Translate this page into:
Evaluation of bacteriophage cocktail on septicaemia caused by colistin-resistant Acinetobacter baumannii in immunocompromised mice model
For correspondence: Dr Gopal Nath, Department of Microbiology, Institute of Medical Sciences, Banaras Hindu University, Varanasi 221 005, Uttar Pradesh, India e-mail: gopalnath@gmail.com
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Since the bacterium, Acinetobacter baumannii (AB) has acquired resistance to almost all commercially available antibiotics, the search for alternative treatment options continues to be need of the hour. Bacteriophage therapy seems to be the most promising amongst various proposed alternatives (e.g. antimicrobial peptides, bacteriocin, probiotics, etc.). The present study, therefore, aimed to evaluate the effect of different dosages of specific phages in immunocompromised rodents in a septicaemia model caused by AB mimicking real clinical situations.
Methods:
The three most active and unique phages (ɸAb4, ɸAb7 and ɸAb14) were selected for this study. A constant dose (100 µl of 108 pfu/ml) of AB was given in all the experiments. Five different sets of experiments were designed: prophylactic administration of phage cocktail in the volume of 100 µl (109 pfu/ml) before and simultaneous with the bacterial challenge; and therapeutic i.e. administration of phage cocktail six, 12 and 24 h after bacterial challenge. Since there were deaths in mice when phage was given 24 h after bacterial challenge, the reduced dosage i.e. 100 µl of 107, 106, 105 pfu/ml of phage cocktail was also evaluated.
Results:
The administration of 100 µl (109 pfu/ml) of phage cocktail after six, 12 and 24 h of the bacterial challenge resulted in the mortality ranging between 20 to 60 per cent. However, no mortality could be observed with simultaneous or prophylactic administration of phages with the bacterial challenge. No mortality was observed with reduced doses of the cocktail (106 and105 pfu/ml).
Interpretation & conclusions:
As per the results of this study, it may be concluded that even if patients with acute infections report late to the hospital, a relatively low dose of the phage cocktail may be therapeutically beneficial.
Keywords
Acinetobacter baumannii
bacteriophage cocktail
colistin-resistant
endotoxins
multidrug resistant
phage therapy
septicaemia
Acinetobacter baumannii (AB) is a member of the ESKAPE group of bacteria (i.e., Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, AB, Pseudomonas aeruginosa, and Enterobacter species) notoriously known for the antibiotic resistance crisis1. AB are opportunistic pathogens that cause a variety of infections involving the respiratory tract, urinary tract, skin and blood (bacteremia) and ventilator-associated pneumonia apart from local wound infections2. AB isolates have been reported to acquire resistance to almost all the antibiotics, including carbapenems as well as colistin12. AB is a part of commensal flora of human body, however, it may also be present in the hospital environment due to its’ capability to resist desiccation and disinfectants2. The infections due to multidrug-resistant (MDR)-AB and extensively drug-resistant (XDR)-AB are reported to be causing high morbidity, and mortality in patients admitted to intensive care units (ICUs). Infections caused by the drug-resistant AB compel for the administration of costly and toxic antibiotics and longer stay in the hospitals, leading to high treatment costs and occasional deaths2. Since many of the strains of this bacterium have achieved superbug state3, it has become an exigency to look for alternatives to antibiotics. Bacteriophage (phage) therapy may be one such alternative for severe infections caused by MDR- and XDR-AB strains4. Phage therapy is highly specific and effective in lysing targeted drug-resistant bacteria4. The pre-clinical evaluation of phage therapy in animal models is, however, an essential step before venturing into clinical trials. Artificial burns, pneumonia, pulmonary infections with various microbes in rodent models have successfully been treated with phages567. Although several AB specific phages have been isolated and characterized in terms of their potential therapeutic applications8910, only a few in vivo studies in animal models are available in literature and majority of these are restricted to local infections1112. One study in animal model regarding systemic use of AB specific bacteriophages showed the use of single phage simultaneously with the bacterial challenge13. On similar lines, Wang et al14 demonstrated good efficacy with simultaneous injection of a single phage in their study. These studies have, however, not simulated the real clinical conditions. In clinical settings, patients with AB septicaemia reporting to ICU may be of variable duration (i.e., ranging from recent infection to as old as many hours/days). A recent report regarding systemic/local use of AB specific in a clinical case with disseminated infection is one of the most highlighted ones in the field of bacteriophage therapy15. However, in this case report also the doses of bacteriophage cocktail were used randomly. While working with the bacteriophage therapy for P. aeruginosa infection in murine burn model during 2008-2011, it was observed that there were deaths in certain situations when fixed dosage of bacterial challenge and phage cocktail were given at different time points of the disease16. It could be speculated that a sudden lysis of bacteria in the systemic circulation might have resulted in an endotoxic crisis due to the optimum concentration of phage versus bacterial load. The other issue is the evolution of resistant mutants during phage therapy when a single bacteriophage is being administered. The use of a single phage has been found to be unsuccessful in solving the above issue17. Also, one cannot be sure regarding the dose of the phage cocktail if the patient reports to the ICU with variable bacterial load because of variations in the reporting time. Currently, no data are available regarding doses and dose schedule in such a situation. Therefore, this study was planned to evaluate the different doses of the cocktail of lytic bacteriophages on septicaemia of variable duration caused by AB in an immunocompromised mouse model.
