Translate this page into:
Evaluation of evidence for pharmacokinetics-pharmacodynamics-based dose optimization of antimicrobials for treating Gram-negative infections in neonates
Reprint requests: Dr Nilima A. Kshirsagar, National Chair Clinical Pharmacology (ICMR), ICMR- National Institute for Research in Reproductive Health, J. M. Street Rd, Parel, Mumbai 400 012, Maharashtra, India e-mail: kshirsagarna@yahoo.in
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Neonates present a special subgroup of population in whom optimization of antimicrobial dosing can be particularly challenging. Gram-negative infections are common in neonates, and inpatient treatment along with critical care is needed for the management of these infections. Dosing recommendations are often extrapolated from evidence generated in older patient populations. This systematic review was done to identify the knowledge gaps in the pharmacokinetics-pharmacodynamics (PK-PD)-based optimized dosing schedule for parenteral antimicrobials for Gram-negative neonatal infections.
Methods:
Relevant research questions were identified. An extensive electronic and manual search methodology was used. Potentially eligible articles were screened for eligibility. The relevant data were extracted independently in a pre-specified data extraction form. Pooling of data was planned.
Results:
Of the 340 records screened, 24 studies were included for data extraction and incorporation in the review [carbapenems - imipenem and meropenem (n=7); aminoglycosides - amikacin and gentamicin (n=9); piperacillin-tazobactam (n=2); quinolones (n=2); third- and fourth-generation cephalosporins (n=4) and colistin nil]. For each of the drug categories, the information for all the questions that the review sought to answer was incomplete. There was a wide variability in the covariates assessed, and pooling of results could not be undertaken.
Interpretation & conclusions:
There is a wide knowledge gap for determining the doses of antimicrobials used for Gram-negative infections in neonates. A different profile of newborns in the developing countries could affect the disposition of antimicrobials for Gram negative infections, necessitating the generation of PK-PD data of antimicrobials in neonates from developing countries. Further, guidelines for treatment of neonatal conditions may incorporate the evidence-based PK-PD-guided dosing regimens.
Keywords
Antimicrobials
dose optimization
Gram-negative infections
neonates
pharmacokinetics-pharmacodynamics
Parenteral antimicrobials are administered largely in inpatient settings, and their optimized use is critical in determining clinical outcomes. Individualization of drug doses based on pharmacokinetic (PK) determinants has proven to be a clinically useful approach1. Parenteral antimicrobials constitute a group of therapeutic agents where the knowledge of covariates affecting PK and pharmacodynamics (PD) is of immense importance in optimizing drug dosage.
The PD outcome such as clinical response to antimicrobial treatment takes time to appear. Hence, other correlates of efficacy and safety outcomes such as time above minimum inhibitory concentration (T>MIC) or peak concentration (Cmax) to MIC ratio or area under the curve (AUC) to MIC ratio (AUC/MIC) and trough levels have been developed.
Neonates with serious infections pose a difficult situation for clinicians for not only making correct decision regarding choice of antimicrobials but also deciding their correct doses. The complex situation arises because of several considerations. The age that needs to be taken into consideration for determination of an important covariate has to be decided from a variety of options such as post-natal age (PNA), post-menstrual age (PMA), gestational age (GA) and/or post-conceptional age (PCA); the weight ranges from a few hundred grams to kilograms; status of drug metabolizing enzymes is changing2; creatine clearance (CLcr) is changing both due to the development process itself and often due to the infective state3. There is high variability in the serum albumin affecting the drug-protein binding. Neonates requiring parenteral antimicrobials are on other medications which may alter PK and/or PD of the administered antimicrobial. In the studies evaluating the effect of these factors as covariates, it becomes difficult to delineate the role of each of these factors. Further, issues such as existence of patent ductus arteriosus (PDA)4, high-frequency oscillatory ventilation (HFOV)5, extracorporeal membrane oxygenation (ECMO)6 and therapeutic hypothermia7 may also contribute to altered PK. Assessment of PD parameters is also difficult in neonates and particularly so in premature neonates.
Conventionally, neonatal sepsis, the condition in which parenteral antimicrobials are lifesaving, has been divided into early- and late-onset sepsis. Both Gram-positive and Gram-negative organisms are responsible for sepsis in neonates. In a study conducted in six countries, it was shown that Gram-negative bacilli accounted for 46.9 per cent of isolates8. An alarming level (>50%) of resistance was seen with second- and third- generation cephalosporins while over 40 per cent of Gram-negative isolates demonstrated resistance to gentamicin8. An important contributor to the development of antimicrobial resistance is inappropriate dosing of antibiotics. Therefore, the current review was aimed at evaluating available literature for PK-PD data of antimicrobials used for Gram-negative infections requiring hospitalization in neonates with the purpose of optimization of dosage.
Material & Methods
A systematic literature search of PubMed (till February 2015), Ovid MEDLINE(R) In-Process and Other Non-Indexed Citations and Ovid MEDLINE(R) (1946 to Present), Embase, Clinical trials, ProQuest dissertations thesis (PQDT) database and the Cochrane Database of Systematic Reviews (Till February, 2015) was performed for all studies evaluating the efficacy, safety, PD and PK of antimicrobials in neonates. The keywords (medical subject headings and free text) newborn, neonate, infant, preterm, imipenem, meropenem, cefotaxime, ceftriaxone, cefoperazone, ceftazidime, cefepime, quinolones-ciprofloxacin, levofloxacin, moxifloxacin, colistin, polymyxin B, aminoglycosides and third- and fourth-generation cephalosporins, pharmacokinetics and pharmacokinetics-pharmacodynamics, clinical trial, meta-analysis, randomized controlled trial, and review were combined. The search limits included studies published in English and conducted in humans. Reference lists of identified articles were also manually screened for additional relevant studies.
Only those studies were included which (i) were undertaken in neonates; (ii) included at least assessment of covariates for determining PK parameters; (iii) evaluation of PD parameters was either a part of study design or at least evaluated while interpreting the results; and (iv) in addition to neonates, studies conducted on infants up to four months were also considered.
Key questions
The key questions included (i) Which were the key PK-PD determinants of treatment outcomes in neonates on parenteral antimicrobials for Gram-negative infections?, (ii) Within the neonatal population, were there any specific considerations for preterm, very low birth weight, small for GA (SGA)?, (iii) How did coexisting PDA, ECMO, parenteral nutrition, therapeutic hypothermia and fluid administration alter the PK-PD of the administered antimicrobial?, (iv) Was there a justification for using a loading dose?, (v) Was there a justification for prolonged infusion? and (vi) How appropriate was the choice of the antimicrobial, its dose and route for administration for CNS infection?
