Translate this page into:
Environmental & lifestyle factors in deterioration of male reproductive health
Reprint requests: Ms Shiva Murarka / Dr Sunil Kumar, Division of Reproductive & Cytotoxicology, National Institute of Occupational Health (ICMR), Meghaninagar, Ahmedabad 380 016, Gujarat, India e-mail: sunilnioh@yahoo.com; sunilnioh@gmail.com
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Male reproductive function in the general population has been receiving attention in recent years due to reports of various reproductive and developmental defects, which might be associated with various lifestyle and environmental factors. This study was carried out to determine the role of various lifestyle and environmental factors in male reproduction and their possible association with declining semen quality, increased oxidative stress as well as sperm DNA damage.
Methods:
Semen samples were obtained from 240 male partners of the couples consulting for infertility problem. Semen analysis was carried out using WHO criteria and subjects were categorized on the basis of self reported history of lifestyle as well as environmental exposure. The oxidative and antioxidant markers; lipid peroxidation (LPO), superoxide dismutase (SOD) and catalase (CAT) as well as DNA damage by acridine orange test (AO) were determined.
Results:
The presence of abnormal semen parameters was significantly higher among the lifestyle and/or environmental exposed subjects as compared to the non-exposed population. Further, the levels of antioxidants were reduced and sperm DNA damage was more among the lifestyle and/or environmental exposed subjects, though the changes were not significant.
Interpretation & conclusions:
These findings indicated that various lifestyle factors such as tobacco smoking, chewing and alcohol use as well as exposure to toxic agents might be attributed to the risk of declining semen quality and increase in oxidative stress and sperm DNA damage.
Keywords
DNA
lifestyle
male infertility
oxidative damage
semen quality
sperm abnormalities
toxicants
Male infertility is the sole or contributing factor in roughly half of these cases and no identifiable cause can be found in over 25 per cent of infertile males1. The risks of alcohol, smoking tobacco, etc. in relation to infertility has also been reported23. The pathophysiology of male infertility could be explained by the cascades of molecular, biochemical and physiological events which are mostly represented by abnormal semen quality parameters. Growing evidence indicates that imbalance between peroxidative and antioxidative substances in semen leads to metabolic and functional disorders of male germ cells and may be a primary cause of some types of infertility4. Spermatozoa are highly sensitive to injuries caused by generations of reactive oxygen species (ROS) concentration such as superoxide anion, hydrogen peroxide, and the hydroxyl radical which can result in the damage to cell membrane5 and lead to deterioration in sperm motility and functional capacity. The imbalance between ROS production and ROS degradation has been hypothesized as a cause of oxidative stress in semen with peroxidative injury to the sperm membrane and a consequent impairment of the related functional properties, such as sperm motility6. Seminal plasma malondialdehyde (MDA) assay, which is the stable lipid peroxidation product, is a simple method to evaluate the effect of lipid peroxidation on sperm7. The source of cytotoxic oxygen radicals is frequently intracellular, as in the case of oligozoospermic males whose spermatozoa generate particularly high levels of ROS8. Superoxide dismutase (SOD) scavenges both intracellular and extracellular superoxide radical and prevents the lipid peroxidation of plasma membrane. It should be conjugated with catalase or glutathione peroxidase to prevent the action of hydrogen peroxide9. Catalase detoxifies both intracellular and extracellular H2O2 to water and oxygen10. Detection of sperm nuclear DNA integrity is potential tool for evaluation of semen samples prior to their use in assisted reproductive techniques11.
Thus, in the present study, the relationship between lifestyle and/ or environmental factors with respect to oxidative stress and semen quality was investigated. Further, the involvement of oxidative as well as DNA damage to sperm was assessed to understand the impairment in the cellular functioning and seminal antioxidant levels as well as morphological alterations.
Material & Methods
The study was carried out in 240 apparently healthy males (age range 20-45 yr) attending the Out Patient Department (OPD) of Obstetrics and Gynecology, Civil Hospital and Institute of Kidney Diseases (IKD), Ahmedabad, Gujarat, India, for the evaluation of infertility during October 2008 to October 2010. Subjects currently on any medication, tonics or antioxidant supplementation and those suffering from any acute infection were excluded. The samples were categorized on the basis of sperm count into three groups: normozoospermic, oligozoospermic and azoospermic, and on the basis of sperm motility, into progressively motile and asthenozoospermic. Similarly, on the basis of sperm morphology they were categorized as normal and teratozoospermic for analysis purpose.
This study was a part of a major research project for which ethical approval was obtained from the Institutional Ethics Committee of NIOH, Ahemdabad. Written informed consent of each subject was also obtained.