Material & Methods
Sample collection: A total of 140 AB isolates comprising of 70 each from clinical and environmental origin, were screened for antibiotic susceptibility. The clinical strains were isolated from non-duplicate specimens of blood, pus, urine, sputum, nasal aspirate, tips of the endotracheal tubes, pericardial and pleural fluids of patients admitted to ICU of a tertiary care hospital of Banaras Hindu University, Varanasi, India. The study was carried out in the department of Microbiology, Institute of Medical Sciences, Banaras Hindu University between December 2014 to July 2017 after seeking clearance from the Institutional Animal Ethics Committee. The 70 environmental AB isolates were from water specimens of sewer, ponds (3 different sites of sewer of University hospital and 3 sites from Assi nala and 6 ponds) and river Ganga (6 different ghats of river Ganga at different times) in Varanasi. The isolation media used was Acinetobacter selective medium (MDR Leeds Acinetobacter medium containing cefsulodin, cephradine and vancomycin as selective components, Himedia, Mumbai).
Bacterial isolates and their identification: All the clinical and environmental isolates were identified as AB by using the standard biochemical and molecular method21. In multiplex PCR, two target regions of the chromosome of AB were selected i.e. recA gene of Acinetobacter species yielding 425 bp which was genus specific and 16S-23S ribosomal DNA intergenic spacer region yielding 208 bp amplicon for AB specific identification following the methods described earlier18. Further study was conducted only on AB confirmed isolates where amplification for both genus-specific and species-specific amplification could be seen.
The minimum inhibitory concentration of imipenem, meropenem, levofloxacin, tigecycline, polymyxin-B and polymyxin-E (colistin) against AB was carried out by broth dilution method following the recommendation of Clinical and Laboratory Standards Institute (2015)19. All the antibiotic powders were procured from HiMedia Pvt. Ltd., Mumbai. Known reference strain of AB ATCC (19606) was used as a positive control. Since minimum inhibitory concentration (MIC) breakpoint for tigecycline against AB was not mentioned in the guideline, the breakpoint mentioned for P. aeruginosa was considered.
Phage isolation and purification: Isolation of bacteriophages was done from different water sources (river, ponds and sewer) by using double agar overlay method with slight modification as described earlier20. In brief, for isolation of bacteriophages, the AB was plated as lawn culture (108 cfu/ml) on Muller-Hinton agar (MHA) (HiMedia). Water specimens from different water bodies were treated with one per cent chloroform (v/v) for 20 min and centrifuged for 15 min at 10,778×g. The supernatant in the volume of one ml was flooded on the five hour old lawn culture growth (log phage) of the AB (isolated strains from different hosts) on 90 mm nutrient agar plate and incubated overnight at 37°C. Next day, the lawn was washed with three ml TMG (Tris-HCl, magnesium sulphate, gelatin pH 7.4) buffer and centrifuged at 10,778×g for 15 min. To the supernatant (1 ml) one drop of chloroform was added and mixed well by vortexing or by inversion for 15 min. Centrifugation was done at 10,778×g for 10 min. The lawn culture in log phase of the host was prepared again, and 100 μl of the supernatant collected as mentioned above was inoculated at 10-12 places to screen for lysis. The surface with clear plaque was cut and collected in one ml of the TMG buffer and propagated further and plaque counting was done by soft agar overlay method21. The single isolated plaque was picked up for further processing. The number of phage particle was increased by the soft agar overlay method. After bulk production, the bacteria were killed with one per cent chloroform and centrifuged. The clear supernatant was preserved at 4°C for further use. For purification (toxin-free) and concentration of phages, the harvested fluid was subjected to membrane dialysis-135 against polyethylene glycol (PEG 6000, HiMedia) (20% in 2.5M NaCl) for overnight and then washed thrice with phosphate buffered saline (PBS) at 4°C. The endotoxin estimation was done using enzyme-linked immunosorbent assay kit for detection (Thermo Scientific™ Pierce™ LAL Chromogenic Endotoxin Quantitation Kit, MA, USA).
