Translate this page into:
Evaluation of cystatin C activities against HIV
Reprint requests: Dr Atmaram Bandivdekar, Department of Biochemistry & Virology, National Institute for Research in Reproductive Health (ICMR), J. Merwanji Street, Parel, Mumbai 400 012, Maharashtra, India e-mail: batmaram@gmail.com
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Several host defense proteins known to possess antimicrobial activities are present on mucosal surfaces and are consequently found in body fluids of vertebrates. Naturally occurring protease inhibitors like cystatins, especially cystatin C (cys C), are abundantly present in human seminal plasma. Although its antiviral activity against herpes simplex virus (HSV) has been demonstrated, the role of this protein against HIV is not well studied. Therefore, the aim of the present study was to evaluate the anti-HIV activities of cys C, which is present innately in the male reproductive tract.
Methods:
Protein-protein interaction of cys C with various HIV proteins was studied using a commercially available HIV blot and specific interaction with HIV protease was studied by dot-blot technique using commercially available cys C. To purify biologically active cys C from human seminal plasma to be used for subsequent experiments, gel-permeation chromatography followed by affinity chromatography was used. The HIV infectivity inhibition activity of the purified cystatin C was tested in TZM-bl cells. To study its activity on HIV protease, time-course enzyme kinetics studies were performed using spectrometric assay.
Results:
Cystatin C reacted with some HIV proteins including HIV protease. Biologically active cys C was purified using gel permeation chromatography followed by affinity chromatography. When tested in TZM-bl cells, purified cystatin C demonstrated HIV-infectivity inhibitory activity (IC50: 0.28 μM). Enzyme kinetic studies demonstrated that it abrogated the action of HIV protease on its substrate.
Interpretation & conclusions:
The present data demonstrate that cystatin C possesses anti-HIV activities. Molecular models need to be designed with this protein which would assist towards prevention/therapeutics against HIV.
Keywords
Affinity chromatography
antimicrobial proteins
gel permeation chromatography
innate immunity
protease inhibitor
Human seminal plasma is known to harbour several antimicrobial polypeptides which are mainly cationic in nature. Although such proteins have been investigated for their anti-bacterial effects, only a few are studied for their antiviral activities especially against HIV1. Naturally occurring protease inhibitors like the serpin, human seminal inhibitor I and cystatins are present in the male reproductive tract2. The cystatins constitute a large group of evolutionary related proteins with diverse biological activities. Initially, these were characterized as inhibitors of cysteine proteases known as cathepsins expressed in the male reproductive tract and other organs3 but subsequently alternative functions of cystatins have been proposed including the potential to modulate immune responses4. The cystatin type-2 family of competitive reversible cysteine protease inhibitors consists of at least 10 members, three of which are expressed predominantly in the male reproductive tract. These are found in a variety of human fluids and secretions, where these appear to provide protective functions5.
Cystatin C (cys c) has a low molecular weight (approximately 13.3 kD); is a non-glycosylated, basic protein with an isoelectric point of 9.3 and is abundantly present in semen6. Although other cystatins like cys 11, cys 8, testatin or CST9 are present in the semen7, previous reports suggest that cys C has microbicidal properties against both bacteria and viruses7. Whole human cys C and its peptides are active against bacteria in the respiratory tract8 and viruses like herpes simplex virus (HSV) and coronavirus9. Though a study using the proteomic approach has indicated that cys C is one of the proteins identified from human seminal plasma to have a putative role in innate host defense mechanisms against HIV10, its role in antiviral activities against HIV is not studied in sufficient details.
Several antiretroviral drugs either interfere with reverse transcriptase or inhibit HIV protease11. Treatment for AIDS in many cases is a combination of two nucleoside analogues of HIV reverse transcriptase and one protease inhibitor11. The combination therapy appears to overcome the ability of the virus to rapidly produce drug resistant mutants12. Inclusion of novel naturally occurring compounds which do not have side effects and which can prevent the emergence of resistant mutants in a ‘combination therapy’ against HIV is becoming increasingly important13. The present study was aimed to demonstrate antiviral activity of cys C against HIV. The interaction of commercially available cys C with various HIV proteins was studied. Biologically active cys C was purified from human seminal plasma and its HIV-infectivity inhibition activity in TZM-bl cells was studied. Further, its effect on the activity of HIV protease was also examined.
Material & Methods
This study was conducted in the Biochemistry and Virology laboratories of National Institute for Research in Reproductive Health (NIRRH), Mumbai, Maharashtra, India, during January 2011 to January 2013. The study protocol was approved by both institutional human ethics committee as well as animal ethics committee.