Data extraction
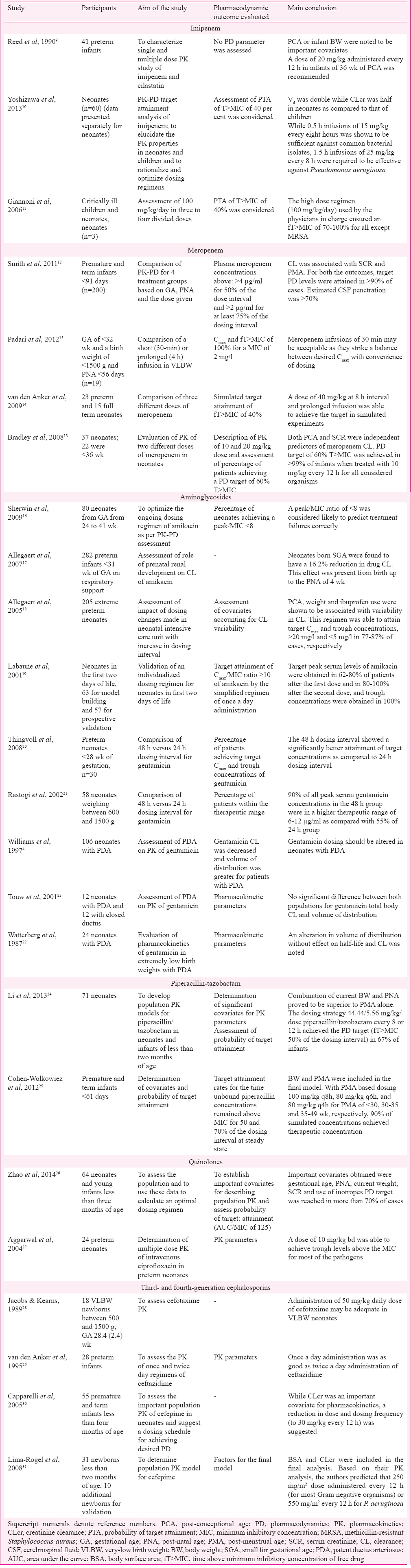
The following data were extracted independently from the included studies: aim of the study, study population, PK-PD parameters, outcomes and important conclusions. The drugs considered were carbapenems (meropenem and imipenem), piperacillin-tazobactam, aminoglycosides (gentamicin and amikacin), quinolones, third- and fourth-generation cephalosporins and polymyxins (colistin and polymyxin B) all used for Gram-negative infections and parenterally for neonates. Within a class, emphasis was laid on antimicrobials found to be more commonly used. A total of 24 studies were found to satisfy the inclusion criteria (Figure). The salient features of these studies are summarised in Table I910111213141516171819202122232425262728293031.
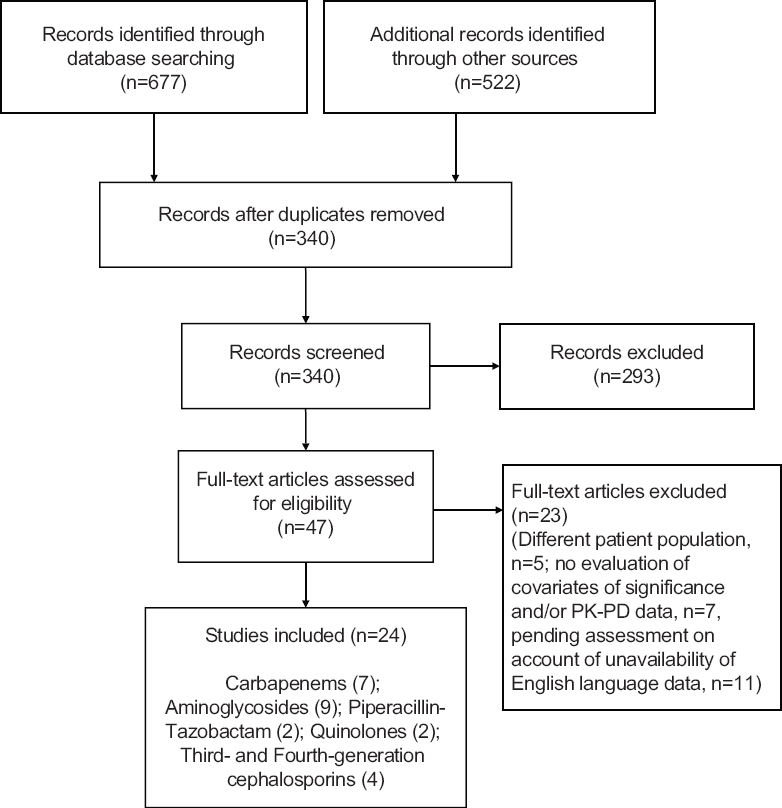
- Flow chart showing the design and inclusion of studies. PK-PD, pharmacokinetics-pharmacodynamics.
Results
Carbapenems
Imipenem: Three studies were included for the analysis91011. Based on an ascending dose study in three neonates, the disposition characteristics of both imipenem and cilastatin were found to be highly variable in premature neonates (GA <37 wk)9. The authors recommended a dose of 20 mg/kg every 12 h in premature infants. On the basis of information obtained from a dataset of 60 participants, it has been shown that 30 min infusions of 15 mg/kg every eight hours and 25 mg/kg every 12 h were sufficient against common bacterial isolates in neonates10. The probability of target attainment (PTA) was described as a fraction that achieved at least 40 per cent T>MIC of free drug (fT>MIC). The PTA for a 0.5 h infusion increased both with the increase in dose (15-25 mg/kg) and the dosing frequency (q12h to 8 h). With an increase in duration of infusion at each of 15 and 25 mg/kg q8h, there was an increase in PK-PD breakpoints. A shift to a higher value of PK-PD breakpoint was noted for a longer duration of infusion at each dose level. These values, respectively, for a 0.5 h infusion and a 1.5 h infusion were 2-4 μg/ml for a 15 mg/kg dose q8h and 4-8 μg/ml for a 25 mg/kg dose q8h. Further, 1.5 h infusions of 25 mg/kg every eight hours (75 mg/kg/day) were required in neonates to be effective against Pseudomonas aeruginosa (MIC for 90% of the isolates was equal to 16 μg/ml)10. Gionani et al11, in their analysis of samples of 19 critically ill children, of whom three were neonates found that administration of a dose of 100 mg/kg/day in three or four divided doses was able to achieve an fT>MIC of 70-100 per cent for all recovered Gram-negative pathogen. No evidence was found for PK-PD evaluation in various subgroups of neonates such as SGA, those receiving ECMO or therapeutic hypothermia or those with PDA.
Meropenem: Four studies satisfied our inclusion criteria12131415. Smith et al12 have undertaken a PK-PD evaluation of dosage schedule which is commonly used in neonatal units. Dosing was done largely based on GA and PNA. They divided the patients into four groups: group 1 (GA <32 wk, PNA <14 days) - 20 mg/kg body weight (BW) every 12 h; group 2 (GA <32 wk, PNA ≥14 days), and group 3 (GA ≥32 wk, PNA <14 days) - 20 mg/kg BW every eight hours and group 4 (GA ≥32 wk, PNA ≥2 wk) - 30 mg/kg BW every eight hours. The PD target exposure level for each infant was defined as plasma meropenem concentrations above >4 μg/ml for 50 per cent of the dose interval and >2 μg/ml for at least 75 per cent of the dosing interval. Trough meropenem concentrations exceeded the therapeutic target of 2 μg/ml in >80 per cent of infants. The PD targets of exposure were achieved in over 90 per cent of patients for both PD target exposure levels. Although the meropenem CSF concentration was variable, nearly 70 per cent of the administered dose penetrated into the CSF samples in the study.
Recommendations from two paediatric studies using Monte Carlo simulation have also emphasized that a different regimen may be used for more resistant organisms such as P. aeruginosa1314. In one of these studies13, two doses of 10 and 20 mg/kg were administered to neonates less than two months of age. Meropenem serum concentrations were measured at specified times during the 24 h post-infusion. Monte Carlo simulations were used for assessing PTA. Thirty seven neonates were enrolled, of whom, 22 were preterm (<36 wk of gestation). Meropenem clearance was related to chronological age and GA, being higher for older chronological age and GA. Serum creatinine and PCA were the best predictors of meropenem elimination. Simulation studies demonstrated that at the dosage levels used, the desired PD targets were attained in over 90 per cent of term and preterm neonates against P. aeruginosa.