Assessment of anti-Acinetobacter baumannii activity of bacteriophages
Bacteriophage host range determination: All the 22 isolated phages were subjected to the assessment of their antibacterial activity on each of 70 clinical and 70 environmental isolates of AB. The lawn culture of AB (1.5×108 cfu/ml) was made on MHA. Each of the phages having a concentration of 109 plaque-forming units (pfu/ml) was spotted on the plate in the volume of 10 μl. The plates were observed for the clear zone after overnight incubation at 37°C. Each phage was tested against all the bacterial strains in duplicate in independent experiments. Lytic activity of these phages onto the lawns formed by clinical isolates of P. aeruginosa, Escherichia coli, Salmonella Typhi, Acinetobacter lwoffii and S. aureus were also tested for.
Isolation of bacteriophage DNA: Isolation of phage DNA was performed with phenol/chloroform and ethanol precipitation method. Briefly, purified phage particles (1010-1012 pfu/ml) were treated with 1 μg of DNase I and RNase A (Bangalore Genei, Bengaluru) at 37°C for 30 min. To the mixture, proteinase K and sodium dodecyl sulphate (SDS) were added at a final concentration of 0.05 mg/ml and 0.5 per cent respectively, and incubated at 56°C. After one hour of incubation, an equal volume of phenol: chloroform was added to remove proteinaceous material. The extraction was repeated thrice with phenol-chloroform-isoamyl alcohol (25:24:1). The nucleic acid was precipitated with chilled ethanol and suspended in 20 μl of TE buffer (10 mM Tris-HCl, pH 7.0, 1.0 mM EDTA, pH 7.0) according to standard procedure21.
Genotyping of bacteriophages by Enterobacterial Repetitive Intergenic Consensus-polymerase chain reaction: All the bacteriophages were subjected to genotyping by Enterobacterial Repetitive Intergenic Consensus (ERIC)-PCR to see whether these were genotypically similar or different. This allowed for picking up of phages which were not only different in antibacterial activity but genotypically also. The primer sequences used for ERIC-PCR conditions were as described earlier22. The ERIC primers were used like Randomly Amplified Polymorphic DNA at the lower temperature of 49°C as annealing temperature rather than 61°C22. PCR was carried out in 25 μl volume using 10 ng of genomic DNA, 1 U of Taq polymerase (Bangalore Genie, Bengaluru), and 15 pmol of each primer (Bangalore Genie), 200 mmol/l (each) deoxynucleotide triphosphate (Bangalore Genie) and 2 mmol/l MgCl2 in standard PCR buffer. Amplification reactions were carried out in a thermal cycler (Biometra, Goettingen, Germany).
Preparation of dendrogram: The size of DNA bands was estimated according to molecular weight markers. Cluster analysis of all the 22 bacteriophages was done based on the fingerprints generated by constructing a dendrogram. For each phage, a haplotype matrix or a binary table was manufactured by linearly composing lysis (1) and no lysis (0), data derived from gel analysis of ERIC-PCR. The resulting similarity matrix was used as the input data for cluster analysis by NTSYS pc2.0 programme of UPGMA23.
Animal model: AB 12 (henceforth, Ab12) strain isolated from endotracheal tubing’s of a patient admitted to ICUs of the University Hospital of Banaras Hindu University, Varanasi was used for animal experimentation. Broth microdilution-based MIC determination revealed that this strain was resistant to imipenem, meropenem, levofloxacin, polymyxin and colistin. Fifty 6-8 wk old Swiss albino mice, weighing 20-25 g each were reared in the departmental animal house.
In the Table I, the data shown is with the bacteriophage cocktail dose of 1×109 pfu/ml (6 groups each comprising of 5 mice). While next 3 groups each consisting of 5 mice were experimented with 1×107 pfu/ml, 1×106 pfu/ml and 1×105 pfu/ml doses. Further 5 mice were put for testing of lethal dose of the bacteria and 5 more mice for testing the safety of the phage cocktail (not shown in the table).