Inclusion/exclusion criteria: Anonymized semen samples from healthy males in the age group of 25-40 yr and having an HIV-negative status were taken for the study. Samples were collected by masturbation after three days of abstinence. After the completion of routine semen analysis, samples were immediately preserved at 4°C. Samples having normal semen parameters and HIV-negative status were used for purification experiments. Seminal plasma from pooled semen was separated from sperms by centrifugation at 1000 g for 10 min and stored at -80° C in batches of 10 ml until used for purification.
Belgium albino rabbits were housed in individual cages in an illumination-controlled room at 25°C. Animals were provided twice daily with standard chow and water was supplied ad libitum.
Cells, viruses and reagents: TZM-bl cells, HIV viruses (HIV-1 93IN101 and HIV-193IN105), HIV protease and peptide substrate for HIV-protease and Darunavir (Prezista, TMC 114) were kind gifts from NIH AIDS Research and Reference Reagent Program (Bethesda; Maryland, USA). TZM-bl cells were maintained in Dulbeco's modified eagle medium (DMEM) (Gibco Invitrogen, USA) supplemented with 10 per cent foetal bovine serum (FBS) (Hi-Media Laboratories Pvt. Ltd, Mumbai, India) and penicillin/streptomycin (Gibco Invitrogen, USA) and passaged upon confluence. Viral stocks were prepared by infecting normal human peripheral blood mononuclear cells (PBMCs), passed through 0.22 µm filter and stored at -80°C until used. TCID50 (50% tissue culture infectivity dose) was calculated using the Spearman-Karber method14. The TZM-bl indicator cell line is a HeLa cell derivative that expresses high levels of CD4, CCR5 and CXCR4. Cells contain HIV long terminal repeat (LTR)-driven β-galactosidase (β-gal) and firefly luciferase reporter cassettes that are activated by HIV infection and subsequent Tat protein expression. Blue-stained HIV-infected cells expressing β-gal can thus be quantified and compared with uninfected cells.
Interaction studies of cystatin C (cys C) with various HIV proteins: A modified method as described earlier1516 was used to study the protein-protein interaction of cys C by Far-Western blot analysis using a commercially obtained ready-to-use HIV protein blot (J. Mitra and Co., New Delhi, India).
Blots were blocked with five per cent skimmed milk powder and two per cent bovine serum albumin (BSA) in 0.01 M phosphate buffer saline (PBS), pH 7.2 (blocking buffer) and incubated overnight (O/N) in 1 ml 0.01M PBS containing 10 μg of cys C (ProSpec-TanyTechnoGeneLtd; Israel). Next day, the blots were washed once with PBS and incubated with 1:200 diluted goat polyclonal antibodies to cys C (Santa Cruz Biotechnology Inc., Shanghai) or normal goat serum (negative control) O/N at 4°C. To ensure the validity of the kit, one blot was treated with HIV- positive serum given along with the kit. The following day, the blots were washed thrice with 0.1 per cent tween-20 in PBS (tween-PBS), incubated for 1.5 h at 37°C in 1:6000 diluted HRP (horse radish peroxidase)-labelled rabbit anti-goat antibody (Bangalore Genie, Bengaluru, India). After washing thrice with tween-PBS, the blots were subsequently visualized by electrochemoluminiscence (ECL plus kit, obtained from GE Healthcare Biosciences, Bengaluru, India) using X-ray films according to the manufacturer's instructions.
Interaction of cys C with HIV protease by dot blot: Specific interaction of cys C with HIV protease was evaluated by dot blot analysis. In brief, 2 µl containing 0.075 and 0.150 µM of HIV protease or an unrelated protein was blotted onto nitrocellulose membranes and allowed to air dry. Following this, the same process for far-Western blotting as described above was performed except that the dots were visualized by incubating the blots in a solution of diaminobenzidene (8 mg in 20 ml PBS) to which 50 µl H2O2 was added. After the dots were visible, the blots were washed thoroughly in PBS and air dried.
