Translate this page into:
Evaluation of interleukin-33 & sST2 levels in type-2 diabetic mellitus patients with or without metabolic syndrome
For correspondence: Dr Rohit Saluja, Department of Biochemistry, All India Institute of Medical Sciences, Bibinagar 508 216, Telangana, India e-mail: drrohitsaluja@gmail.com
-
Received: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Diabetes mellitus (DM) is characterized by increase in blood glucose levels due to defective insulin secretion or insulin sensitivity. Interleukins (ILs) are known to play an important role in the pathogenesis of DM. The aim of this study was to investigate the serum concentration of IL-33 and its receptor soluble ST2 (sST2) in patients with diabetes and draw a correlation between their serum levels and different standard glycaemic indices of patients affected with type-2 diabetes with or without metabolic syndrome.
Methods:
Thirty type-2 diabetic individuals and 30 healthy controls were recruited for this study. Serum and plasma were separated by centrifugation of blood for quantitative measurement of IL-33, sST2 and other biochemical parameters.
Results:
It was observed that serum IL-33 levels were significantly less and sST2 levels were significantly high in type-2 diabetic individuals as compared to healthy controls. A significant correlation between the serum IL-33 concentration and fasting plasma glucose (FPG) and postprandial plasma glucose (PPG) levels were also found. Additionally, data also elucidated that serum levels of high-density lipoprotein, low-density lipoprotein or triglyceride in type-2 diabetics did not influence the serum levels of IL-33 and sST2, thereby excluding these factors as the major drivers of changes in serum IL-33 and sST2 concentration.
Interpretation & conclusions:
This study demonstrated alteration in serum levels of IL-33 and sST2 in type-2 diabetic individuals. Further mechanistic studies, focusing on the progression of type-2 diabetes could elucidate the involvement of IL-33 in the cellular acquisition of insulin resistance as observed in type-2 diabetics.
Keywords
Diabetes mellitus
fasting plasma glucose
interleukin-33
postprandial plasma glucose
soluble ST2
Diabetes mellitus (DM) is a disorder characterized by the abundance of glucose due to a defect in insulin secretion or action. It affects 422 million people worldwide1. Depending on the pathophysiology, DM has been classified into type-1 DM (T1DM) and type-2 DM (T2DM). T1DM is insulin dependent and mainly occurs due to autoimmune destruction of pancreatic insulin producing beta cells. T2DM is mainly due to insulin resistance and is more common in relatively older age groups. Genetic predisposition is also responsible for both T1DM and T2DM2. Higher levels of inflammatory biomarkers [interleukin (IL)-1β, IL-6 and tumour necrosis factor (TNF-α)] were detected in patients diagnosed with T1DM3,4. Serum levels of TNF-α and C-reactive protein were also found to be significantly higher in T2DM5. IL-1 superfamily (IL-1β, IL-18 and IL-33) has an important function in DM-associated pathogenesis through alteration of immune and inflammatory responses6. In addition, IL-1 modulates the level of blood glucose, blood pressure and basic metabolic rate. The IL-1 family consists of both pro- and anti-inflammatory cytokines7. IL-33 is a recently discovered novel cytokine of the IL-1 superfamily that binds with two isoforms-soluble ST2 (sST2) and transmembrane form ST2L8. IL-33 is responsible for inducing Th2-mediated response9. It is reported that IL-33 downregulates the expression of resistin10. The role of IL-33 and sST2 in diabetes is least explored as compared with IL-1β and IL-18. Previous studies have already reported the role of IL-33 in type-1 diabetes11-13. Recently, a study suggested that IL-33 and sST2 show various correlation profiles with different metabolic biomarkers14. To the best of our knowledge, this is the first pilot study in the central Indian population to investigate the role of IL-33 and sST2 in metabolic syndrome. The present study was aimed to evaluate the serum levels of IL-33 and sST2 in T2DM individuals as well as healthy controls and to establish a correlation of IL-33 with different biochemical parameters such as fasting plasma glucose (FPG), postprandial plasma glucose (PPG), HbA1c, high-density lipoprotein (HDL), low-density lipoprotein (LDL) and triglyceride (TG).