| Group A: Bacteriophage cocktail given in the volume of 100 µl containing 109 pfu/ml | Observation made of in hours after intervention with bacteriophage cocktail | |||||
|---|---|---|---|---|---|---|
| 6 | 12 | 24 | 48 | 72 | 96 | |
| A1) Simultaneous administration of bacteriophage and Acinetobacter baumannii challenge | 2+2+2+2+2 (2) | 2+3+2+3+2 (2.4) | 3+3+2+3+2 (2.4) | 3+2+2+2+2 (1.8) | 1+1+1+1+1 (1.0) | 1+1+1+1+1 (1.0) |
| A2) Bacteriophage cocktail six hours before bacterial challenge | 2+2+2+2+2 (2) | 2+2+3+3+2 (2.4) | 2+2+2+2+2 (2) | 1+1+1+1+1 (1.0) | 1+1+1+1+1 (1.0) | 1+1+1+1+1 (1.0) |
| A3) Bacteriophage cocktail six hours after bacterial challenge | 2+2+2+2+2 (2) | 3+2+3+3+2 (2.6) | 3+3+4+3+3 (3.2) | 2+2+5+2+2 (2.6) | 1+1+5+1+1 (1.8) | 1+1+5+1+1 (1.8) |
| A4) Bacteriophage cocktail 12 h after bacterial challenge | 3+2+2+3+3 (2.6) | 4+3+3+3+3 (3.2) | 4+4+3+4+2 (3.6) | 5+3+2+3+2 (3.0) | 5+2+2+2+2 (2.6) | 5+1+1+1+1 (1.8) |
| A.5) Bacteriophage cocktail 24 h after bacterial challenge | 2+2+2+2+2 (2) | 3+4+3+3+3 (3.2) | 4+4+4+4+3 (3.4) | 5+5+4+5+4 (3.8) | 5+5+2+5+2 (3.8) | 5+5+1+5+1 (3.4) |
| Bacteriophage therapy at lower dosage | ||||||
| A5.1) Bacteriophage cocktail 24 h after bacterial challenge (dose 107 pfu/ml) | 3+3+3+3+3 (3.0) | 4+4+3+3+4 (3.6) | 4+4+4+4+4 (4.0) | 4+4+4+3+5 (4.0) | 3+2+3+2+5 (3.0) | 1+1+1+1+5 (1.8) |
| A5.2) Bacteriophage cocktail 24 h after bacterial challenge (dose 106 pfu/ml) | 3+3+3+3+3 (3.0) | 3+3+4+3+3 (3.2) | 4+4+4+4+4 (4.0) | 4+3+4+3+4 (3.6) | 3+3+3+2+2 (2.6) | 1+1+1+1+1 (1.0) |
| A5.3) Bacteriophage cocktail 24 h after bacterial challenge (dose 105 pfu/ml) | 3+3+3+3+3 (3.0) | 3+3+3+3+3 (3.0) | 4+4+4+4+4 (4.0) | 4+4+3+4+4 (3.8) | 3+3+3+3+2 (2.6) | 1+1+1+1+1 (1.0) |
Grading of diseases. 1, normal; 2, slight illness, lethargy, ruffled fur; 3, moderate illness, severe lethargy, ruffled fur and hunched back; 4, severe illness with above sign, exudative accumulation around eyes; 5, death; figure in parenthesis shows the average of the signs of all the 5 mice in a particular study group
Phage cocktail preparation: The three most potent bacteriophage ɸ4, ɸ7 and ɸ14 were purified to minimize the endotoxins with membrane dialysis (dialysis membrane-135) and three washings with phosphate buffer saline (PBS pH 7.4) made the composition free of endotoxin to the level of 0.05 EU/ml. We gave only 100 µl of phage cocktail delivering 0.005 EU/ml of the endotoxin. The recommendation for an animal model is the same as for human beings i.e. 5 EU/kg of body weight. Thus for 25 g of mouse, the phage cocktail dose having endotoxin up to 0.125 EU is a permissible limit. Phage cocktail containing equal concentration and volume of the above three phages were titered at 1×109 pfu/ml was prepared.
Immunocompromised septicemia mice model: To develop a bacteraemia mice model, immunosuppression of the mice was done by administration of cyclophosphamide (200 mg/kg) and cortisone acetate (250 mg/kg) before two days and after three days (−2 to +3 days) of the days of the introduction of infection24. Cyclophosphamide and cortisone acetate (Zydus, Hyderabad) administered intraperitoneally and subcutaneously, respectively.
Determination of LD100: A group of five mice were fed antibiotic-free diet and were immunocompromised as described above. On the third day, mice were challenged through intraperitoneal (I/P) injection of 100 μl of Ab12 suspension containing 1×108 cfu/ml. The mice were kept under observation in an ambient atmosphere. It was observed that all the mice died between 36 and 72 h of the infection. Liver, spleen, heart and peritoneum fluids of dead mice were collected postmortem. Individual organs were weighed and suspended in 2 ml of PBS. These were then homogenised using Wheaton overhead stirrers. The homogenate was plated on MH agar plate to see the bacterial count.
Safety of bacteriophage cocktail: A group of five mice were taken and 100 μl bacteriophage cocktail (1×109 pfu/ml) was injected intraperitoneally without immunosuppression. These mice were observed for a month for any disease development. None of the mice was seen with any sickness and thus excluding the presence of endotoxin in the composition of phage cocktail.
Assessment of microbiological and clinical efficacy of the phage cocktail: The phage cocktail was used for prophylactic as well as therapeutic purposes. The mice experiments were set up in groups five. The sickness of the mice was graded on the basis of following features: (i) normal (no abnormality detected); (ii) slight illness (lethargy, ruffled fur); (iii) Moderate illness (severe lethargy, ruffled fur and hunched back); (iv) severe illness (along with above signs, exudative accumulation around eyes); (v) death; (Table I).
The bacterial and bacteriophage quantifications were done in sacrificed/dead mice.