Purification of cys C from human seminal plasma by immunoaffinity chromatography: to obtain bioactive cyc C in sufficient amounts from human seminal plasma the following purification procedure was carried out:
-
Fractionation of seminal plasma proteins by gel-permeation chromatography - Human seminal plasma proteins were clarified by centrifugation at 3000 g for 10 min. The supernatant thus obtained was diluted 1:3 (v/v) with 0.02 M PBS, pH 7.2 and fractionated using HiLoad 16/60 Superdex-200 column (GE Healthcare Biosciences, Bengaluru, India) on AKTA Explorer Protein Purification System (GE Healthcare Biosciences, Bengaluru, India) using the same buffer. The eluted fractions were dialyzed against distilled water to remove excess salt and lyophilized. The concentration of the proteins was estimated by Bradford's method17 and the fractions visualized by SDS-PAGE (sodium dodecyl sulphate polyacrylamide gel electrophoresis) on 12 per cent polyacrylamide gel.
-
Detection of the presence of cys C in the partially purified fractions obtained by gel permeation chromatography - To detect the presence of cys C, proteins in the fractions were separated on 15 per cent SDS-PAGE and Western blot was performed as described. In brief, proteins separated on SDS-PAGE were transferred onto a nitrocellulose membrane and blocked O/N with blocking buffer for 2 h. Next day, the blots were washed thrice with tween-PBS and incubated in antibodies to cys C (1:2000) for 1.5 h. HRP-labelled secondary antibody (1:3000) was added after washing the blots thrice with tween-PBS. The blots were visualized by ECL method as described before.
-
Purification of bioactive cys C from fraction-4 purified by gel permeation chromatography from human seminal plasma proteins - antibodies to commercially obtained cys C were raised in rabbits as described earlier18. The titre obtained was 1:8000 as observed by indirect ELISA. Immunoaffinity chromatography was carried out using the AminoLink® Plus Immobilization kit (Thermo-Scientific Pierce, Mumbai, India) according to the manufacturer's instructions
Immunoglobulins (IgG) were purified from serum of rabbit immunized with cys C19 and were immobilized on the AminoLink Plus column provided in the kit. In brief, immunoglobulins (10 mg/2ml coupling buffer, pH 10) were loaded on the column and incubated overnight. (All column centrifugations were performed at 1000 g for 1 min using 15 ml collection tubes). The column was washed with the coupling buffer and sodium cyanoborohydride (coupling agent) was added and incubated O/N at 4°C. Next day, reactants and uncoupled IgG were washed off with wash buffer (1M NaCl) and the column stored at 4°C until used.
The IgG-coupled column was equilibrated with binding buffer (PBS, pH 7.2). Fraction-4 (20mg/2ml/run) of the seminal plasma proteins (containing cys C) purified using Superdex-200 column was added to the column and subsequently washed 3 to 4 times with binding buffer to remove unbound proteins. cys C bound to the column was eluted using elution buffer (0.1 M glycine-HCl, pH 2.5). The resulting eluate was dialyzed against distilled water and protein content estimated. The yield of cys C was 25 μg/ml of seminal plasma.
Demonstration of the inhibitory activity of cys C on the binding of HIV protease to its substrate: The inhibitory activity of cys C on the binding of HIV protease to a chromatophoretic peptide substrate was studied by spectrometric assay20. The oligopeptide (H-Arg-Lys-Ile-Leu-Phe(NO2)-Leu-Asp-Gly-NH2) is based on the reverse transcriptase-endonuclease cleavage site in the Pr160 Gag-pol of HIV-1, with chromophoric Phe (NO2) residue located in the P1’ position. This peptide is cleaved by protease between the Leu and Phe (NO2) residues. A continuous decrease of absorbance at 310 nm occurs during cleavage.
In brief, 10 μl containing 0.150 μM of HIV protease was added to 350 μl assay buffer (0.8 mM NaCl and 1 mM EDTA dissolved in 20 mM PBS, pH 7.0) and mixed with 50 μl of 0.75 and 1.50 μM cystatin C each in test tubes and kept at room temperature for 10 min. The mixture was subsequently added to a UV quartz cuvette containing 100 μM/50 μl of peptide substrate. Darunavir, a non peptidic protease inhibitor, and an unrelated protein treated similarly were taken as positive control and negative controls, respectively. Readings were taken continuously at 310 nm at intervals of 60 sec in a spectrophotometer (BioRad, SmartSpec 3000, USA) for 1800 sec and a graph of absorbance versus time (in seconds) plotted.
