIV-infected was withdrawn from the follow up as on day 2, the case was identified as having mixed infection. There was 100 per cent adequate clinical and parasitological response (ACPR) after treating with AS+SP combination therapy, both in malaria cases of HIV-infected and uninfected individuals (Table). Fig. 1 shows the proportion of cases having parasitaemia on different days of follow up in the two groups.
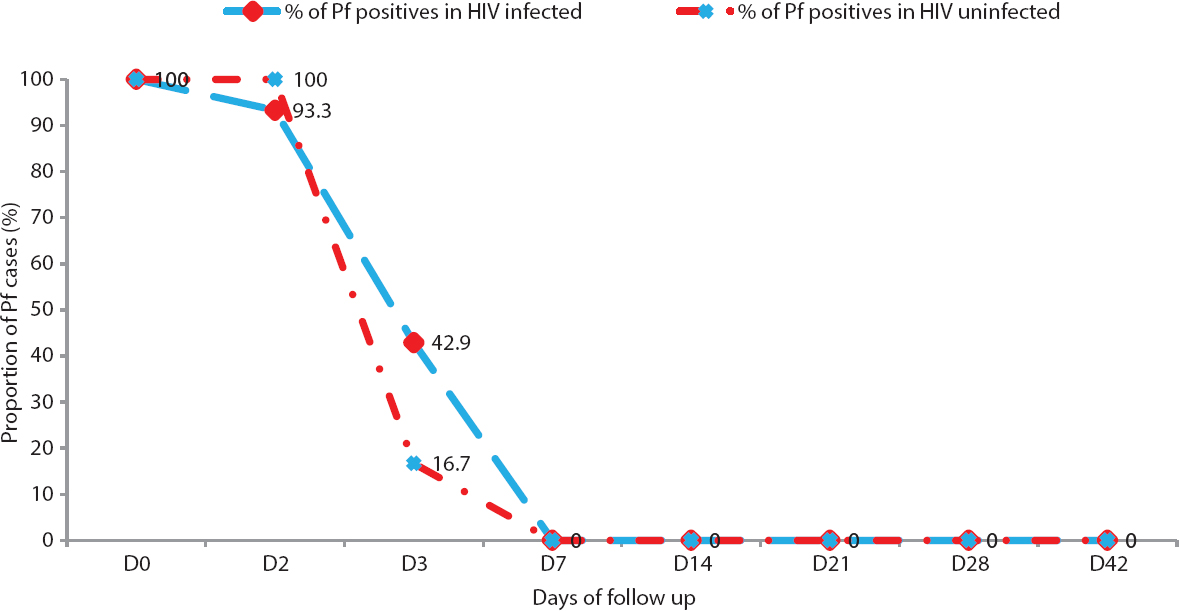
- Proportion of Plasmodium falciparum (Pf)-positive HIV-infected and HIV-uninfected individuals showing clearance of asexual parasitaemia on different days during 42 days follow up in Mizoram and Manipur (2010 - 2011).
Of the 20 malaria cases who showed ACPR, in six cases (42.8%) amongst the HIV-infected (all from Mizoram) and in one (16.7%) of the HIV-uninfected cases parasites were present on day 3, whereas in all other cases, parasite clearance was on or before day 2 (Fig. 1). Parasitaemia on day 3 was <25 per cent, and patients did not have fever and any other symptoms. Although the number of cases with parasitaemia on day 3 was higher in HIV-infected than in HIV-uninfected, the difference in number of cases was non-significant. For the three cases of P. vivax detected among HIV-infected in Mizoram, chloroquine and primaquine were given as per the national drug policy. No HIV-uninfected P. vivax cases could be included for comparison. All three cases were followed for 28 days, and in all three cases, ACPR was observed.
Viral load estimation in HIV-infected individuals during therapeutic efficacy study: Viral load in HIV-infected P. falciparum cases on D0 ranged from 1110 to 147,000 copies/ml blood. A decline in the HIV viral load was observed from day 0 to day 7. From day 7, there was a continuous increase in the viral loads till day 28 and again a decline was observed from day 28 to 42. However, the log transformation of the above geometric means revealed no significant difference in viral load on different days of follow up (Fig. 2).
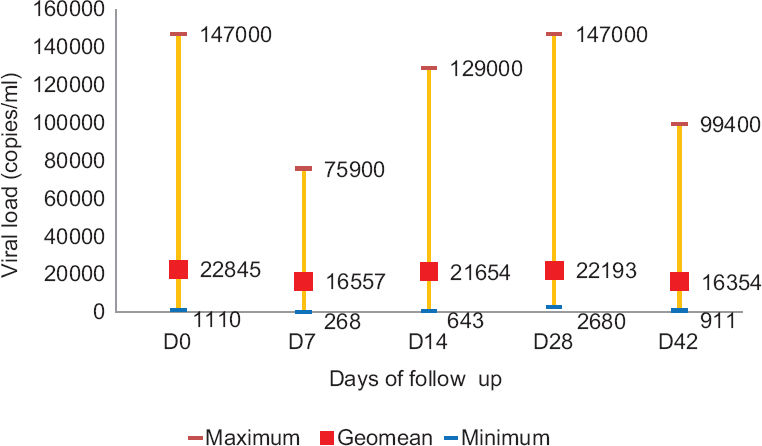
- Variation in geometric mean of HIV viral load during therapeutic efficacy follow up of Plasmodium falciparum cases in Mizoram and Manipur.