In another study14, meropenem was administered in different doses (10, 20 and 40 mg/kg) as short (30 min) or prolonged infusion (four hours) to 23 preterm and 15 full-term neonates. In their study, 40 per cent fT>MIC was taken as PD target for running the simulations. For both the doses of 20 and 40 mg/kg, PTA was better achieved with the prolonged infusion (four hours). The authors showed that a dose of 40 mg/kg administered as a prolonged infusion produced target attainments in excess of 90 per cent to a MIC of 8 mg/l, irrespective of GA.
In a special population of neonates (preterm and weighing <1.2 kg), Padari et al15 compared the steady-state PK of meropenem when given as a short (30 min) or prolonged (4 h) infusion given in a dose of 20 mg/kg q12. A short infusion resulted in a higher mean drug concentration in serum than a prolonged infusion (89 vs. 54 mg/l). Neither PNA nor PMA influenced the clearance of meropenem. For an MIC of 2 mg/l, both short and prolonged infusions were able to achieve fT>MIC in more than 80 per cent of cases. A value of fT × 6.2 × MIC was designated as a cut-off for prevention of resistance to P. aeruginosa. This target was again achieved in over 80 per cent of patients in both infusion groups (Table I).
No study could be found addressing PK-PD of meropenem in special sub-groups of neonates.
Aminoglycosides
Nine studies41617181920212223 satisfied the inclusion criteria. Sherwin et al16, conducted a study in 80 neonates with a sizeable population of preterm neonates with extremely low birth weight, and demonstrated that PMA and current weight were important determinants of clearance of amikacin. Based on their PK-PD data, the authors showed that the main predictor of treatment failure was an amikacin peak to MIC ratio of less than eight. This led to redefining of dose schedule as follows - 15 mg/kg at 36 h interval, 14 mg/kg at 24 h interval and 15 mg/kg at 24 h intervals for neonates with PMA of ≤28 wk, 29-36 wk and ≥37 wk, respectively.
The importance of weight at the time of birth as an important determinant of renal clearance of amikacin has been demonstrated. It has been shown that for SGA, the renal clearance may be decreased by 16 per cent as compared to appropriate for GA neonates17.
In view of sepsis occurring within the first two days of life, it may be required to start aminoglycosides within the first two days. Extended-interval dosing for amikacin has ranged from 24 to 42 h, viz. PCA<28 wk, 20 mg/kg per 42 h; PCA 28-30 wk, 20 mg/kg per 36 h; PCA 31-33 wk, 18.5 mg/kg per 30 h; PCA 34-37 wk, 17 mg/kg per 24 h; PCA >37 wk, 15.5 mg/kg per 24 h with additional six hours of increase if the neonate had received ibuprofen or had suffered hypoxia. This regimen was able to attain target Cmax and trough concentration >20 and <5 mg/l in 77-87 per cent of cases, respectively18. The variation in clearance was accounted for largely by PCA and current weight of the neonates. Taking these two factors into account the authors predicted a dosing regimen for extreme preterm neonates. The recommended doses ranged from 6 mg/kg at a dosing interval of 44 h for a neonate of 24 wk PCA and weight of 0.5 kg to a dose of 15.7 mg/kg at a dosing interval of 29 h for a neonate weighing 1.3 kg and PCA of 30 wk18.
Once a day individualized amikacin dosing in neonates was proposed based on population PK model19. A dosing chart was made following the data obtained from PK parameter values of the reference population. These were previously estimated using a non-parametric algorithm on 63 newborns treated with amikacin for two days and divided into three groups of 30, 30-34 and >34 wk. The authors generated a detailed Table for dosing based on GA and birth weight, and recommended the following daily dosage regimen (mg/kg/day) for the first injection: GA <30 wk, 4.09; GA=30-34 wk, 9.31 and GA 134 wk, 12.84. The findings of this study were validated in 57 individuals who were administered the drug in the recommended dosage. More than two-thirds and nearly all patients achieved target peak serum levels of amikacin after first and second doses, respectively, and trough concentrations were achieved universally. It has been shown that for preterm neonates <28 wk of gestation, a 48 h dosing interval for gentamicin may be superior to 24 h interval20. The 48 h dosing group had a higher mean gentamicin peak (9.43 vs. 6.0 µg/ml) and lower mean gentamicin trough (1.08 vs. 1.54 µg/ml), compared with the 24 h dosing group. Compared to the 24-h group, a peak level <6 µg/ml was seen in a small percentage (43 vs. 7%) in the 48 h group. The same fact was earlier evaluated for extremely low birth weight neonates (600-1500 g)21.
Effect of simultaneous existence of PDA on the PK parameters of aminoglycosides has been evaluated in three studies42223. While two of these studies2223 indicated a need for alteration of dosing in neonates with gentamicin, one study4 did not show any alteration in PK variables.
Piperacillin-tazobactam
Two studies were included in the analysis2425. In a population PK study conducted in children less than two months of age, it was shown that a dose of 44.44/5.56 mg/kg every 8 or 12 h may not be enough for controlling infection in neonates and infants less than two months of age in the Neonatal Intensive Care Unit. The authors suggested a higher dose or more frequent regimens in this group of patients24. Further, considering the probability of target attainment (PTA) for extended spectrum beta lactamase (ESBL), the same group conducted simulation studies. Considering a PK-PD correlate best associated with favourable outcome (fT>MIC% >50%), a wide variation was noted in the dose requirement for infants. While those with a BW of 1 kg and PNA of three days, a dose of 10 mg/kg every eight hours was found to be adequate; it was suggested that the dose needed to be increased to 100 mg/kg given every six hours in an infant with a BW of 4.5 kg and a PNA of seven days24.
Another study done in infants <32 wk GA who were <120 days old and who were receiving i.v. piperacillin or piperacillin-tazobactam for routine medical care were evaluated25. A PD target of T>MIC of 50 and 75 per cent of the dosing interval for 16 and 64 mg/l was evaluated. Only 60 per cent of all infants achieved piperacillin concentrations >16 mg/l for 50 per cent of the dosing interval. Piperacillin concentration of >64 mg/l was found to be inadequate for infants in the 30-32 wk age group. The percentage of patients achieving target PD concentration for >75 per cent of the dosing interval was even lesser.
Quinolones
Two studies2627 were included for evaluation. As a part of ‘Treat Infection in Neonates’, 60 infants <120 wk of age were enrolled in population PK analysis of ciprofloxacin26. The median PMA of participating neonates was 35.7 wk. For quinolones, a PD target, AUC/MIC of 125 was considered. A dosing regimen of 7.5 mg/kg twice daily for infants with PMA of <34 wk and a dose of 12.5 mg/kg for the rest were decided. The MIC susceptibility breakpoint of 0.5 mg/l was considered. Monte Carlo simulations showed that for the aforementioned dosing regimen and PD targets, more than 90 and 84 per cent of new-borns with PMA <34 and >34 wk, respectively, achieved the desired target. The associated risks of overdose for the proposed dosing regimen were <8 per cent. The concentrations of ciprofloxacin in six CSF samples were highly variable (187-1650 ng/ml). The median value of CSF/serum concentration ratio was 0.32. CSF collection time correlated with CSF/serum concentration ratio in a manner, suggesting a slower elimination from the CSF as compared to that from systemic circulation. Ciprofloxacin clearance decreased with the co-administration of inotropic drugs.
