Translate this page into:
Functional response analysis of Anisops sardea (Hemiptera: Notonectidae) against Culex quinquefasciatus in laboratory condition
Reprint requests: Dr Goutam Chandra, Department of Zoology, Mosquito & Microbiology Research Unit Parasitology Laboratory, Burdwan Universiry, Burdwan 713 104, India e-mail: goutamchandra63@yahoo.co.in
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Culex quinquefasciatus is the principal vector of lymphatic filariasis (LF). Application of alternative vector control methodologies are aimed at reduction of mosquito breeding sites and biting activity through the use of biological control methods. In the present study, functional response of aquatic Hemipteran backswimmer, Anisops sardea was assessed against Cx. quinquefasciatus larvae in laboratory bioassay.
Methods:
The functional respons of A. sardea was assessed against IIIrd instar larvae of Cx. quinquefasciatus. Respective handling times and coefficient of attack rates were determined by a non linear polynomeal regression equation.
Results:
The results of rates of predation in variable prey densities exhibited a ‘linear rise to plateau curve’, associated with ‘Type -II’ functional response. The logistic regression estimated a significant negative linear parameter (P1<0) which also supported the same observation. Associated ‘attack rates’ and ‘handling times’ were also calculated using the Holling Disc Equation.
Interpretation & conclusions:
The results of present experiments indicate that A. sardea can be used as a biocontrol agent against the larval forms of Cx. quinquefasciatus in temporarily available breeding places of mosquito with relatively clear water. However, a detailed field study has to be done to confirm these findings.
Keywords
Anisops sardea
consumption rate
functional response
habitat modification
mosquito larva
Mosquitoes are vectors of many protozoan, viral, and nematode-associated diseases that affect public health. Culex quinquefasciatus is the principal vector of lymphatic filariasis (LF). A current estimate reveals that about 120 million people in 83 countries are infected with LF parasites and more than 20 per cent of the world population are at risk of acquiring filarial infection. In India it is estimated that about 554.2 million people are at risk of LF infection in 243 districts1.
Vector control strategies (either as adulticides or larvicides) include application of chemical, environmental, genetical, biological or integrated control avenues. Considering the better health of ecosystems, biodegradable nature, negligible evidences of resistance development in target species, minimum adverse effect on non-target organisms including human beings and to preserve the faunal biodiversity, the biological control methodology is now widely accepted and practiced throughout the world. There are various options available for biocontrol of immature mosquitoes in temporary and permanent water bodies, such as use of amphibian tadpoles, fishes, predatory insects, cyclopoid copepods, helminthes, etc. Among the predatory insects, the nymphal forms of dragonfly (Odonata: Zygoptera) and damselfly (Odonata: Anisoptera) are widely used in mosquito control studies due to their wide distribution, species diversity and trophic interactions234.
Anisops sardea Herrich-Schaeffer (Hemiptera: Notonectidae) is a small-bodied aquatic backswimmer found in high densities in many temporary pools and permanent water bodies throughout India. Laboratory based predatory experiments have revealed that this species have a high predation rate against larval Culex mosquitoes56. The size selective prey preference of A. sardea against Daphnia was reported by Lindholm and Hessen7. Its effects on oviposition habitat selection of mosquitoes and other dipterans and on community structure have been studied8. Before recommendation of any species in field, a detailed numerical analysis of the predator species against variable prey densities and suitable habitat condition is necessary. Therefore, in the present study an attempt was made to analyze the functional responses of this species against Cx. quinquefasciatus, the most common filarial vector in South East Asia.
Material & Methods
This study was conducted in the department of Zoology, Bankura Sammilani College, Bankura, West Bengal, India.