Discussion
All malaria-positive cases detected were from HIV-infected who visited the hospital with fever. Later, during the follow up period, no malaria cases were recorded. Increase in viral load was observed in HIV-infected during malaria episode, but there was no systematic and significant increase in viral load. Studies in sub-Saharan Africa have documented increase in HIV-1-RNA during malaria episodes in HIV-infected individuals although this effect was limited to individuals with advanced immune suppression6. This finding was not documented in Indian settings probably because the immune status of the HIV-infected individuals was different with CD4 cell counts being higher (>250). Observations on parasite density in malaria-positive HIV-infected individuals in this study also differed from those in African studies. Parasite density in uncomplicated malaria was significantly higher in HIV-infected cases than in HIV-uninfected cases on day 0 in South Africa, Uganda and Kenya,71920 whereas in our study, it was significantly lower in HIV-infected individuals. Van Geertruyden et al21 reported that the geometric mean parasite density in uncomplicated malaria was related to their immune status. In Manipur and Mizoram study sites, the mean CD4 counts were much higher with a geometric mean of 453 and 443 counts, respectively. It is also possible as demonstrated by Migot et al22 that some of the components of the specific human immune responses to P. falciparum parasites may not be modified during HIV infection in spite of strong cellular alterations induced by HIV. It has been suggested that not all effector cells involved in the immune responses directed against malaria are affected by concurrent HIV-infection, and that important CD4 independent mechanisms of protections against malaria are conserved and HIV infection may be causing selective depletion of T-cell subsets that are not implicated in the antimalarial immune response22. These observations suggest that the phenomenon of increase of HIV viral load and also higher parasite density among malaria-positive HIV-infected individuals needs examination in a larger sample and with CD4 counts <200 cells/µl.
In this study efficacy of antimalarial in the treatment of malaria-positive HIV-infected individuals was studied. As all HIV-infected P. falciparum cases showed ACPR similar to the malaria-positive HIV-uninfected individuals, AS+SP combination therapy seemed effective for the treatment of P. falciparum malaria in HIV-infected cases. Similarly, HIV-infected individuals with P. vivax infection showed ACPR to chloroquine in the northeast. Though the absolute number of cases of delayed parasite clearance in P. falciparum cases was higher among HIV-infected than in HIV-uninfected cases, the analysis did not show any significant association between HIV status and delay in parasite clearance time. The cases of delay in parasite clearance in both HIV positive and negative individuals suggest that parasite in this area may be developing tolerance to the combination drug. In the northeastern States, the drug for uncomplicated P. falciparum has been changed to artemether (20 mg) - lumefantrine (120 mg) combination from 201323, but in other parts of the country artesunate, and sulphadoxine (25 mg/kg body weight) plus pyrimethamine combination continues to be used. Resistance in P. falciparum to sulphadoxine-pyrimethamine has been observed in several parts of the country, but resistance to artemisinin has not been reported earlier24. However, there are reports from Thai-Kampuchea border and Cambodia of P. falciparum developing resistance to artemisinin2526. Even though the drug combination has been changed in the northeastern region, delayed parasite clearance on day 3 in both HIV-infected and uninfected calls for more studies to establish the correct status of artemisinin resistance in P. falciparum in Mizoram and Manipur States. All the malaria-positive cases detected among HIV-infected were at the time of enrolment, and no re-infections or new cases of malaria were found during the follow up. Inclusion of malaria-positive HIV-uninfected controls in the study was also challenging as this required malaria-positive individuals undergoing HIV testing for determining HIV status. Only six malaria-positive individuals agreed to undertake HIV testing. Due to the design of the study and due to low malaria endemicity in the study area during the study period, less number of cases has been assessed for therapeutic efficacy. Thus, there is a need to carry out more studies involving larger samples in a multicentric mode to cover different epidemiological situations in the country and also to include individuals having CD4 counts <200 to resolve the aspects related to co-infection of HIV and malaria.
In conclusion, this study gave an idea that malaria episodes in India did not alter the viral load in HIV-infected persons as seen in Africa. It also showed that the treatments recommended for malaria by the National Malaria Control Programme were effective in HIV-infected persons. Considering the burden of malaria in certain pockets in India, more systematic studies are needed to determine the role that HIV infections might play in different epidemiological settings.
Acknowledgment
Authors acknowledge the encouragement and support given by the Secretary, Department of Health Research and Director General, Indian Council of Medical Research, New Delhi, throughout this study. Authors thank the doctors from community health centres (CHC) in Moreh who helped in the clinical examinations of HIV-infected persons, and acknowledge the field staff of this project and the technical support given by the staff of all the participating institutes.