Demonstration of anti-HIV activity of cys C in TZM-bl cells: TZM-bl cells (2x104)/200 µl/well were cultured for 24 h in 96-well culture plates in medium containing DMEM, 10 per cent FBS, penicillin and streptomycin (10,000 U/ml and 100 µg/ml, respectively) (designated as complete medium) at 5 per cent CO2 at 37°C. Next day, 50 µl [approximately 200 pg as determined by p24 assay carried out by an ELISA kit (XpressBio Life Sciences products, USA)] of the virus in complete medium containing 0.1 per cent DEAE-dextran (Sigma-Aldrich, India), at a multiplicity of infection (m.o.i.) of 0.15, were added to the wells with different concentrations of cys C and incubated further for 24 h. PBS was added as vehicle control instead of the fractions in ‘virus only control’ wells.
β-galactosidase assay (Bangalore Genie, Bengaluru, India) was performed according to the manufacturer's instructions. In brief, wells were washed with wash buffer, incubated with 100 µl fixative for 5 min and further washed thrice with wash buffer. Thereafter, 100 µl of staining solution was added and plates incubated for 4 h at 37°C. Wells were washed once with rinse solution and 100 µl wash buffer added. HIV-infected (blue-stained) cells in treated wells were counted under an inverted microscope (Nikon India Pvt Ltd, India) and compared with ‘virus only control’ cells.
Cell viability of the TZM-bl cells in presence of cys C was checked using an MTT assay kit (Krishgen Biosystems, India) according to the manufacturers’ instructions. In brief, TZM-bl cells were grown for 24 h and next day increasing amounts of cys C (50 μl) was added to the wells along virus as described above. The wells to which cys C was not added served as control. The following day, the medium was removed and replaced with 100 μl of fresh medium. Freshly prepared MTT solution (10 µl) was added to the wells and incubated for 3 h after which 100 μl of SDS-HCl solution was added and incubation was carried for 4 h. Absorbance was read at 570 nm.
Statistical analysis: Results are represented as mean ± SD from three independent estimates. Data were analyzed using GraphPad Prism version 4.0 (La Jolla, CA, USA). Difference between test groups was examined statistically by one-way ANOVA and Dunnett's multiple post-test comparison.
Results
interaction of cys C with HIV proteins: Cys C interacted with various HIV proteins like gp160, gp120, p31 and p24 (Fig. 1A). An unrelated protein did not bind to any of bands on the blot when treated similarly as test sample (Fig. 1B). All HIV proteins were visible on the blot when treated with HIV-positive serum only (Fig. 1C). Cys C also interacted with HIV protease using the dot-blot technique (Fig. 1D).
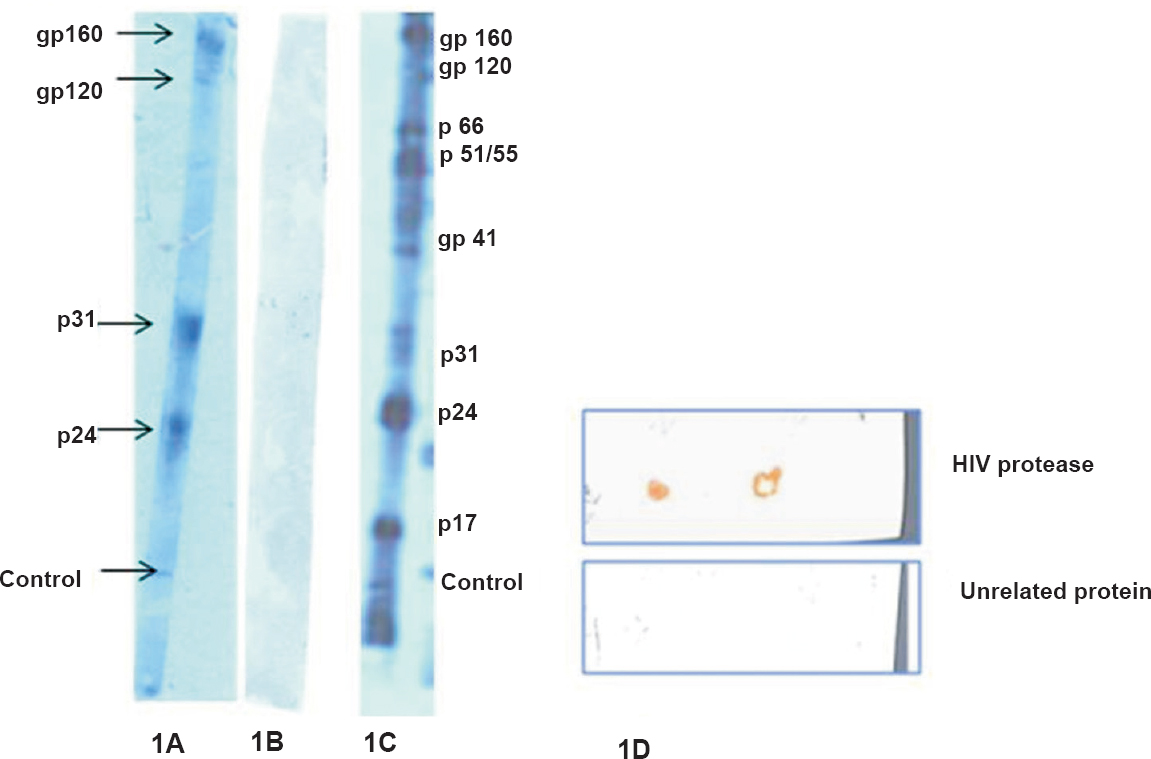
- (A). Bands of HIV proteins (gp160, gp120, p31, p24) interacting with cys C. (B). No bands were seen with an unrelated protein treated similarly (C). Bands visualized with HIV-positive serum only are shown (D). Interaction of cys C with HIV protease by dot blot. No binding was observed with an unrelated protein.