Phage cocktail in prophylaxis and therapy at constant dose:
Simultaneous administration of bacteria and phage cocktail: A challenge dose of 100 μl of AB12 (1×108 cfu/ml) was given simultaneously with 100 μl phage cocktail (1×109 pfu/ml) administered at the opposite flank. Both the injections were given IP. The mice were observed for 96 h.
Administration of phage cocktail 6 h before AB infection: Initially, 100 μl bacteriophage cocktail (1×109 pfu/ml) injected IP to a group of the five immunosuppressed mice. The bacterial challenge in the volume of 100 μl (1×108 cfu/ml) was given 6 h later. The mice were observed for 96 h.
Administration of phage cocktail 6 h after AB infection: In this group, the same concentration of bacteria as described above was given IP to 5 immunosuppressed mice. The bacteriophage cocktail was given IP six hours after the bacterial challenge.
Administration of phage cocktail 12 h after AB infection: The same volume (100 μl) of bacteria (1×108 cfu/ml) was given IP to 5 immunosuppressed mice. A volume of 100 μl of bacteriophage cocktail (1×109 pfu/ml) was given 12 h after the bacterial challenge through IP route. The mice were observed for 96 h.
Administration of phage cocktail 24 h after AB infection: In this group, 100 μl bacteriophage cocktail (1×109 pfu/ml) was injected IP to all the five immunosuppressed mice 24 h after the challenge dose of AB and observed for 96 h.
Administration of phage cocktail in decreasing dosage: Three groups of immunosuppressed mice comprising five in each were challenged with 100 μl of bacterial suspension containing 1×108 cfu/ml. After 24 h of bacterial challenge, 100 μl of phage cocktail containing 1×107 pfu/ml (Group A 5.1), 1×106 pfu/ml (Group A 5.2) and 1×105 pfu/ml (Group A 5.3), were given intraperitoneally.
Results
The MIC determination of the recommended antibiotic for clinical use revealed that Ab12 was resistant to all the antibiotics tested, which include imipenem, meropenem, levofloxacin, polymyxin and colistin. Ab12 was used for further experiments.
There were three types of plaques seen on the lawn during the primary isolation. The plaques were either circular with a well-defined margin or having an irregular margin. There was variation in the plaque size also. Overall, 22 bacteriophages were isolated from different water sources against 22 clinical MDR-AB and designated as ɸAb1 to ɸAb22.
Bacteriophage host range determination: Table II shows the percentage of the bacterial isolates lysed after spotting the 22 bacteriophages. When compared with the environmental isolates, the AB from clinical origin were observed to be more susceptible. Furthermore, with the exception ɸAb9 that lyzed A. lwoffii, none of the other phages lysed any other bacterial species tested.
| Serial number | Bacteriophage | Percentage susceptibility; environmental isolates (n=70) | Percentage susceptibility; clinical isolates (n=70) |
|---|---|---|---|
| 1 | ɸAb1 | 17.14 (12/70) | 25.72 (18/70) |
| 2 | ɸAb2 | 21.42 (15/70) | 18.57 (13/70) |
| 3 | ɸAb3 | 25.71 (18/70) | 34.28 (24/70) |
| 4 | ɸAb4 | 15.71 (11/70) | 48.57 (34/70) |
| 5 | ɸAb5 | 14.28 (10/70) | 42.85 (30/70) |
| 6 | ɸAb6 | 22.85 (16/70) | 38.57 (27/70) |
| 7 | ɸAb7 | 15.72 (11/70) | 52.86 (37/70) |
| 8 | ɸAb8 | 8.58 (6/70) | 45.72 (32/70) |
| 9 | ɸAb9 | 12.85 (9/70) | 40.00 (28/70) |
| 10 | ɸAb10 | 14.28 (10/70) | 21.42 (15/70) |
| 11 | ɸAb11 | 12.85 (9/70) | 30.00 (21/70) |
| 12 | ɸAb12 | 24.28 (17/70) | 28.57 (20/70) |
| 13 | ɸAb13 | 8.58 (6/70) | 38.57 (27/70) |
| 14 | ɸAb14 | 5.72 (4/70) | 47.15 (33/70) |
| 15 | ɸAb15 | 8.58 (6/70) | 32.85 (23/70) |
| 16 | ɸAb16 | 5.72 (4/70) | 18.57 (13/70) |
| 17 | ɸAb17 | 7.15 (5/70) | 30.00 (21/70) |
| 18 | ɸAb18 | 10.00 (7/70) | 35.72 (25/70) |
| 19 | ɸAb19 | 11.42 (8/70) | 27.14 (19/70) |
| 20 | ɸAb20 | 12.85 (9/70) | 35.72 (25/70) |
| 21 | ɸAb21 | 10.00 (7/70) | 21.42 (15/70) |
| 22 | 4.38 pt | 27.14 (19/70) | 28.57 (20/70) |
When a dendrogram was prepared based on the susceptibility pattern of 20 randomly selected AB isolates of clinical as well as environmental origin, 22 bacteriophages showed two major clusters I and II. Interestingly, only two pairs of bacteriophage (11 & 18 and 15 & 19) were found to have identical lytic patterns. Rest of the viruses had completely distinct lytic patterns and differed from each other (data not shown).