Conflicts of Interest: None.
References
- Malaria attribuTable to the HIV-1 epidemic, Sub-Saharan Africa. Emerg Infect Dis. 2005;11:1410-9.
- [Google Scholar]
- Dual infection with HIV and malaria fuels the spread of both diseases in Sub-Saharan Africa. Science. 2006;314:1603-6.
- [Google Scholar]
- A prospective study of bloodstream infections as cause of fever in Malawi: Clinical predictors and implications for management. Trop Med Int Health. 2004;9:928-34.
- [Google Scholar]
- Effect of HIV-1 and increasing immunosuppression on malaria parasitaemia and clinical episodes in adults in rural Uganda: A cohort study. Lancet. 2000;356:1051-6.
- [Google Scholar]
- Increasing rates of malarial fever with deteriorating immune status in HIV-1-infected Ugandan adults. AIDS. 2001;15:899-906.
- [Google Scholar]
- Delayed clearance of Plasmodium falciparum in patients with human immunodeficiency virus co-infection treated with artemisinin. Ethiop Med J. 2002;40(Suppl 1):17-26.
- [Google Scholar]
- Effect of HIV-1 infection on antimalarial treatment outcomes in Uganda: A population-based study. J Infect Dis. 2006;193:9-15.
- [Google Scholar]
- Malaria and HIV interactions and their implications for public health policy Report of a technical consultation. Geneva, Switzerland: World Health Organization; 2004.
- The effect of Plasmodium falciparum malaria on HIV-1 RNA blood plasma concentration. AIDS. 1999;13:487-94.
- [Google Scholar]
- Effect of Plasmodium falciparum malaria on concentration of HIV-1-RNA in the blood of adults in rural Malawi: A prospective cohort study. Lancet. 2005;365:233-40.
- [Google Scholar]
- 2011. National AIDS Control Organization. Annual Reports 2009-2010. :1. Available from http://www.naco.gov.in
- National Vector Borne Disease Control Programme. Director General of Health Services, Ministry of Health and Family Welfare, Government of India. Malaria. Available from http://www.nvbdcp.gov.in/malaria3.html
- [Google Scholar]
- Human immunodeficiency virus type 1 infection in patients with severe falciparum malaria in urban India. J Postgrad Med. 2003;49:114-7.
- [Google Scholar]
- HIV and malaria co-infection in Mumbai, Western India. J Vector Borne Dis. 2011;48:155-8.
- [Google Scholar]
- Correlates of HIV and malaria co-infection in Southern India. Malar J. 2012;11:306.
- [Google Scholar]
- Guidelines for management of HIV-infected adults and adolescents including post-exposure prophylaxis, National AIDS Control Organization. Ministry of Health and Family Welfare, Government of India 2007
- [Google Scholar]
- Operational Guidelines for Integrated Counselling and Testing Centres. National AIDS Control Organization. Ministry of Health & Family Welfare. Government of India 2007
- [Google Scholar]
- World Health Organization. 2003. Assessment and monitoring of antimalarial drug efficacy for the treatment of uncomplicated falciparum malaria. Geneva: World Health Organization; Available from http://www.whqlibdoc.who.int/hq/2003/WHO_HTM_RBM_2003.50.pdf?ua=1
- [Google Scholar]
- HIV infection as a cofactor for severe falciparum malaria in adults living in a region of unsTable malaria transmission in South Africa. AIDS. 2004;18:547-54.
- [Google Scholar]
- HIV immunosuppression and antimalarial efficacy: Sulfadoxine-pyrimethamine for the treatment of uncomplicated malaria in HIV-infected adults in Siaya, Kenya. J Infect Dis. 2006;194:1519-28.
- [Google Scholar]
- The impact of HIV-1 on the malaria parasite biomass in adults in Sub-Saharan Africa contributes to the emergence of antimalarial drug resistance. Malar J. 2008;7:134.
- [Google Scholar]
- Selected P. falciparum specific immune responses are maintained in AIDS adults in Burkina Faso. Parasite Immunol. 1996;18:333-9.
- [Google Scholar]
- National Drug Policy for Malaria 2013. National Vector Borne Disease Control Programme. 2013. Director General of Health Service, Ministry of Health and Family Welfare, Government of India. Available from http://nvbdcp.gov.in/Doc/National-Drug-Policy-2013.pdf
- [Google Scholar]
- Antimalarial drug resistance of Plasmodium falciparum in India: Changes over time and space. Lancet Infect Dis. 2011;11:57-64.
- [Google Scholar]
- Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361:455-67.
- [Google Scholar]
- Artemisinin-resistant Plasmodium falciparum in Pursat province, Western Cambodia: A parasite clearance rate study. Lancet Infect Dis. 2012;12:851-8.
- [Google Scholar]