In a study conducted in 24 preterm neonates, ciprofloxacin was administered in a dose of 10 mg/kg 12 hourly27. The mean peak concentrations ranged from 2.3 to 3.0 µg/ml and mean trough levels ranged from 0.7 to 1.0 µg/ml. The trough levels were above the MIC90 of most clinically important pathogens, except Staphylococcus aureus and P. aeruginosa. In case of the latter organism, the peak values were adequate, but the AUC above the MIC90 was estimated to be inadequate. Although the authors did not employ population PK-PD model for their analysis, the report may be considered first evidence for providing important data regarding determination of PK profile of ciprofloxacin in this subgroup of neonates.
No studies were identified for specific subgroups of neonates.
Third- and fourth-generation cephalosporins
Among the third-generation cephalosporins, cefotaxime is most commonly used in neonates. There is an increasing use of cefoperazone in this patient population; hence, a literature search for the same was also undertaken. Cefepime, a fourth-generation cephalosporin, is also used for Gram-negative infection in neonatal population and hence included in this review.
Third-generation cephalosporins: Four studies were included for evluation28293031. In a group of very low birth weight (VLBW) neonates28, a single 50 mg/kg/day dose of cefotaxime was able to achieve concentrations of cefotaxime and its active metabolite desacetyl-cefotaxime was sufficient to achieve CSF sterilization within 24-48 h (Table I).
Twenty preterm infants received 25 mg/kg once or twice daily29. Twice-daily dosing with ceftazidime led to high serum trough concentrations (42.06 vs. 13.4 mg/l). Although once-daily dose resulted in significant reduction in mean trough concentrations, the smallest of these concentrations (8.1 mg/l) was above MIC for major neonatal pathogens such as Streptococcus agalactiae and E. coli.
Fourth-generation cephalosporins: In a landmark study, Capparelli et al30 based on their population PK study, were able to suggest that cefepime clearance was closely associated with serum creatinine. The authors further suggested that cefepime, if administered at a dose of 30 mg/kg/dose every 12 h for infants <14 days, regardless of GA, should provide antibiotic exposure equivalent to or >50 mg/kg every eight hours in older infants and children.
In a population PK study of cefepime in neonates31, it was administered at a dose of 50 mg/kg of BW as 30 min infusion at a dosing interval of 8 or 12 h. The authors identified two important correlates, BSA and CLcr, of plasma concentration.
No studies could be included for addressing the issues of special population of neonates.
Colistin
No study could be included for colistin in the current review.
Discussion
Carbapenems
Although within this group, meropenem, imipenem, doripenem and ertapenem are included, for the current review, only imipenem and meropenem have been evaluated as these are commonly used in neonates.
Imipenem: Imipenem is largely distributed in the extracellular fluid and is removed predominantly by glomerular filtration. Though for the current review, only three studies were included for PK-PD data, 12 studies in foreign language were categorized as ‘pending assessment’. One study was reported in French32 and 11 studies were reported in the Japanese language3334353637383940414243.The early works carried out in neonates formed the basis of dosing of imipenem in neonates4445. The background work for describing the PK of imipenem-cilastatin was earlier undertaken in 30 neonates44. No accumulation of either imipenem and cilastatin, was noted. A marked inter-subject variability, particularly for cilastatin, was noted.
In view of the findings in this review, there is a need to revisit the dosing schedules described in standard references46. However, it is important to note that the direct evidence available so far for premature infants is from the study done by Reed et al9, according to which a 20 mg/kg/dose at q12 h provides a desirable PK profile.
The PD response to treatment may vary with the condition of the neonate. The earlier study carried out in 104 premature and new-born infants belonging to high-risk group with extreme prematurity, perinatal asphyxia and amnion infection as well as various malformations, relied on retrospective data on peak and trough imipenem concentrations47. A dose of 50 mg/kg/day in two divided doses was used. The PK parameters were expressed in terms of peak and trough serum concentrations. With imipenem following time-dependent kinetics, relying on peak and trough serum concentrations for PD assessment may be inappropriate. Six deaths were reported, of which five were due to cause unrelated to sepsis47. Later on, a need for higher dose in children with more severe infections was highlighted. In case reports of two neonates who failed to respond to imipenem given in recommended doses of 60 or 75 mg/kg/day, the trough levels were found to be undetecTable48. These findings were thought to be the cause of non-responsiveness of the patients despite the presence of imipenem susceptible strains of bacteria and administration of recommended doses. These findings were corroborated in the study done by Giannoni et al11. An important concern regarding the effect of a broad-spectrum antimicrobial on the faecal flora of children was addressed in a study49. It was noted that the faecal flora of treated neonates was largely unaffected. A possible explanation could be the fact that glomerular filtration accounted for the majority of drug elimination. Inclusion of outcomes which reflect upon alteration of microbiological flora and development of resistance could help in evaluation of results of PK-PD studies in a more comprehensive manner.
Keeping in view the evidence obtained from the literature search, a paucity of data to guide therapy was noted for imipenem. No evidence was found for PK-PD evaluation in various subgroups of neonates such as SGA, those receiving ECMO or therapeutic hypothermia or those with PDA.
Meropenem: Meropenem is largely distributed in extracellular water and is excreted by glomerular filtration. As, in a neonate, percentage of body water and renal functions changes rapidly, the disposition of meropenem is likely to get influenced by virtue of these changes. The half-life of meropenem in infants is substantially longer than that in adults50. In the study conducted by Smith et al12 although patients with elevated creatinine were excluded, creatinine was still retained as a significant covariate in the final analysis. PNA was found to be best associated with changes in renal function, and as meropenem is excreted by glomerular filtration, PNA emerging as an important covariate for dosing was expected. Meropenem is often used for CNS infections, a slower elimination of meropenem was noted from the CSF as compared to systemic circulation. A potential role of meningeal inflammation in meropenem CSF concentrations was also noted, however, the conclusion was made from only nine CSF samples in the study.
Recommendations from two paediatric studies using Monte Carlo simulation have also emphasized that a different regimen may be used for more resistant organisms such as P. aeruginosa1314. However, one needs to draw the conclusions with caution as the assessment of PTA was largely based on simulation study. Further, in practice, the use of PCA for guiding therapy may not be a practical option as PCA can be correctly measured only for the neonates in whom assisted fertilization is undertaken.
Although, it has been suggested that a longer duration of infusion of meropenem may be useful14, an important concern with the use of meropenem is its potential to degrade following reconstitution51. Though meropenem infusions of 30 min are accepTable in very low birth weight neonates, this may not hold true for intermediate or resistant microorganisms (with meropenem MICs of >2 mg/l) such as Acinetobacter spp. and P. aeruginosa.
Meropenem has shown good CSF penetrability and offers a good option for use in neonatal meningitis. However, there is a dearth of data in these patients. The results of pharmacokinetic evaluations done in NEOMERO projects (undertaken by European multicenter network), done with the aim of evaluating pharmacokinetics, safety and efficacy of meropenem in infants below three years of age, once published will be able to throw a better light. The reports of this work may be able to answer some of the pending unanswered PK-PD queries. However, it seems unlikely that the issue of special subgroups of neonates would still be addressed.