Purification of bioactive cys C from human seminal plasma by immunoaffinity chromatography: Bioactive cys C was purified from human seminal plasma for further evaluation of this protein in anti-HIV activities. Five major fractions of human seminal plasma proteins were obtained by gel permeation chromatography (Fig. 2A). Maximum number of proteins in the range of 6-15 kDa, in which cys C, a protein of approximately 14 kDa was likely to be present, were seen to be concentrated in fraction-4 (Fig. 2B, C). Figure 3A demonstrates the presence of cys C in fraction-4 (Fr. 4) of the fractions purified by gel permeation chromatography by Western blot. Figure 3B depicts the band(s) of cys C purified by immunoaffinity chromatography.
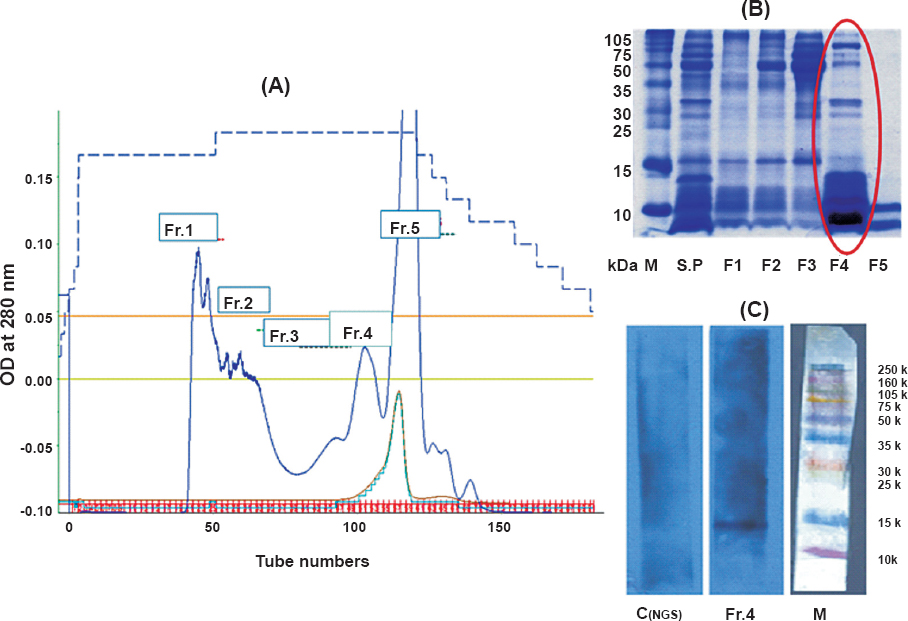
- (A). depicts the chromatogram of human seminal plasma proteins purified by gel permeation chromatography, the protein profile of which is shown in (B). The presence of cys C in Fr.4 is indicated in (C). (M, mol wt. marker; Control, blot treated with pre-immune serum; test, Blot treated with cys C antibody).