Collection of prey and predator species: Adult predator species, viz. A. sardea, were collected from the different water bodies of Bankura, West Bengal, India, during May-June 2008, with an insect net having 200-μm mesh size. From the collected predator populations, 50 organisms of almost similar size and morphology were separated and kept in five glass beakers (1 l capacity) containing pond water in the laboratory of Department of Zoology, Bankura Sammilani College. From each of these beakers one organism was collected randomly and altogether five adult organisms were identified from the Zoological Survey of India (ZSI, Voucher specimen No. 62/2008). Each of the specimens was identified as A. sardea by ZSI based on available scientific literatures. However, male/female identifications were not carried out. Specimens of aquatic weeds and gravels were placed inside the aquarium to produce natural conditions. The average body length of A. sardea used in the experiments was 0.6-0.7 mm.
Mosquito larvae were collected from cemented drains of the same area at regular intervals during the experiments as required. After each collection, the third instars larvae of C. quinquefasciatus were separated from the other larval instars based on length and maturity and kept within enamel trays in the laboratory with an adequate amount of food. Third instars larvae were used during the experiments as they are easily visible that make count easy and there is no chance to metamorphose into a pupa unlike fourth instars. The specific instars were identified following the key of Chandra (2000)9 which was prepared based on Christophers (1933)10 and Barraud (1934)11.
The predatory insects were collected 10 days before the commencement of the experiments and were maintained in the laboratory for acclimatization with Cx. quinquefasciatus larvae as food.
Functional response analysis: To each adult specimen of A. sardea, IIIrd instar larvae of Cx. quinquefasciatus were supplied at various prey densities (10, 20, 30, 40, 50, 60, 70, 80 and 90 larvae per 500 ml of pond water) in glass beakers (1l) and were allowed to predate for a period of 24 h within a BOD incubator (Eastern Instrument, India 180cft volume) at a temperature of 34°C (temperature found in the laboratory during the study period), humidity of 80-86%, and photoperiod of 14h L: 10h D.
The analysis of the functional responses was carried out in two steps according to Juliano12 : (i) determination of the type of functional response by nonlinear polynomial logistic regression equation between proportions of prey consumed and given initial prey density; and (ii) estimation of the functional response parameters. The nonlinear polynomial regression equation13 used was as follows:
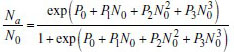
Where Na is the number of prey eaten, N0 is the initial number of prey given, P0, P1, P2, and P3 being the intercept, linear, quadratic and cubic coefficients, respectively. If the linear coefficient is significant and negative i.e., P1<0, then the data describe a type II functional response curve13.
In the second step, the functional response parameters were estimated through a nonlinear regression procedure (NLR; SPSS 2006; IBM). The Holling's Disc equation14 was used to estimate the parameters. The associated equation was
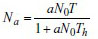
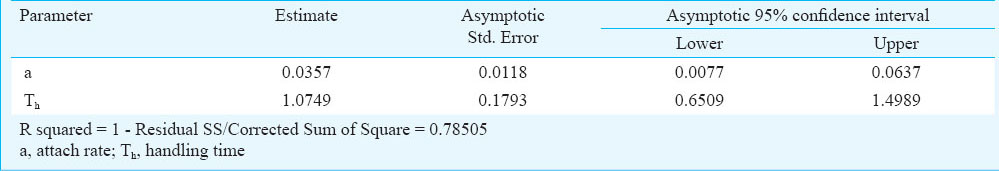
where Na is the number of prey eaten, N0 is the initial number of prey, a is the attack constant, T is the total time available (24 h) and Th is the handling time per prey13.
Nine replicates for each of the prey densities were carried out with nine different adult morphs with similar size for determination of the rate of predation and the type of functional response. During each of the experiments the predators were fed to satiation and then starved for 24 h before their utilization in the experiments to equalize the hunger level. The pond water was collected from aquatic habitats common to predators.
Results & Discussion
The results of rates of predation in variable prey densities are presented in the Figure. Associated regression equation and ‘R’ value are also presented in the same figure. The results exhibited a ‘linear rise to plateau curve’ as the estimated rate of predation increased with initial prey density until it reached the upper asymptote and thereafter remained nearly unchanged or showed inverse density-dependence. Associated intercept, linear, quadratic and cubic coefficient values were given in Table I. The predator species exhibited a ‘Type-II’ functional curve as the logistic regression estimated a significant negative linear parameter (P1<0). Type-II nature of the functional curve was further confirmed from the Figure, where proportion of prey consumed was plotted against given initial prey density. So, the Holling Disc Equation can be used for the estimation of instantaneous attack rate (a) and handling time (Th). The associated attack rates and handling times are presented in Table II.