Material & Methods
Study design: This was a hospital-based case–control study conducted at the department of Biochemistry, All India Institute of Medical Sciences, Bhopal, after approval by the Institution Human Ethics Committee (IHEC-LOP/2016/EF0030). The target population for this study were patients clinically diagnosed with DM as cases and healthy adults visiting the department of Medicine at AIIMS, Bhopal, as controls. The criteria for the diagnosis of diabetes were based on the following biochemical parameters, FPG ≥126 mg/dl and/or PPG ≥200 mg/dl and/or HbA1c ≥6.5 per cent (any one of this). A detailed written informed consent was taken from the healthy controls and diabetic patients. The selection of diabetic patients for the study was done according to the American Diabetes Association guidelines15. Controls included those individuals without T2DM and were also free from any other non-communicable, autoimmune and inflammatory diseases. Controls comprised those individuals who visited the department for a routine check up.
Inclusion criteria for the study included newly detected diabetic individuals within one year of diagnosis. Exclusion criteria for the study comprised those who were suffering from any known comorbidities such as hypertension, ischaemic heart disease and COPD or any other known complication of diabetes (neuropathy, nephropathy, retinopathy, stroke, ischaemic heart diseases and peripheral vesicular diseases). The study period was from July 2016 to February 2017.
According to the National Cholesterol Education Program Adult Treatment Panel (NCEP ATP III)16, the diagnosis of metabolic syndrome was made based on the following criteria when any three of the following were present: (i) Fasting triglyceride (TG)>150 mg/dl; (ii) HDL cholesterol (HDL-C)<40 mg/dl in men and <50 mg/dl in women; (iii) FPG >110 mg/dl; (vi) waist circumference >102 cm in male and >88 cm in female; (v) systolic blood pressure >130 mmHg or diastolic blood pressure >85 mmHg.
About 2-3 ml of blood was collected in a vacutainer and serum was separated by centrifugation for the quantitative measurement of IL-33, sST2 and other biochemical parameters such as HDL, LDL and TG. For fasting and post-prandial glucose, blood was collected in a vial containing sodium fluoride and for HbA1c estimation, blood was collected in potassium EDTA vials.
Measurement of serum cytokine levels: The serum levels of IL-33 (Human IL-33 Duoset ELISA, R and D Systems, USA) and sST2 (Quantikine ELISA Human ST2/IL-33R Immunoassay, R and D Systems) were checked by sandwich ELISA as per the kit protocol. The lowest detection limit for IL-33 is 23.4 pg/ml and for sST2 is 31.3 pg/ml. For spectrophotometric measurement, ELISA reader was used (EON + Elx 50/8 OR 12, BioTek, USA).
Measurement of biochemical parameters: All biochemical parameters (FPG, PPG, HbA1c, Serum HDL, LDL and TGs) were estimated using an autoanalyzer (Beckman Coulter, USA).
Statistical analysis: For the sample size estimation, G Power 3.1 software (Heinrich Heine University Dusseldorf, Germany) was used. For sample size calculation, we expected an effect size of 0.8 between the two groups (diabetic vs. healthy) and with 95 per cent confidence interval (CI) power 85 per cent, allocation ratio of 1. The calculated sample size was found to be 30 in each group. 30 type-2 diabetic patients and 30 age and sex matched healthy controls were included in this study. Data were expressed as mean ± Standard error of mean (SEM) values and analyzed using Student’s t test, after ascertained homogeneity of variance. Pearson correlation was determined using Graph pad prism 6 software (La Jolla, CA, USA). P < 0.05 was considered significant.