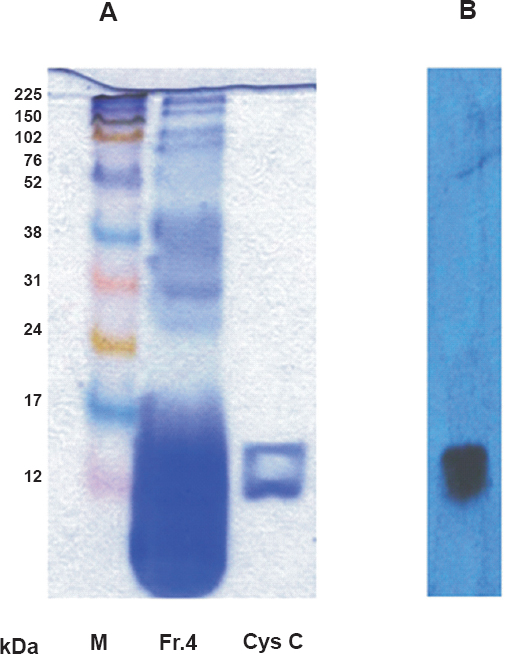
- (A). demonstrates the band(s) of purified cystatin C from fraction 4 of human seminal plasma proteins purified by gel-permeation chromatography and by western blotting using specific antibodies to cys C (B).
Demonstration of the inhibition by cys C on HIV protease activity: HIV protease cleaved the peptide substrate resulting in a continuous decrease of absorbance at 310 nm (Fig. 4). Since 0.75 μM cys C (test) abrogated the activity of HIV protease on the peptide substrate, there was no decrease in the absorbance. Similar activity was obtained with Darunvir, a known protease inhibitor (positive control). An unrelated protein (negative control) demonstrated a continuous decrease in the absorbance (Fig. 4).
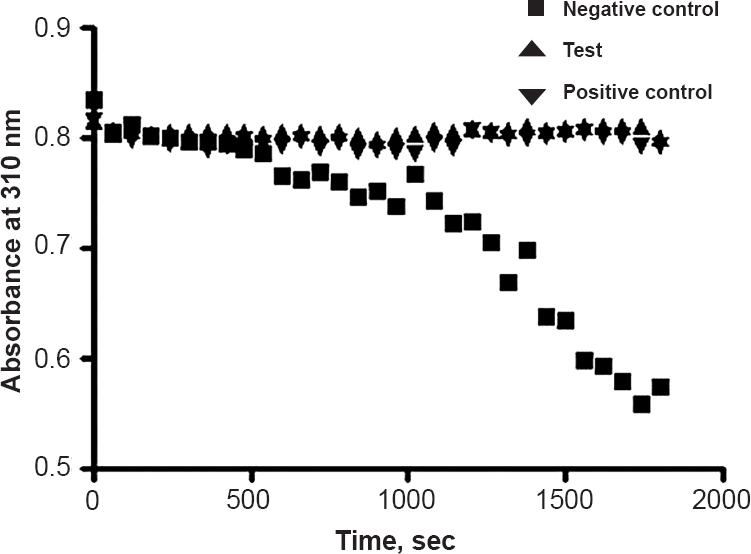
- A representative graph depicting the activity of 0.75 μM cys C (test), Darunavir (positive control) and an unrelated protein (negative control) on the action of HIV protease on its peptide substrate. A continuous decrease in absorbance at 310 nm was observed with unrelated protein whereas cys C and Darunavir did not show decrease in absorbance.
Demonstration of HIV-infectivity inhibition activity of cys C in TZM-bl cells: Cys C demonstrated a dose-dependant decrease in HIV-infected cells as compared to virus only controls (Fig. 5). Inhibitory concentration 50 per cent (IC50) was calculated by plotting HIV-infected cells versus concentration curves using non-linear regression curve. The IC50 for cys C was 0.280 μmoles. The cell viability of the TZM-bl cells was not affected in spite of treatment with high concentration of cys C (4 μM) indicating that it was not cytotoxic to the cells (Fig. 6).