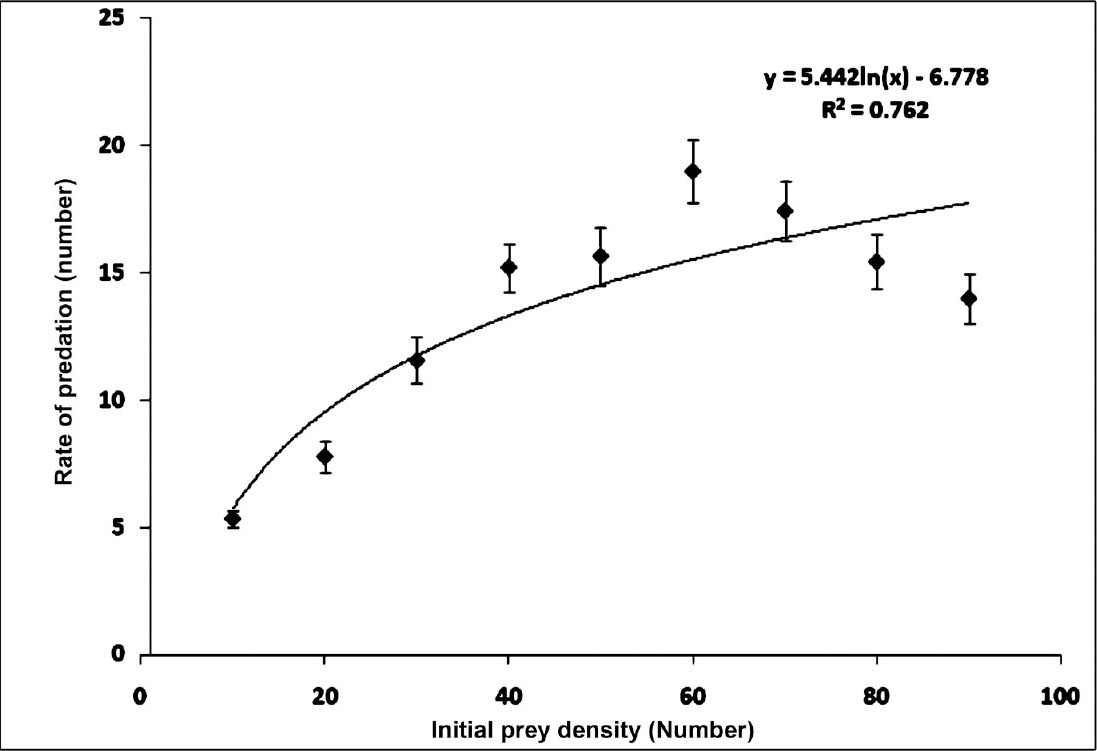
- Predation rate (mean ± standard error) of Anisops sardea against Culex quinquefasciatus (n=9 experiments) with logarithmic trend line.
The functional response of predators against prey describes how the consumption rate of individual consumers changes with respect to resource density over a given time interval1516. The functional responses showed by predators against variable density of prey are of three basic types. It may of Type I, which represents an increasing linear relationship, Type II, that exhibits a decelerating curve, or Type III, that shows a sigmoidal relationship. In most of the single predator-single prey experiments functional response curves associated with insect predators’ exhibits a Type II response615. Functional response has multi dimensional significance from the biological point of view. It works as a principal instrument for understanding the ecology between different kinds of predators and their prey items and depends upon the habitat complexity of an ecosystem. The present study revealed that A. sardea predated a good number of larvae in laboratory condition and exhibited a Type-II response like other insects.