Results & Discussion
In this study diabetic individuals (40±7 pg/ml) were found to have significantly lower IL-33 concentration as compared to healthy controls (458±112 pg/ml) (Figure 1A). However, the levels of sST2 among the diabetics (41±2 ng/ml) were significantly elevated as compared to healthy controls (18±1 ng/ml) (Fig. 1B). Alteration in plasma cytokine levels is known to be associated with diabetes onset and progression17. In contrast, diabetes itself can affect the plasma level of many cytokines including IL-17, IL-23 and TGF-β18. The findings of this study suggest that IL-33 and sST2 are inversely regulated among diabetics similar to other studies19,20. In another study, significantly elevated levels of IL-33 were reported in T1DM patients as compared to healthy controls. sST2 levels positively correlated with the predictors of diabetes such as glucose and triglyceride. T2DM individuals having elevated level of sST2 level were found to be affected with LVDD (left ventricular diastolic dysfunction). It has been reported previously that a higher concentration of sST2 were found in different diseases such as sepsis, systemic lupus erythematous (SLE), heart failure, liver cirrhosis, hepatocellular carcinoma and asthma21-24.These results suggest that IL-33 and sST2 may represent novel biomarkers for diabetes. Serum levels of IL-33 and sST2 in metabolic syndrome positive and negative individuals were also analyzed, however, no significant changes were observed (Fig. 2A and B). Among the diabetics, IL-33 showed indicative correlation with FPG (r=0.65, P=0.0002) as well as PPG (r=0.417, P=0.030) as shown in Figure 3A and B. However, sST2 failed to show any correlation with FPG (r=0.209, P=0.316) and PPG (r=0.016, P=0.934, Figs 3C and D). Furthermore, no correlation of HbA1c was found with either IL-33 (r=0.302, P=0.117) or sST2 (r=0.042, P=0.834) levels as shown in Figures 3E and F. We also performed the correlation analysis by using the ratio of sST2/IL-33 levels with a different glycaemic index such as FPG, PPG and HbA1c, however, failed to establish any significant correlation (Fig. 4A-C). So far, there are only a few published articles on the role of IL-33 and sST2 in T1DM and T2DM. However, to the best of our knowledge, there is no published study from India that shows the correlation between the IL-33 and sST2 with FPG, PPG and HbA1c. Diabetes is a multifactorial disease and our results indicate that IL-33 is well correlated with the FPG and PPG. An in vivo study demonstrated that administration of rIL-33 to genetically obese diabetic (ob/ob) mice was responsible for reducing FPG and adiposity and improving insulin tolerance10. Similar findings have been highlighted in murine (Apo E-/-) mice models suggesting the protective role of IL-33. A recent study25 has also reported a protective role of IL-33 on β-cell mass, insulin content and release demonstrating a beneficial role of IL-33 in protecting the insulin-producing cells of the islets. The level of IL-33 in that study was found to be significantly low in both the groups (diabetes with or without metabolic syndrome) as compared to healthy controls (P<0.05). However, in the present study there was no significant difference in IL-33 levels in between diabetics with or without metabolic syndrome was recorded in Figure 5A-C. The level of sST2 was found to be significantly high in both these categories as compared to healthy controls while there was no significant difference between diabetics with or without metabolic syndrome (Fig. 5D-F). These results suggest that the level of IL-33 and sST2 are not affected by metabolic syndrome. In comparison with Hasan et al18, the present study showed a strong positive correlation among IL-33 and FPG and only a moderate correlation with PPG and no correlation with HbA1c and sST2. Furthermore, the levels of IL-33 and sST2 in diabetic patients are not affected by other associated clinical parameters such as HDL, LDL and TG.
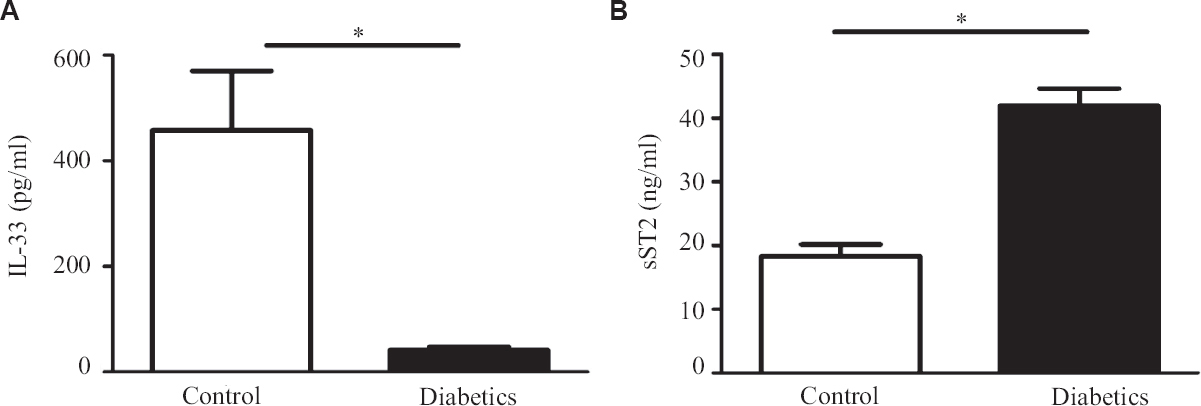
- (A) Serum levels of IL-33 in control and type-2 diabetics (T2DM) individuals (B) Serum levels of sST2 in control and T2DM. P *<0.05 was considered as significant between control and T2DM. IL, Interleukin; sST2, soluble ST2.