Genotyping of bacteriophages by ERIC-PCR: ERIC PCR-based whole-genome analysis of 22 phages produced an average of 1-9 bands per phage ranging between 100-4500 bp. When dendrogram was prepared based on the presence and absence of banding pattern in the gel, it was found that only two pairs (1 & 11 and 13 & 15) had an identical banding pattern (data not shown). Rest of the viruses were observed to have unique sets of bands. There were two well-defined clusters I and II where branching occurred at the similarity level of 25 per cent, and the cluster II practically had quite unrelated bacteriophages with a similarity level of 25 per cent. Interestingly, three phages observed to be in the IInd cluster were common by both the methods of differentiation (ERIC-PCR and bacteriophage host range determination) of phages i.e. ɸ2, ɸ4, and ɸ5 (data not shown).
It was further observed that the six most virulent phages (ɸAb4, ɸAb5, ɸAb7, ɸAb8, ɸAb9 and ɸAb14) of the clinical AB were dissimilar to each other by both the methods, i.e., lytic activity as well as genotyping. Therefore, for pre-clinical evaluation randomly three phages ɸAb4, ɸAb7, ɸAb14 out of the above six phages were selected for the cocktail preparation.
Animal model: The three most active phages lysing 48.6, 52.9 and 47.1 per cent of the clinical isolates respectively, were selected for the in vivo evaluation of their efficacy in treating septicaemia caused by AB in the immunocompromised mouse model. The LD100 could be observed to be 1×108 cfu/ml of Ab12. Bacteriophage cocktail was administered at a constant dose of 100 µl containing 109 pfu/ml.
In group A1 where simultaneous administration of bacteriophage and AB challenge, no mortality occurred and maximum level of illness went up to grade 3, i.e., moderate illness, severe lethargy, ruffled fur and hunched back in three mice after 24 h. These signs improved by 48 h and all the five recovered by 72 h (Table I and Figure). In case of group A2 where bacteriophage cocktail was administered six hours before the bacterial challenge, severe sickness was observed at 12 h and by 24 h post cocktail administration slight illness, lethargy, ruffled fur remained. By 72 h here too all mice recovered (Table I and Figure).
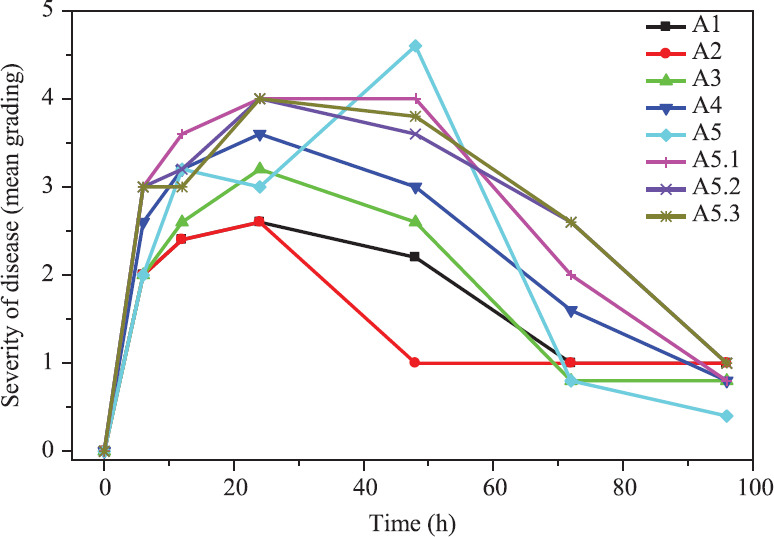
- The outcome of the phage therapy on Acinetobacter baumannii septicaemia at different time points and dosage. Details of study groups: A1- bacterial challenge and phage cocktail given simultaneously, A2-phage cocktail given six hours before bacterial challenge, A3 phage cocktail given six hours after bacterial challenge, A4-phage cocktail given 12 h after bacterial challenge; A5-phage cocktail given 24 h after bacterial challenge with 1x108 cfu/ml, A5.1, A5.2 and A5.3 all with bacterial challenge dose 108 cfu/ml and phage cocktail administered consisted of 107, 106, and 105 cfu/ml.
In group A3 where bacteriophage cocktail was administered six hours after the bacterial challenge, at 24 h, all the five mice were quite ill and mice no. 3 had exudative accumulation around eyes along with, severe lethargy, ruffled fur and hunched back. This mouse died when examined at 48 h, however, at 72 h the remaining four mice were normal (Table I and Figure). In group A4, where bacteriophage cocktail was administered 12 h after the bacterial challenge, the severity of sickness was quite high, with the average grade point of 2.6 at six hours after the start of therapy. The severity of illness was seen increasing between 12-48 h from the start of the therapy with one death at 48 h. Later at 72 h the rest of the mice showed improvement and fully recovered by 96 h (Table I and Figure).