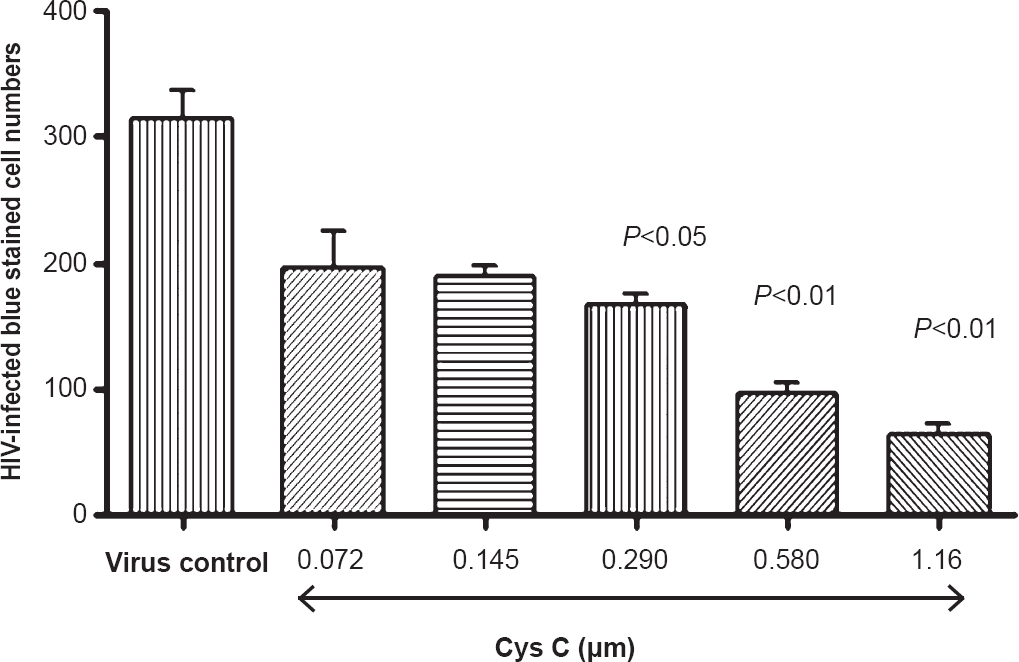
- Cys C exhibited a dose-dependant decrease of blue-stained HIV-infected cells as compared to virus only control.

- No significant difference observed in the absorbance with increasing concentrations of cys C as compared to control in MTT assay.
Discussion
The male reproductive tract is known to harbour protease inhibitors like the active site inhibitor serpin, human seminal inhibitor I and the cysteine protease inhibitor, cystatin C2. The cystatin superfamily comprises a large group of the cystatin domain containing proteins, present in a wide variety of organisms, including humans. Cystatin inhibitory activity is vital for the delicate regulation of normal physiological processes by limiting the potentially highly destructive activity of their target proteases such as the papain family, including cathepsins21.
In the present study the anti-HIV activities of cys C were investigated. Experimental evidence suggests that cys C binds to various HIV proteins including HIV protease. The function of HIV protease is to cleave newly synthesized polyproteins at the appropriate places to create mature functional protein components of an infectious HIV virion22. Cystatins are thought to be non-selective and inactivate proteases reversibly and competitively by indirect blockage of catalytic centers and form very stable bimolecular complexes with proteases23. Our findings demonstrated that cys C was able to abrogate the action of HIV protease on its peptide substrate, suggesting that it might prevent the normal functioning of HIV protease which in turn would potentially prevent viral replication and transmission. Human seminal plasma is known to contain cys C in abundant amounts. Hence, biologically active cys C was purified from seminal plasma employing gel permeation chromatography followed by immunoaffinity chromatography techniques. Two bands of cys C were obtained on SDS-PAGE, both of which reacted with the specific antibodies to cys C, thus leading to the conclusion that the low molecular weight band might be a truncated form of cys C. Previously, Martellini et al10 have identified fragments of cys C besides the whole cys C in human seminal plasma.
The ability of the purified protein to inhibit HIV infectivity was tested using TZM-bl cells. Our findings indicated that cys C was able to significantly reduce the HIV-infectivity in TZM-bl cells. Further, high amounts of cys C did not affect the cell viability as exhibited by MTT assay suggesting it to be non-cytotoxic. Cys C was found to bind to various HIV proteins including HIV envelope proteins like gp120 and gp160. Therefore, it would be of interest to examine whether the binding of cys C to these proteins prevents the fusion of HIV virus with CD4+ cells thus potentially preventing entry within the cells.
In preliminary studies the ability of this protein to suppress the cytotoxic activity manifested as formation of syncytia (giant cells) in TZM-bl cells infected with syncytium-inducing (SI) HIV virions has been tested; which would be an indirect indication of its fusion and entry inhibiting activity24. Although we did find a significant syncytia-inhibiting activity with this protein (data not shown), in vitro experiments to demonstrate whether cys C could inhibit binding of CD4 to gp120 would further strengthen these observations.
Inhibition of HIV protease represents an important avenue for antiviral therapy25. Our data suggested that cys C might play a physiological role as inhibitor of HIV replication. Preventing HIV-1 from establishing a persistent infection after sexual contact may be more effective than treating HIV-1-seropositive patients with antiretroviral medicines26.