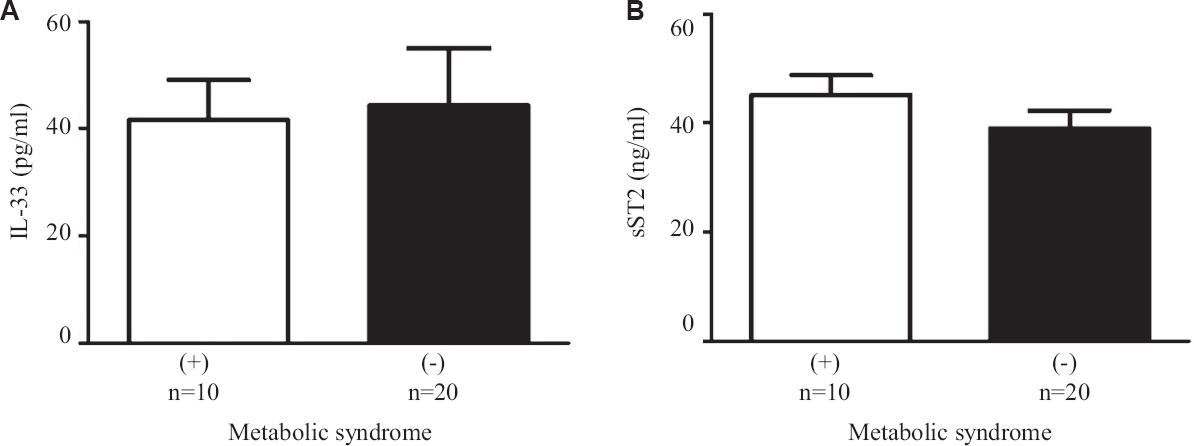
- Serum levels of (A) IL-33, and (B) sST2 in metabolic syndrome positive and metabolic syndrome negative patients.
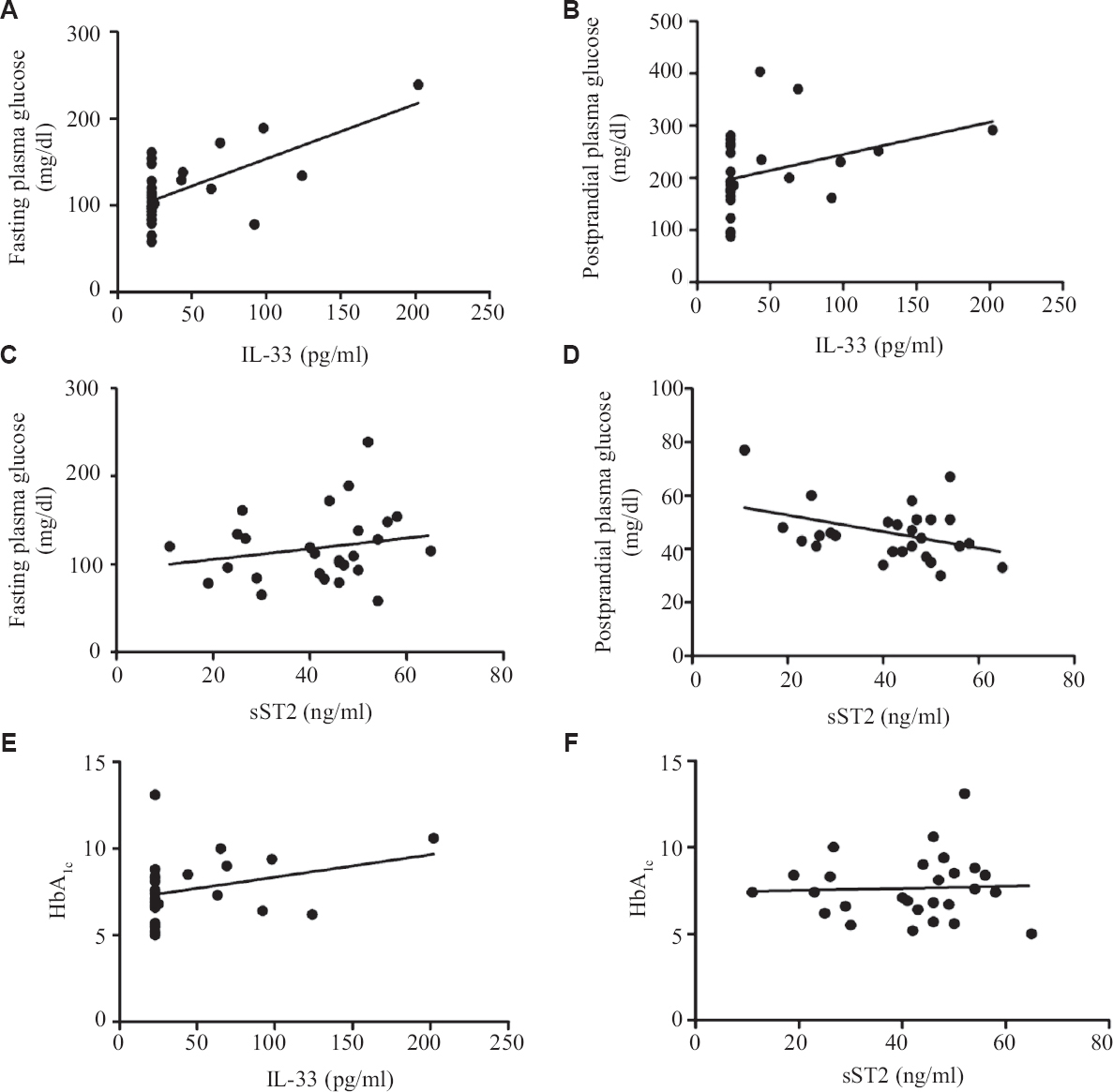
- Line scatter graph showing correlation (A) IL-33 positively correlated with FPG, (B) IL-33 positively correlated with PPG, (C) sST2 not correlated with FPG, (D) sST2 not correlated