Aminoglycosides
A remarkable change in aminoglycosides therapeutics has been seen based on PK-PD studies. From the earlier practice of two to three times dosing, a practice of once a day dosing has become a norm for adults. This was based on application of knowledge regarding the relevance of concentration-dependent kinetics, post-antibiotic effect and the phenomenon of adaptive resistance demonstrated by aminoglycosides. Even in neonates, a greater probability of target Cmax and trough level attainment with extended interval dosing of gentamicin52. A systematic review of gentamicin concluded in favour of extended interval dosing in neonates52. Eleven studies52 were included in this review with a total sample size of 574. All infants in both ‘once a day’ as well as ‘multiple doses a day’ regimen showed adequate clearance of bacteraemia. ‘Once a day’ dosing of gentamicin was superior as compared to ‘multiple doses a day’ regimen for other PK parameters. On the lines of gentamicin, amikacin extended interval dosing was found to achieve a higher PTA and extended interval is now a routine practice.
Some key developments included the identification of PMA and current weight rather GA and birth weight as guides for dosing regimens. This was based on observation that treating GA and PNA as separate covariates did not give usable information about renal clearance in neonates more than 72 h of age. PMA was considered to be more closely related to the renal clearance53.
SGA neonates are an important subgroup requiring antimicrobial therapy. Growth restriction by itself has a negative impact on the normalized weight of the kidney, on the number of nephrons, on glomerular filtration rate (GFR) and on tubular function54. The importance of weight at the time of birth as an important determinant of renal clearance of amikacin has been demonstrated53. It was shown that clearance of gentamicin was lesser for SGA as compared to AGA neonates if the drug was administered within seven days of birth or when the PMA was less than 32 wk55. This report has reemphasized the need for elucidating the PK parameters in SGA neonates.
Since, complicated dose regimens with dosing intervals ranging from 18 to 48 h, have been described for aminoglycosides, Labaune et al19 took into consideration the fallouts of such strategy in practice setting and designed their study with once a day regimen and a shorter duration of infusion.
Aminoglycosides are one class of agents for which effect of simultaneous existence of PDA on the PK parameters has been evaluated in three studies42223. It would make the information more useful if concomitantly administered drugs such as ibuprofen are taken into account.
Change in PK of gentamicin in neonates receiving high-frequency oscillatory ventilation (HFOV) was evaluated5. The evidence for this has not been considered in this review because the study was conducted with 12 hourly dosing interval, a practice which is not followed anymore. HFOV is often resorted to intensive care settings and in future it may be interesting to explore the evidence for this subgroup of patients as well.
Before the adoption of extended interval dosing, it was shown that loading doses might be needed for aminoglycosides. Subsequent to adoption of extended dosing, the need for loading dose, especially in preterm infants with birth weight <1500 g who required amikacin therapy, was evaluated56. Although, a loading dose of 10 mg/kg followed by 7.5 mg/kg/24 h administered in preterm neonates, was found to have more than 80 per cent PTA, the principle has not come into standard practice.
Although aminoglycosides have been evaluated most extensively for refining the dosage regimen based on PK-PD data in neonates, important points of consideration are the inclusion of resistance parameters in the PD outcomes, the effect of co-administration of potentially nephrotoxic drugs such as vancomycin and amphotericin-B, which is sometimes required in these patients. Further, with different studies having used algorithms based on PNA or PCA for evaluation of PD parameters, it becomes difficult to arrive at a conclusion based on results of good quality studies. Valitalo et al57 used datasets from previously done studies to generate a dataset of 5000 virtual patients. Monte Carlo simulations on the basis of validated models were undertaken to evaluate the attainment of following targets for gentamicin and tobramycin - peak (5-12 mg/l) and trough (<0.5 mg/l) concentrations. They found a discrepancy in attainment of peak and trough concentrations when different guidelines were used. While the Dutch National Formulary for Children, the British National Formulary for Children and NeoFax resulted in adequate trough but inadequate peak concentrations; the case with Red Book was reverse with peak concentrations being less than 75 per cent below target. They proposed a dose of 4.5 mg/kg of gentamicin with dosing intervals determined by birth weight and PNA. The PNA considered were 5, 6-10, 11-20 and >20 days, while three categories of birth weight were considered <1, 1 and 2 and >2 kg. The dosing intervals ranged from 24 to 72 h. The authors predicted attainment of adequate peak concentrations with only 33-38 per cent of the trough concentrations above target57. This regimen needs to be validated. Moreover, it did not take into consideration the covariates such as existence of PDA and use of concomitant antimicrobials.
Issues related to inhaled route of tobramycin have not been covered since its use in intensive care setting is still restricted.
Piperacillin-tazobactam
Piperacillin-tazobactam is used for multiple infective conditions in inpatient settings. There is a paucity of PK data in neonatal age group. The PD correlate of piperacillin-tazobactam is f T>MIC of approximately 50 per cent of the dosing interval for Gram-negative bacteria24.
In a study the patients received dosing as per NeoFax (100 mg/kg every 8-12 h) and The Harriet Lane Handbook (75 mg/kg every 8-12 h)25. The dosage schedule recommended in these handbooks is probably derived from a study conducted in infants and children, wherein the authors based on their study findings had noted that a dose of 100 mg of piperacillin and 12.5 mg of tazobactam per kg of BW administered as a fixed-dose combination every six to eight hours may be appropriate for attaining clinical efficacy in infants and children58. Piperacillin clearance increased with allometrically scaled BW, and it decreased proportionally with increasing serum creatinine. However, the results must be interpreted with caution as the results are based on scavenged samples. Since the enrolled participants ranged from two months to 12 yr, this study has not been included in the current review.
Quinolones
Among the quinolones, ciprofloxacin, norfloxacin and levofloxacin are frequently used in neonates. In the inpatient setting, parenteral ciprofloxacin is commonly used. A wide variability exists in the dose of ciprofloxacin used ranging from ≤10 to ≥21 mg/kg/day59. It was noted that ciprofloxacin clearance decreased with the co-administration of inotropic drugs26. A possible explanation is that inotropic agents are often given when there is decreased blood pressure, which will result in decreased GFR.
In a case series of six neonates a good penetration of ciprofloxacin in CSF was reported60. This information may be useful for CNS infections showing susceptibility to ciprofloxacin. A single case report of moxifloxacin use for a case of Mycoplasma hominis meningitis has been described61. Moxifloxacin was administered at a dose of 5 mg/kg q24h. Moxifloxacin was infused over one hour, and blood samples were collected around the seventh and fourteenth doses of moxifloxacin at 0.7, 1.9, 17.5 and 22.1 h after the end of infusion. The Cmax/MIC ratio was approximated as 27. The levels of moxifloxacin metabolites were higher in infant than in adults. The implication of higher levels of inactive metabolites was not evaluated in this single patient report. As these metabolic processes may be compromised in neonates, it may be a prudent exercise to evaluate the same in a larger cohort of neonates.
Third- and fourth-generation cephalosporins
Among the third-generation cephalosporins, cefotaxime is most commonly used in neonates. Although the antimicrobial profile of ceftriaxone is similar to that of cefotaxime, the use of ceftriaxone is associated with concern in case of neonates with hyperbilirubinemia62. Further, administration of ceftriaxone and calcium-containing solutions or products may lead to fatal reactions with calcium-ceftriaxone precipitates in lung and kidney. There is an increasing use of cefoperazone in this patient population; hence, a literature search for the same was also undertaken. Cefepime, a fourth-generation cephalosporin, is also used for Gram-negative infection in neonatal population and hence included in this review.
The first PK study of cefotaxime in neonates concluded that a dose of 50 mg/kg administered every 12 and 8 h, respectively, in neonates less than seven days and those between 7 and 28 days would be appropriate63. There was no PD evaluation undertaken in this study. Further, in the study, the comparison between groups was made for the current weight of the neonate. However, the dosing recommendations were made based on PNA.