In group A5, where bacteriophage cocktail was 24 h after administered the bacterial challenge, though the severity of illness was less at six hours after the start of therapy, but it increased with time, and at 48 h three mice were found dead. However, the remaining mice fully recovered by 96 h (Table I and Figure).
The three different dosages i.e. 107, 106, 105 pfu/ml were given to A5.1, A5.2 and A5.3 groups of mice respectively, and it was observed that after six hours of administration of phage cocktail, all the five mice of the three groups were sick uniformly having moderate illness, severe lethargy, ruffled fur and hunched back.
In group A5.1, where a dose of 100 μl having 107 pfu/ml, bacteriophage cocktail was given, three of the five mice had increased severity and developed the additional sign of exudative accumulation around the eyes. Also mouse no. 5 was found dead at 48 h. In the rest of the mice, severe illness persisted at 72 h, however, they recovered by 96 h (Table I and Figure). In case of group A5.2 which received the bacteriophage cocktail dose of 100 μl having 106 pfu/ml, severe illness persisted up to 72 h with the peak at 24 h. In this group also severe illness continued up to 72 h. However, all of them recovered by 96 h (Table I and Figure).
The group A5.3 received 105 pfu/ml in 100 μl of the cocktail. All the mice had all the signs of severe infection at 24 h after the start of the therapy. The sickness persisted up to 72 h with complete recovery at 96 h (Table I and Figure).
The quantification of bacteriophage and AB bacteria carried out in the dead mouse after therapy showed only phage, not the bacteria. The peritoneal fluid, liver, spleen, heart blood were found to have only phages. None of the organs of the dead mice treated with phage cocktail yielded AB bacteria.
Discussion
This study was aimed to look at the efficacy of phage therapy as an alternative to antibiotics in case of septicaemia caused MDR AB in an immunocompromised mouse model. This work was carried out after considering the safety of the phage concentration employed based on a previous study form our laboratory which determined the optimum dose (volume and concentration) of the phage cocktail in the treatment of septicaemia caused by P. aeruginosa16. In that study, a dose of 100 µl of the bacteriophage cocktail in the concentration of 1012 pfu/ml was employed empirically at different time points after bacterial challenge and mortality was observed only when the phage cocktail was given six hours before and after the bacterial challenge. The mortality, however, decreased to nil with the decreasing concentration of the phage cocktail to 109 pfu/ml. By simulating the actual clinical scenario in ICU using the in vivo mouse model, 100 µl of the phage cocktail at the concentration of 109 pfu/ml was administered simultaneously, and also six, 12 and 24 h after the bacterial challenge. This concentration of the phage caused mortality in 60 per cent of the mice receiving the phage cocktail 24 h after the bacterial challenge. It could be speculated that this concentration of phage cocktail reached the optimum level for sudden lysis of the bacteria similar to the zone phenomenon. It has been reported that when antibiotics inhibiting cell-wall synthesis (penicillins, cephalosporins and carbapenems) are administered as a bolus, sudden lysis occurs in cases of gram-negative bacterial septicaemia2425. Bacteriophages are also known to disrupt the cell wall of bacteria26. In this study, when the concentration of phage cocktail was decreased to 106 and 105 pfu/ml, no mortality could be observed in the groups of mice challenged with bacteria 24 h before.
A report by Schooley et al15 in 2017 demonstrated the potentials of personalized bacteriophage-based therapeutic cocktail in an elderly patient with disseminated MDR AB having the inexorable downhill clinical course for three months duration. This report discussed the different aspects of phage therapy. Amongst many, there is special mention regarding the possibility of a sudden massive release of endotoxin during therapy. Notably, in a clinical case, the treating physicians have good access to monitor the vitals and decide the dosage of phage cocktail accordingly.
There are many reports regarding the role of bacteriophages against AB mostly limited to in vitro evaluation27282930. However, there are studies on the evaluation of AB specific phage therapy in rodent models such as on wounds29 and in mouse pneumonia model30 administering the phages by the nasal route14. There is a report showing the efficacy of bacteriophages in BALB/c septicaemia model as well30. In this study, the phage therapy was initiated two hours after the bacterial challenge and showed the significantly reduced mortality30. The limitation of this study was, however, that they used a single experimental setup.
Based on the results of the present study alongwith our previous findings studies, it can be deduced that in septicaemia treatment with phages, the therapy should be started with a low dose of the phage cocktail. However, there are many questions which still need to be addressed including: (i) whether further lower doses need to be considered, if yes then how low; (ii) whether lower but multiple doses of the phage cocktail can be administered and; (iii) whether low dosage in the form of slow intravenous infusion are safe?