with PPG, (E) IL-33 not correlated with HbA1c, (F) sST2 not correlated with HbA1c. PPG, postprandial plasma glucose; FPG, fasting plasma glucose.
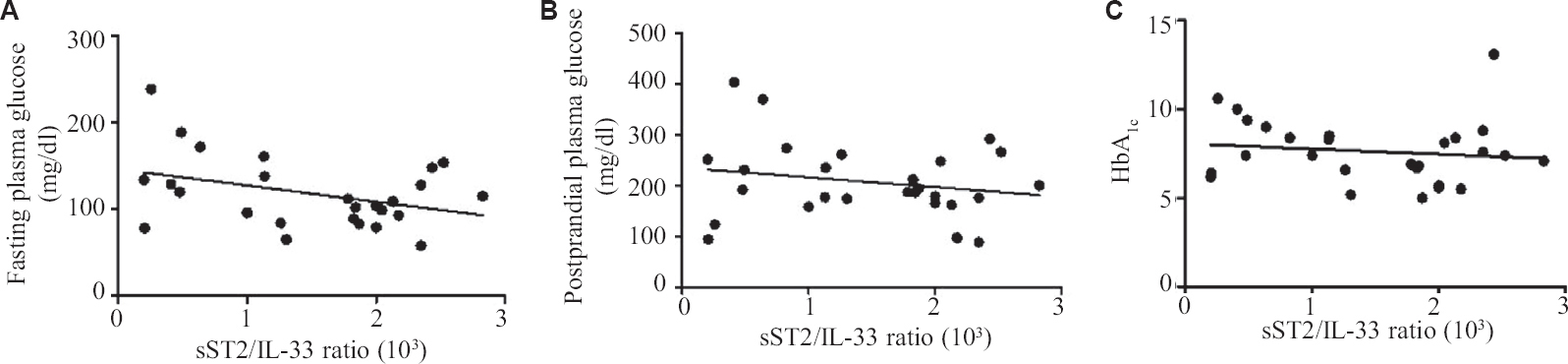
- Line Scatter graph showing correlation (A) sST2/IL-33 ratio is not correlated with FPG, (B) sST2/IL-33 ratio is not correlated with PPG, (C) sST2/IL-33 ratio is not correlated with Hb A1c.