In a study on PK of ceftriaxone in neonates the authors concluded that the half-life of drugs was related to the current weight of neonates with it being longer (7.7-8.4 h) for neonates weighing <1500 g64. It was further observed, that accumulation of ceftriaxone might occur for those neonates who received the drug at a frequency greater than once a day. However, based on another PK study, a dose of 50 mg/kg given every 12 h was suggested for neonates less than seven days old65. Another PK study in very low birth weight neonates66 showed a significant linear correlations between GA and weight and cefotaxime half-life and total body clearance. A dose of 50 mg/kg every 24 h was considered appropriate for this group. Kearns et al67 proposed in his review that either a cefotaxime dosage of 50-75 mg/kg every eight hours or 75 mg/kg every 12 h should provide adequate antimicrobial concentrations of desacetyl-cefotaxime in serum, especially if the combination of cefotaxime and desacetyl-cefotaxime is synergistic. None of the studies included in this review could be included for our analysis since a simulation for PD parameters was not undertaken.
An important use of these agents is in neonatal meningitis owing to reliable penetration of both the agents in CSF. It has been shown that for ceftriaxone, the CSF concentrations achieved with 50 and 75 mg/kg were usually 10-100 times greater than the MIC for recovered bacteria68. Based on the study results, a tentative dosage schedule of 100 mg/kg given as i.v. infusions over 24 h was proposed. Cefotaxime has shown a good CSF penetration with an ability to achieve sterilization as early as 36 h69. However, this study was carried out in children over one month of age and cefotaxime was administered in a dose of 200 mg/kg/day in four divided doses.
Cefoperazone offers the advantage of longer half-life than other third-generation cephalosporins and crossing the blood-brain barriers such as cefotaxime and ceftriaxone. A pharmacokinetic study of cefoperazone has shown that cefoperazone administered in doses of 12.5 mg/kg every 12 h by i.v. or intramuscular (i.m.) routes may have similar pharmacokinetics70. The levels achieved in blood during 15-30 min after the dose were 12-100 times the MIC for 90 per cent of the strains of pathogens such as E. coli and Proteus mirabilis and 17-30 times more than necessary to inhibit 90 per cent of the strains of S. aureus. However, the results would need to be interpreted with caution since these were based on blood levels obtained soon after drug administration. No standard methodology for determination of covariates was used in this study and no criteria were mentioned for PD target attainment. However, the study may be considered as foundation for further studies.
The activity of ceftazidime against P. aerugionosa makes it particularly useful in infections due to Pseudomonas which is not uncommon. It was conventionally administered as twice daily dose in neonates. The requirement of an antimicrobial to maintain a concentration above MIC for most of the dosing interval is met in adults by either intermittent administration or by continuous administration. However, lower clearance in preterm neonates leads to once-daily administration of a low dose resulting in concentrations of cefatzidime that are above the MICs for major neonatal pathogens during the complete 24-h dosing interval71. The authors based this study on their previous observation in which they noted that ceftazidime clearance increased significantly with increasing GFR72. Prenatal exposure to indomethacin resulted in significantly lower GFR values and ceftazidime clearances72.
A fourth-generation cephalosporin, cefepime, has been shown to have broader antibacterial spectrum than third-generation cephalosporins, including activity against aerobic Gram-positive organism. Its stability to beta-lactamase hydrolysis provides an additional advantage73. While cefepime has been evaluated by an optimized dosing regimen based on PK-PD data, such studies are not available for other cephalosporins.
Colistin
Colistin is often used in extensively drug- or multidrug-resistant organisms in neonatal population. The near-absence of PK and PD data in the neonatal age group compels clinicians to depend on the data derived from other, often paediatric patients. The drug has poor CNS penetrability. However, intraventricular or intrathecal routes have been used successfully for CNS infections74. For pulmonary infections, administration via inhalational route may ensure more concentration of the drug in pulmonary parenchyma75. The PK-PD data on colistin in neonates are not available. The situation is complicated further by unavailability of cost-effective methods of analysis of plasma concentration of polymyxins in blood. Very similar to the situation of colistin is the case of polymyxin B.
The present systematic review highlights the paucity of literature on the subject reviewed. Furthermore, a lot of practices are based on studies done in the period where PK-PD correlates of antimicrobials were not properly established. Conventional reporting of PK data does not throw light on PD responses. The review has certain limitations. Full-text articles of literature available in foreign language could not be analyzed and only abstracts had to be relied upon. However, this was largely seen for only one drug (imipenem). Pooling of data could not be undertaken for want of more than one study for a particular outcome.
Conclusions
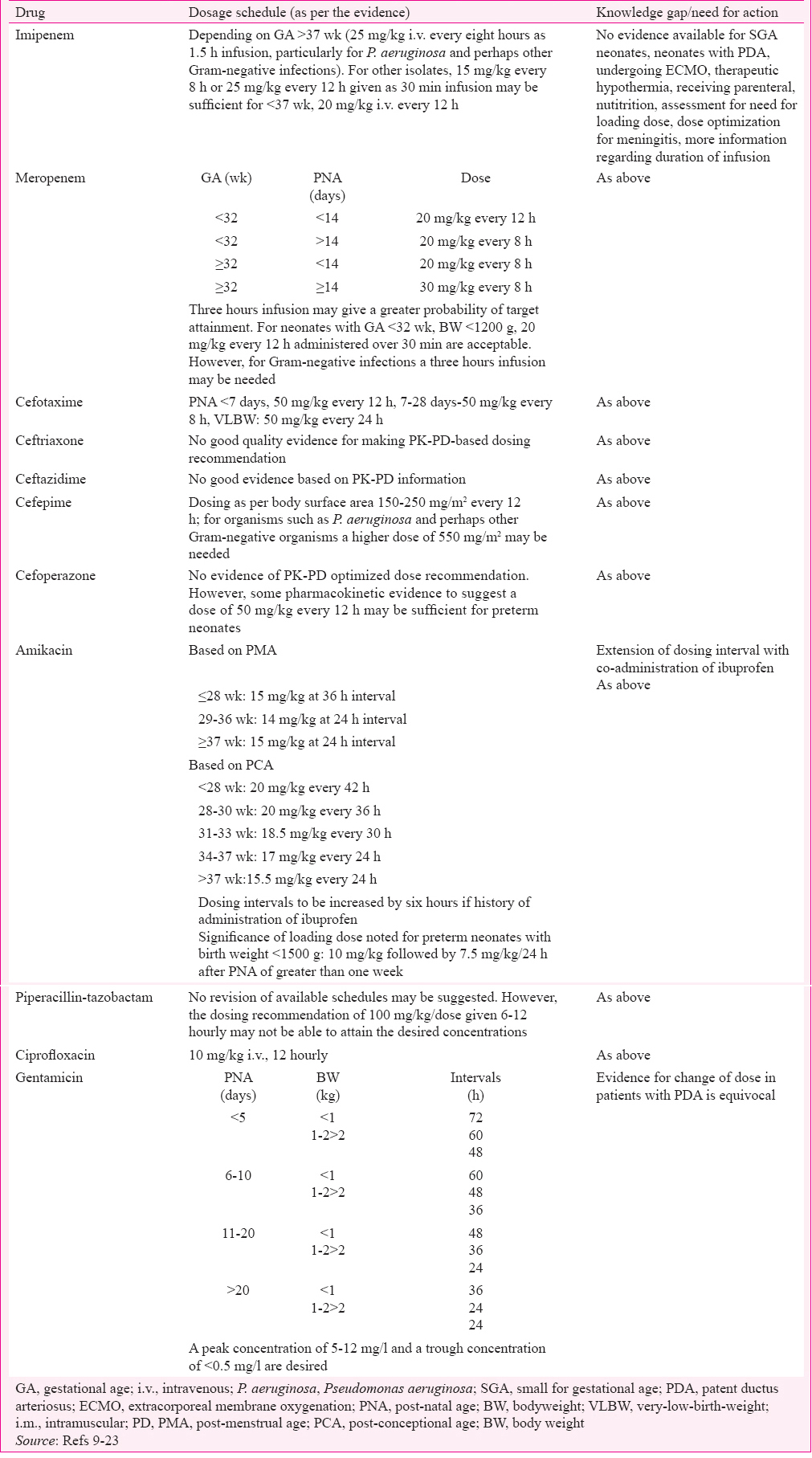
The armamentarium of antimicrobials available for treatment of Gram-negative infections is running out. The available agents have to be used judiciously and every measure aimed at improving efficacy and reducing toxicity with the use of antimicrobials for Gram-negative infections should be undertaken. An optimized dose based on PK-PD studies is one of the tools available to address this situation. Like in many centres world over, it is important to develop a collaborative effort to identify knowledge gaps for guiding antimicrobial therapy in neonatal infections caused by Gram-negative organisms. A good model for such collaborative exercise is being set by the Prato polymyxin consensus76. Determination of important covariates for PK and development of models based on the same, validation of these models in prospective studies, assessment of PK-PD parameters in various subgroup of neonates, particularly SGA, assessment of PK-PD parameters in case of different comorbidities and therapeutic modalities, validation of PD parameters of efficacy in neonatal population, incorporation of PD parameters of resistance development are some of the areas for research. While these activities are initiated, it may be suggested at this point that guidelines for the management of infective conditions of neonates, such as protocol for management of neonatal sepsis77 as developed by Neonatology Forum of India, could consider incorporating evidence-based dosing regimens for antibiotics. Table II summarizes the findings comparing the dosing schedules based on PK-PD data available.