In conclusion, this study supports the systemic use of bacteriophages as therapeutic agents to combat MDR, XDR and PDR AB infections in patients. Further pre-clinical studies are, however, needed to determine the safety of the dosing schedule.
Financial support & sponsorship: The authors acknowledge financial support by the Indian Council of Medical Research (ICMR) and University Grant Commission (UGC), New Delhi, India, in the form of Junior Research Fellowship to SRP and Postdoctoral Fellowship to CBP.
Conflicts of Interest: None.
References
- Spread of carbapenem-resistant international clones of Acinetobacter baumannii in Turkey and Azerbaijan:A collaborative study. Eur J Clin Microbiol Infect Dis. 2016;35:1463-8.
- [Google Scholar]
- An increasing threat in hospitals:Multidrug-resistant Acinetobacter baumannii. Nat Rev Microbiol. 2007;5:939-51.
- [Google Scholar]
- Phage therapy of staphylococcal chronic osteomyelitis in experimental animal model. Indian J Med Res. 2016;143:87-94.
- [Google Scholar]
- Phage therapy of Pseudomonas aeruginosa infection in a mouse burn wound model. Antimicrob Agents Chemother. 2007;51:1934-8.
- [Google Scholar]
- Bacteriophages can treat and prevent Pseudomonas aeruginosa lung infections. J Infect Dis. 2010;201:1096-104.
- [Google Scholar]
- Treatment of experimental infections of mice with bacteriophages. J Med Microbiol. 1992;37:258-61.
- [Google Scholar]
- Isolation and characterization of a virulent bacteriophage AB1 of Acinetobacter baumannii. BMC Microbiol. 2010;10:131.
- [Google Scholar]
- Isolation and characterization of wide host range lytic bacteriophage AP22 infecting Acinetobacter baumannii. FEMS Microbiol Lett. 2012;332:40-6.
- [Google Scholar]
- Bacteriophages as potential new therapeutics to replace or supplement antibiotics. Trends Biotechnol. 2010;28:591-5.
- [Google Scholar]
- Phage Abp1 rescues human cells and mice from infection by pan-drug resistant Acinetobacter baumannii. Cell Physiol Biochem. 2017;44:2337-45.
- [Google Scholar]
- Intranasal treatment with bacteriophage rescues mice from Acinetobacter baumannii-mediated pneumonia. Future Microbiol. 2016;11:631-41.
- [Google Scholar]
- Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant Acinetobacter baumannii infection. Antimicrob Agents Chemother. 2017;61:e00954-17.
- [Google Scholar]
- Bacteriophage therapy:An alternative to antibiotics –An experimental study in mice. Ann Natl Acad Med Sci. 2019;55:151-8.
- [Google Scholar]
- Polymerase chain reaction assay for the detection of Acinetobacter baumannii in endotracheal aspirates from patients in the intensive care unit. J Microbiol Immunol Infect. 2011;44:106-10.
- [Google Scholar]
- Methods for Dilution antimicrobial susceptibility tests for bacteria that grow aerobically;Approved standard. In: CLSI Document M07-A10 (10th ed). Wayne, PA: CLSI; 2015.
- [Google Scholar]
- Enumeration of bacteriophages by double agar overlay plaque assay. Methods Mol Biol. 2009;501:69-76.
- [Google Scholar]
- Molecular Cloning:A Laboratory Manual. (2nd ed). Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989.
- [Google Scholar]
- Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 1991;19:6823-31.
- [Google Scholar]
- Cluster Analysis for Researchers. Morrisville, NC: Lulu.com; 2004.
- Antibiotic induced endotoxin release and clinical sepsis:A review. J Chemother. 2001;1:159-72.
- [Google Scholar]
- Evaluation for endotoxemia in patients receiving penicillin therapy for secondary syphilis. JAMA. 1986;256:388-90.
- [Google Scholar]
- Phage lytic proteins:biotechnological applications beyond clinical antimicrobials. Critical Reviews in Biotechnology. 2016;36:542-52.
- [Google Scholar]
- Characterization of newly isolated lytic bacteriophages active against Acinetobacter baumannii. PLoS One. 2014;9:e104853.
- [Google Scholar]
- Efficacy of Acinetobacter baumannii bacteriophage cocktail on Acinetobacter baumannii growth. Afr J Microbiol. 2015;9:2159-65.
- [Google Scholar]
- Characterization and testing the efficiency of Acinetobacter baumannii Phage vB-GEC_Ab-M-G7 as an antibacterial agent. Front Microbiol. 2016;7:1590.
- [Google Scholar]
- Efficacy of ϕkm18p phage therapy in a murine model of extensively drug-resistant Acinetobacter baumannii infection. Infect Drug Resist. 2018;11:2301-10.
- [Google Scholar]