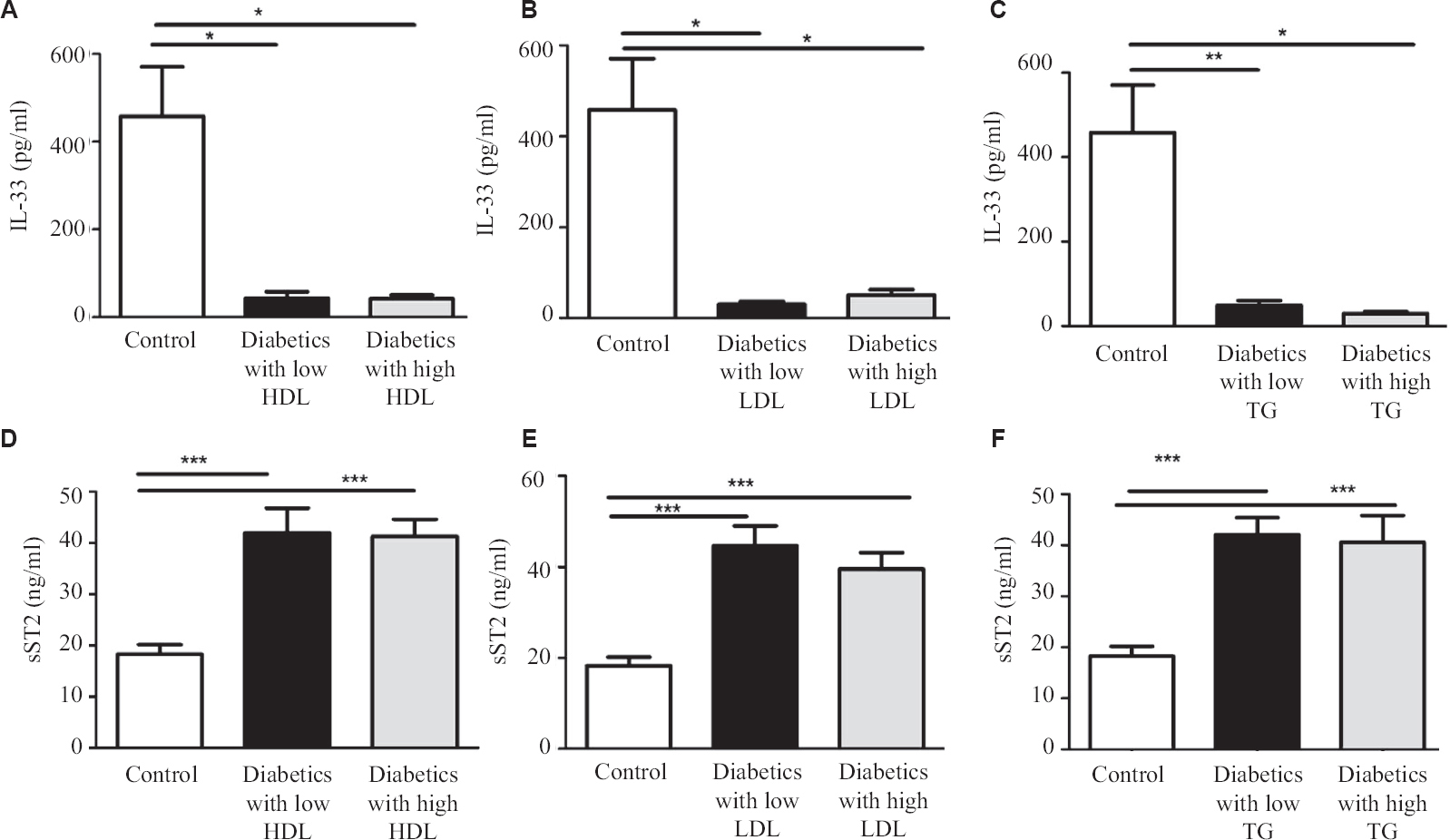
- Serum level of IL-33 and sST2 in healthy controls as compared to type-2 diabetics. (A) IL-33 levels with low and high level of HDL, (B) IL-33 levels with low and high level of LDL, (C) IL-33 levels with low and high level of TG, (D) sST2 levels with low and high level of HDL, (E) sST2 levels with low and high level of LDL, (F) sST2 levels with low and high level of TG. P <0.05 was considered significant. LDL, low density lipoprotein; HDL, high density lipoprotein; TG, triglyceride.
In the present study, we found significantly reduced levels of IL-33 in both the groups (Diabetic with or without metabolic syndrome) as compared to healthy controls. However, we also found significantly elevated levels of sST2 in both the groups as compared to healthy control.
The limitations of this study were the small sample size and the study setting was limited to a hospital setup. However, we believe this study has the potential of a better understanding of the pathophysiology of T2DM and can change the management plan in time to come. We have not looked for the socio-economic status of patients.
Overall, this study shows the protective role of IL-3 in T2DM and suggests that IL-33 could be a novel molecule in the molecular orchestration of the pathophysiology of diabetes.