Conflicts of Interest: None.
References
- Towards evidence-based dosing regimens in children on the basis of population pharmacokinetic pharmacodynamic modelling. Arch Dis Child. 2014;99:267-72.
- [Google Scholar]
- The ontogeny of drug metabolism enzymes and implications for adverse drug events. Pharmacol Ther. 2008;118:250-67.
- [Google Scholar]
- Serum creatinine concentration, urinary creatinine excretion and creatinine clearance during the first 9 weeks in preterm infants with a birth weight below 1500 g. Eur J Pediatr. 1996;155:815-9.
- [Google Scholar]
- Gentamicin pharmacokinetics in neonates with patent ductus arteriosus. Crit Care Med. 1997;25:273-5.
- [Google Scholar]
- Gentamicin pharmacokinetics in term newborn infants receiving high-frequency oscillatory ventilation or conventional mechanical ventilation: A case-controlled study. J Perinatol. 2003;23:559-62.
- [Google Scholar]
- Morphine metabolite pharmacokinetics during venoarterial extra corporeal membrane oxygenation in neonates. Clin Pharmacokinet. 2006;45:705-14.
- [Google Scholar]
- Effect of hypothermia and extracorporeal life support on drug disposition in neonates. Semin Fetal Neonatal Med. 2013;18:23-7.
- [Google Scholar]
- Etiology of bacteremia in young infants in six countries. Pediatr Infect Dis J. 2015;34:e1-8.
- [Google Scholar]
- Clinical pharmacology of imipenem and cilastatin in premature infants during the first week of life. Antimicrob Agents Chemother. 1990;34:1172-7.
- [Google Scholar]
- Population pharmacokinetic-pharmacodynamic target attainment analysis of imipenem plasma and urine data in neonates and children. Pediatr Infect Dis J. 2013;32:1208-16.
- [Google Scholar]
- Prospective determination of plasma imipenem concentrations in critically ill children. Antimicrob Agents Chemother. 2006;50:2563-8.
- [Google Scholar]
- Population pharmacokinetics of meropenem in plasma and cerebrospinal fluid of infants with suspected or complicated intra-abdominal infections. Pediatr Infect Dis J. 2011;30:844-9.
- [Google Scholar]
- Meropenem pharmacokinetics, pharmacodynamics, and Monte Carlo simulation in the neonate. Pediatr Infect Dis J. 2008;27:794-9.
- [Google Scholar]
- Meropenem pharmacokinetics in the newborn. Antimicrob Agents Chemother. 2009;53:3871-9.
- [Google Scholar]
- Short versus long infusion of meropenem in very-low-birth-weight neonates. Antimicrob Agents Chemother. 2012;56:4760-4.
- [Google Scholar]
- Individualised dosing of amikacin in neonates: A pharmacokinetic/pharmacodynamic analysis. Eur J Clin Pharmacol. 2009;65:705-13.
- [Google Scholar]
- Renal drug clearance in preterm neonates: Relation to prenatal growth. Ther Drug Monit. 2007;29:284-91.
- [Google Scholar]
- Limited predictability of amikacin clearance in extreme premature neonates at birth. Br J Clin Pharmacol. 2005;61:139-48.
- [Google Scholar]
- Once-a-day individualized amikacin dosing for suspected infection at birth based on population pharmacokinetic models. Biol Neonate. 2001;80:142-7.
- [Google Scholar]
- Observational trial of a 48-hour gentamicin dosing regimen derived from Monte Carlo simulations in infants born at less than 28 weeks'gestation. J Pediatr. 2008;153:530-4.
- [Google Scholar]
- Comparison of two gentamicin dosing schedules in very low birth weight infants. Pediatr Infect Dis J. 2002;21:234-40.
- [Google Scholar]
- Effect of patent ductus arteriosus on gentamicin pharmacokinetics in very low birth weight (less than 1,500 g) babies. Dev Pharmacol Ther. 1987;10:107-17.
- [Google Scholar]
- Gentamicin pharmacokinetics in preterm infants with a patent and a closed ductus arteriosus. Pharm World Sci. 2001;23:200-4.
- [Google Scholar]
- Population pharmacokinetics of piperacillin/tazobactam in neonates and young infants. Eur J Clin Pharmacol. 2013;69:1223-33.
- [Google Scholar]
- Population pharmacokinetics of piperacillin using scavenged samples from preterm infants. Ther Drug Monit. 2012;34:312-9.
- [Google Scholar]
- Population pharmacokinetics of ciprofloxacin in neonates and young infants less than three months of age. Antimicrob Agents Chemother. 2014;58:6572-80.
- [Google Scholar]
- Multiple dose pharmacokinetics of ciprofloxacin in preterm babies. Indian Pediatr. 2004;41:1001-7.
- [Google Scholar]
- Cefotaxime pharmacokinetics and treatment of meningitis in neonates. Infection. 1989;17:338-42.
- [Google Scholar]
- Once-daily versus twice-daily administration of ceftazidime in the preterm infant. Antimicrob Agents Chemother. 1995;39:2048-50.
- [Google Scholar]
- Population pharmacokinetics of cefepime in the neonate. Antimicrob Agents Chemother. 2005;49:2760-6.
- [Google Scholar]
- Population pharmacokinetics of cefepime in neonates with severe nosocomial infections. J Clin Pharm Ther. 2008;33:295-306.
- [Google Scholar]
- Clinical and pharmacokinetic study of imipenem/cilastatin in children and newborn infants. Pathol Biol (Paris). 1989;37:485-90.
- [Google Scholar]
- Bacteriological and clinical evaluations of imipenem/cilastatin sodium in neonates and premature infants. Jpn J Antibiot. 1988;41:1692-703.