In conclusion, cystatin C may be considered as a potential candidate microbicide to prevent replication of HIV-1 transmitted via the sexual route. In future, peptides designed on naturally occurring compounds such as cys C could be potentially used along with other anti-HIV formulations in a ‘combination therapy’ to provide an effective regimen to prevent HIV transmission though the sexual route.
Acknowledgment
The first author (VV) acknowledges the Department of Science and Technology, for providing funds under Women Scientists (DST, WOS-A) scheme. Authors acknowledge the ‘National Institute for Health (NIH) AIDS Research and Reference Reagent Program, USA’ for gifting some of the reagents used for the study and Dr Nilesh Shah, Metropolis Healthcare Ltd., Mumbai, India, for providing semen samples.
References
- Mucosal immunity to HIV: a review of recent literature. Curr Opin HIV AIDS. 2008;3:541-7.
- [Google Scholar]
- Endogenous mucosal antiviral factors in the oral cavity. J Infect Dis. 1999;179(Suppl 3):431-4.
- [Google Scholar]
- Human cystatin C, an amyloidogenic protein, dimerizes through three-dimensional domain swapping. Nat Struct Biol. 2001;8:316-20.
- [Google Scholar]
- Cystatin 11: a new member of the cystatin type 2 family. Endocrinology. 2002;143:2787-96.
- [Google Scholar]
- Synthesis and antibacterial properties of peptidyl derivatives and cyclopeptides structurally based upon the inhibitory center of human cystatin C. Dissociation of antiproteolytic and antibacterial effects. APMIS. 2000;108:473-81.
- [Google Scholar]
- Cystatin C, a human proteinase inhibitor, blocks replication of Herpes simplex virus. J Virol. 1990;64:941-3.
- [Google Scholar]
- Cationic polypeptides contribute to the anti-HIV-1 activity of human seminal plasma. FASEB J. 2009;23:3609-18.
- [Google Scholar]
- Protease inhibitors: a new weapon and a new strategy against HIV. J Assoc Nurses AIDS Care. 1996;7:57-71.
- [Google Scholar]
- AIDS and other immunodeficiencies. In: Goldsby RA, Kindt TJ, Osborne BA, Kuby J, eds. Immunology (5th ed). New York: WH Freeman and Co; 2007. p. :429-59.
- [Google Scholar]
- ACTG virology manual for HIV laboratories. (2nd ed). Bethesda, Md, USA: National Institute of Allergy and Infectious Diseases, National Institute of Health; 1993.
- [Google Scholar]
- Identification of CD4-independent HIV receptors on spermatozoa. Am J Reprod Immunol. 2003;50:322-7.
- [Google Scholar]
- Detecting protein-protein interactions by Far-western blotting. Nat Protoc. 2007;2:3278-84.
- [Google Scholar]
- Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principal of protein-dye binding. Anal Biochem. 1976;72:248-54.
- [Google Scholar]
- Studies with synthetic peptides of 80kDa human sperm antigen (80 kDA HSA) Am J Reprod Immunol. 2004;51:106-11.
- [Google Scholar]
- Antifertility effect of passive administration of antibodies to 80kDa human sperm antigen and its synthetic peptides in male and female rats. Am J Reprod Immunol. 2005;54:332-41.
- [Google Scholar]
- Chromatophoric peptide subtrates for the spectrophotometric assay of HIV-1 protease. Biochem Biophys Res Commun. 1990;168:274-8.
- [Google Scholar]
- Cystatins: biochemical and structural properties, and medical relevance. Front Biosci. 2008;13:5406-20.
- [Google Scholar]
- HIV-1 assembly, budding, and maturation. Cold Spring Harb Perspect Med. 2012;2:a006924.
- [Google Scholar]
- Cathepsins: Fundamental effectors of endolysosomal proteolysis. Indian J Biochem Biophys. 2008;45:75-90.
- [Google Scholar]
- Amino functionalized novel cholic acid derivative include HIV-1 replication and syncytia formation in T cells. J Med Chem. 2006;4:2652-5.
- [Google Scholar]
- Drugs in traditional drug classes (nucleoside reverse transcriptase inhibitor/nonnucleoside reverse transcriptase inhibitor/protease inhibitors) with activity against drug-resistant virus (tipranavir, darunavir, etravirine) Curr Opin HIV AIDS. 2009;4:507-12.
- [Google Scholar]
- Lactoferrin prevents dendritic cell-mediated human immunodeficiency virus type 1 transmission by blocking the DC-SIGN-gp120 interaction. J Virol. 2005;79:3009-15.
- [Google Scholar]