The frequencies of mosquito borne diseases are ever increasing in the tropical countries associated with high mortality and morbidity. The highest incidence of these diseases mainly occurs during the rainy season because of creation of temporary water logging aquatic habitats in nature and household areas that act as a breeding ground of many mosquito species. In the clear aquatic habitats mainly, Anopheles mosquitoes breed, whereas polluted water logged areas act as breeding ground of Culex mosquitoes. Anisops species co-exist with Anopheles mosquitoes in clear aquatic habitats as well as field survey reported its existence with Culex mosquitoes16. Therefore, the augmentative release of the predator species in temporary habitats with polluted water where Culex mosquitoes breed is beneficial as evident from the result of the present study. It is also reported that Anisops releases a Kairomone that prevents oviposition of other mosquitoes17.
In conclusion, the findings of our study show that the application of A. sardea can be effectively used in temporary or permanent water logged habitat of different species of mosquitoes. However, a detailed field study is necessary before its wide application in mosquito control programme.
Acknowledgment
Authors acknowledge the help received from The Director, Zoological Survey of India, Kolkata, West Bengal for identification of the predator species, and Shri Anindya Sen, Assistant Professor in English, Bankura Christian College, West Bengal for revising and improving the quality of the article. Authors also acknowledge the University Grant Commission, New Delhi, for providing financial support to the second author (AG).
References
- Mass durg administration for elimination of lymphatic filariases: Recent experiences from a district of West Bengal, India. J Trop Parasitol. 2013;3:67-71.
- [Google Scholar]
- Predation by odonates depresses mosquito abundance in water filled tree holes in Panama. Oecologia. 1997;112:244-53.
- [Google Scholar]
- Eco-friendly control of mosquito larvae by Brachytron pratense nymph. J Environmental Health. 2007;69:44-8.
- [Google Scholar]
- Biocontrol efficiency of odonate nymphs against larvae of mosquito, Culex quinquefasciatus Say, 1823. Acta Tropica. 2008;106:109-14.
- [Google Scholar]
- The biology of the notonectid Anisops sardea H.S., an active mosquito predator in Egypt. Bull Entomol Soc Egypt. 1986;66:117-26.
- [Google Scholar]
- Competition and niche partitioning in a floodplain ecosystem: a cladoceran community squeezed between fish and invertebrate predation. Afr Zool. 2007;42:158-64.
- [Google Scholar]
- Effects of Anisops sardea (Hemiptera: Notonectidae) on oviposition habitat selection by mosquitoes and other dipterans and on community structure in artificial pools. Hydrobiologia. 2002;485:183-9.
- [Google Scholar]
- The fauna of British India, including Ceylon and Burma. Diptera. In: Family Culicidae. Vol iv. London: Tribes Anophelini. Taylor and Francis; 1933. p. :371.
- [Google Scholar]
- The fauna of British India, including Ceylon and Burma. Diptera. In: Family Culicidae. Vol V. London: Tribes Megarhinini and Culicini. Taylor and Francis; 1934. p. :463.
- [Google Scholar]
- Nonlinear curve fitting. Design an Analysis of Ecological Experiments. New York: Oxford University Press; 2001.
- [Google Scholar]
- Functional responses of coccinellid predators: An illustration of a logistic approach. J Insect Sci. 2005;5:1-6.
- [Google Scholar]
- The functional response of invertebrate predators to prey density. Mem Entomol Soc Can. 1966;48:1-86.
- [Google Scholar]
- Functional responses of Laccotrephes griseus (Hemiptera: Nepidae) against Culex quinquefasciatus (Diptera: Culicidae) in laboratory bioassay. J Vector Borne Dis. 2011;48:72-7.
- [Google Scholar]
- A mosquito predator survey in Townsville, Australia, and an assessment of Diplonychus sp. and Anisops sp. predatorial capacity against Culex annulirostris mosquito immature. J Vector Ecol. 2007;32:16-21.
- [Google Scholar]
- Can adults of the New Zealand mosquito Culex pervigilans (Bergorth) detect the presence of a key predator in larval habitats? J Vector Ecol. 2010;35:100-5.
- [Google Scholar]