- [Google Scholar]
- Clinical and pharmacokinetic evaluation of imipenem/cilastatin sodium in neonates and young infants. Jpn J Antibiot. 1988;41:1650-6.
- [Google Scholar]
- Pharmacokinetic and clinical studies on imipenem/cilastatin sodium in neonates and premature infants. Jpn J Antibiot. 1988;41:1671-91.
- [Google Scholar]
- Pharmacokinetics and clinical evaluation of imipenem/cilastatin sodium in pediatric surgery. Jpn J Antibiot. 1988;41:1721-30.
- [Google Scholar]
- Evaluation of imipenem/cilastatin sodium in neonatal infections. Jpn J Antibiot. 1988;41:1715-20.
- [Google Scholar]
- Pharmacokinetic and clinical evaluation of imipenem/cilastatin sodium in neonates and premature infants. A study of imipenem/cilastatin sodium by a perinatal co-research group. Jpn J Antibiot. 1989;42:953-72.
- [Google Scholar]
- Pharmacokinetic and clinical studies with imipenem/cilastatin sodium in neonates. Jpn J Antibiot. 1988;41:1657-70.
- [Google Scholar]
- Pharmacokinetics and clinical efficacy of imipenem/cilastatin sodium in neonates. Jpn J Antibiot. 1988;41:1704-14.
- [Google Scholar]
- Pharmacokinetic and clinical evaluations of imipenem/cilastatin sodium in neonates and premature infants. Jpn J Antibiot. 1989;42:1102-24.
- [Google Scholar]
- Pharmacokinetic, bacteriological and clinical studies on imipenem/cilastatin sodium in neonates. Jpn J Antibiot. 1989;42:1087-101.
- [Google Scholar]
- Pharmacokinetic, bacteriological and clinical studies on imipenem/cilastatin sodium in neonates. Jpn J Antibiot. 1989;42:1077-86.
- [Google Scholar]
- Pharmacokinetics of imipenem-cilastatin in neonates. Antimicrob Agents Chemother. 1985;27:431-5.
- [Google Scholar]
- Single-dose pharmacokinetics of imipenem-cilastatin in neonates. Antimicrob Agents Chemother. 1985;27:511-4.
- [Google Scholar]
- Therapeutic guidelines for prescribing antibiotics in neonates should be evidence based: A French National Survey. Arch Dis Child. 2015;100:394-8.
- [Google Scholar]
- Pharmacokinetic and clinical evaluation of serious infections in premature and newborn infants under therapy with imipenem/cilastatin. Infection. 1999;27:299-304.
- [Google Scholar]
- Imipenem levels are not predicTable in the critically ill patient. J Trauma. 2004;56:111-7.
- [Google Scholar]
- The effect of imipenem/cilastatin on the aerobic faecal flora of children. J Antimicrob Chemother. 1986;18(Suppl E):121-5.
- [Google Scholar]
- Pharmacokinetics of meropenem in preterm neonates. Ther Drug Monit. 2001;23:198-201.
- [Google Scholar]
- Stability and degradation kinetics of meropenem in powder for injection and reconstituted sample. J Pharm Biomed Anal. 2006;41:1363-6.
- [Google Scholar]
- One dose per day compared to multiple doses per day of gentamicin for treatment of suspected or proven sepsis in neonates. Cochrane Database Syst Rev. 2011;11:CD005091.
- [Google Scholar]
- Nonparametric population pharmacokinetic analysis of amikacin in neonates, infants, and children. Antimicrob Agents Chemother. 2002;46:1381-7.
- [Google Scholar]
- Long term circulatory and renal consequences of intrauterine growth restriction. Acta Paediatr. 2005;94:817-24.
- [Google Scholar]
- Impact of small-for-gestational age (SGA) status on gentamicin pharmacokinetics in neonates. J Clin Pharmacol. 2014;54:39-45.
- [Google Scholar]
- Evaluation of an amikacin loading dose for nosocomial infections in very low birthweight infants. Acta Paediatr. 2004;93:356-60.
- [Google Scholar]
- Novel model-based dosing guidelines for gentamicin and tobramycin in preterm and term neonates. J Antimicrob Chemother. 2015;70:2074-7.
- [Google Scholar]
- Single-dose pharmacokinetics of piperacillin and tazobactam in infants and children. Antimicrob Agents Chemother. 1994;38:2817-26.
- [Google Scholar]
- Wide intra- and inter-country variability in drug use and dosage in very-low-birth-weight newborns with severe infections. Eur J Clin Pharmacol. 2013;69:1031-6.
- [Google Scholar]
- Ciprofloxacin in neonatal Enterobacter cloacae septicaemia. Arch Dis Child. 1989;64:1388-91.
- [Google Scholar]
- Pharmacokinetics of moxifloxacin in an infant with Mycoplasma hominis meningitis. Pediatr Infect Dis J. 2012;31:197-9.
- [Google Scholar]
- Safety of ceftriaxone sodium at extremes of age. Expert Opin Drug Saf. 2008;5:515-23.
- [Google Scholar]
- Pharmacokinetics of cefotaxime in newborn infants. Antimicrob Agents Chemother. 1982;4:683-4.
- [Google Scholar]
- Ceftriaxone pharmacokinetics in newborn infants. Antimicrob Agents Chemother. 1983;23:341-3.
- [Google Scholar]
- Cefotaxime and desacetylcefotaxime pharmacokinetics in very low birth weight neonates. J Pediatr. 1989;114:461-7.
- [Google Scholar]
- Cefotaxime dosage in infants and children. Pharmacokinetic and clinical rationale for an extended dosage interval. Clin Pharmacokinet. 1992;22:284-97.
- [Google Scholar]
- Pharmacokinetics of ceftriaxone in pediatric patients with meningitis. Antimicrob Agents Chemother. 1983;23:191-4.
- [Google Scholar]
- Pharmacokinetics and clinical evaluation of cefotaxime in children suffering with purulent meningitis. J Antimicrob Chemother. 1984;14(Suppl B):161-5.
- [Google Scholar]
- Pharmacokinetic evaluation of cefoperazone in infants. Antimicrob Agents Chemother. 1985;28:149-50.
- [Google Scholar]
- Ceftazidime pharmacokinetics in preterm infants: Effect of postnatal age and postnatal exposure to indomethacin. Br J Clin Pharmacol. 1995;40:439-43.
- [Google Scholar]
- Ceftazidime pharmacokinetics in preterm infants: Effects of renal function and gestational age. Clin Pharmacol Ther. 1995;58:650-9.
- [Google Scholar]
- Activity of cefepime against ceftazidime- and cefotaxime-resistant gram-negative bacteria and its relationship to beta-lactamase levels. Antimicrob Agents Chemother. 1989;33:498-502.
- [Google Scholar]
- Intraventricular colistin use in neonatal meningitis caused by Acinetobacter baumanii . Ann Fr Anesth Reanim. 2011;30:854-5.
- [Google Scholar]
- Colistin inhalation monotherapy for ventilator-associated pneumonia of Acinetobacter baumannii in prematurity. Pediatr Pulmonol. 2014;49:381-8.
- [Google Scholar]
- Framework for optimisation of the clinical use of colistin and polymyxin B: The Prato polymyxin consensus. Lancet Infect Dis. 2015;15:225-34.
- [Google Scholar]
- Evidence Based Clinical Practice Guidelines: National Neonatology Forum. Available from http://www.nnfi.org/images/pdf/nnf_cpg_consolidated_file-january102011.pdf