Financial support and sponsorship
This study received funding support from Department of Biotechnology sponsored Ramalingaswami fellowship (BT/RLF/Re-entry/53/2013).
Conflicts of interest
None.
References
- Prevalence of hypertension, diabetes, and associated risk factors among geriatric population living in a high-altitude region of rural Uttarakhand, India. J Family Med Prim Care. 2018;7:1527-36.
- [Google Scholar]
- Oxidative stress and interleukin-6 secretion during the progression of type 1 diabetes. Arq Bras Endocrinol Metabol. 2012;56:441-8.
- [Google Scholar]
- Serum IL-1beta, IL-2, and IL-6 in insulin-dependent diabetic children. Mediators Inflamm. 2006;2006:59206.
- [Google Scholar]
- Levels of inflammatory cytokines in type 2 diabetes patients with different urinary albumin excretion rates and their correlation with clinical variables. J Diabetes Res. 2013;2013:138969.
- [Google Scholar]
- Interleukin-1 (IL-1) family of cytokines:role in type 2 diabetes. Clin Chim Acta. 2012;413:1163-70.
- [Google Scholar]
- Targeting the IL-1 family members in skin inflammation. Curr Opin Investig Drugs. 2010;11:1211-20.
- [Google Scholar]
- The role of IL-33 and mast cells in allergy and inflammation. Clin Transl Allergy. 2015;5:33.
- [Google Scholar]
- IL-33 promotes the induction and maintenance of Th2 immune responses by enhancing the function of OX40 ligand. Allergol Int. 2014;63:443-55.
- [Google Scholar]
- Interleukin-33 induces protective effects in adipose tissue inflammation during obesity in mice. Circ Res. 2010;107:650-8.
- [Google Scholar]
- IL-33 prevents MLD-STZ induction of diabetes and attenuate insulitis in prediabetic NOD mice. Front Immunol. 2018;9:2646.
- [Google Scholar]
- Interleukin-33 prevents the development of autoimmune diabetes in NOD mice. Int Immunopharmacol. 2019;70:9-15.
- [Google Scholar]
- Inflammatory cytokines and the risk to develop type 2 diabetes: Results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes. 2003;52:812-7.
- [Google Scholar]
- Correlation profile of suppression of tumorigenicity 2 and/or interleukin-33 with biomarkers in the adipose tissue of individuals with different metabolic states. Diabetes Metab Syndr Obes. 2020;13:3839-59.
- [Google Scholar]
- Standards of medical care in diabetes-2019 abridged for primary care providers. Clin Diabetes. 2019;37:11-34.
- [Google Scholar]
- Circulation. 2002;106:3143-421.
- Serum IL-17, IL-23, and TGF-βlevels in type 1 and type 2 diabetic patients and age-matched healthy controls. Biomed Res Int. 2014;2014:718946.
- [Google Scholar]
- IL-33 is negatively associated with the BMI and confers a protective lipid/metabolic profile in non-diabetic but not diabetic subjects. BMC Immunol. 2014;15:19.
- [Google Scholar]
- Distribution and clinical association of plasma soluble ST2 during the development of type 2 diabetes. Diabetes Res Clin Pract. 2016;118:140-5.
- [Google Scholar]
- Soluble ST2 plasma concentrations predict mortality in severe sepsis. Intensive Care Med. 2010;36:630-7.
- [Google Scholar]
- Serum levels of IL-33 and soluble ST2 and their association with disease activity in systemic lupus erythematosus. Rheumatology (Oxford). 2010;49:520-7.
- [Google Scholar]
- Measurement of the interleukin family member ST2 in patients with acute dyspnea: Results from the PRIDE (Pro-Brain Natriuretic Peptide Investigation of Dyspnea in the Emergency Department) study. J Am Coll Cardiol. 2007;50:607-13.
- [Google Scholar]
- High serum levels of the interleukin-33 receptor soluble ST2 as a negative prognostic factor in hepatocellular carcinoma. Transl Oncol. 2013;6:311-8.
- [Google Scholar]
- Association between adipose tissue interleukin-33 and immunometabolic markers in individuals with varying degrees of glycemia. Dis Markers. 2019;2019:7901062.
- [Google Scholar]
- Interleukin-33-activated islet-resident innate lymphoid cells promote insulin secretion through myeloid cell retinoic acid production. Immunity. 2017;47:928-42. e7
- [Google Scholar]