Semen samples were obtained by masturbation in a sterile container within 3-7 days of sexual abstinence. Routine semen analysis (liquefaction time, volume, pH, viscosity, sperm count, motility and morphology) was carried out according to the WHO guidelines12. The motility characteristics of the sperm cells were classified into four groups as fast progressive, slow progressive, non-progressive motility and non-motile. Total progressive sperm motility was defined as the spermatozoa with fast progressive motility plus those with slow or sluggish progressive motility. Sperm morphology was recorded as the percentage of normal forms and other anomalies such as total head, midpiece and tail defects. Sperm count was carried out in duplicate using haemocytometer and the mean was expressed in millions/ml.
Biochemical assays: Liquefied semen was centrifuged at 3000 g for 15 min and seminal plasma was separated and processed for further analysis without freezing thawing. SOD was measured according to the method of Marklund and Marklund13. The increase in absorbance was measured at 420 nm and expressed as U/min/l of seminal plasma. CAT activity was measured spectrophotometrically according to the method of Aebi14 by measuring the decrease in the concentration of hydrogen peroxide at 240 nm. The catalase activity was expressed as specific activity (U/min/ml of seminal plasma). The lipid peroxide level in the seminal plasma was measured using a thiobarbituric acid reactive substances assay, which monitors MDA (malondialdehyde) production15. The MDA intensity was measured at 535 nm and expressed as nanomoles/ml of seminal plasma.
Acridine orange (AO) staining: Sperm DNA damage was assessed by acridine orange staining test as per the method of Tejada et al16. Normal DNA takes up intercalating dyes like acridine orange in the monomer form and produces green fluorescence while the affinity for intercalation is low in relaxed DNA and is lost in fragmented DNA that gives red fluorescence.
Statistical analysis: All statistical analyses were conducted using SPSS 16.0 for windows (SPSS, Chicago, Illinois, USA). The parameters/characteristics were compared among different groups based on lifestyle and/or toxicant exposure using students ‘t’ test. Analysis of variance (ANOVA) was performed using Tukey's test to identify significant differences. For two-group comparison independent samples ‘t’ test was used.
Results
The mean age of the study population was 31.91 ± 0.37 yr and most of the subjects were residing in the urban areas. The subjects having the habit of tobacco smoking/ chewing and/or alcohol were considered as lifestyle exposed whereas the subjects exposed to toxic harmful substances such as metals, pesticides, solvents, etc. based upon reported history were considered as toxicant exposed. From the collected history of the subjects in this study, 63.3 per cent of the subjects were non-exposed to toxicants and 57.5 per cent were indulged in any of the three lifestyle habits i.e. tobacco chewing, smoking and alcohol (Table I). Considering both lifestyle and toxicant exposure, about 31.7 per cent of the subjects were non-exposed to toxicant and/or having lifestyle habits whereas 68.3 per cent of the subjects were having lifestyle habits and/or the exposure of toxicant factors (Table I). Among the subjects having both lifestyle habits and/or toxicant exposure, 26 (15.9%) subjects were only toxicant exposed while 76 (46.3%) subjects were indulged in only lifestyle habits. The remaining 62 (37.8%) subjects were exposed to both lifestyle as well as toxicant factors.
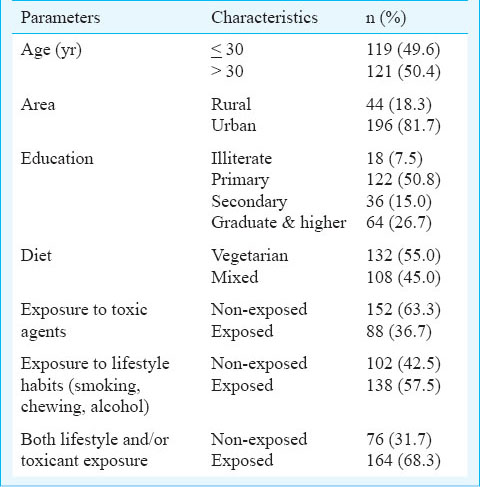
Among the study subjects, 19 (7.9%) were azoospermic (not analysed), 63 (26.3%) had oligozoospermic and the remaining 158 (65.8%) had normal sperm count. Eighty one (36.7%) subjects were asthenozoospermic and 140 (63.3%) had progressively motile sperm. Similarly, 29.6 per cent subjects were teratozoospermic and 70.4 per cent were morphologically normal (Table II).
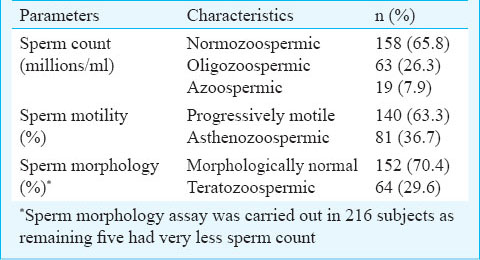
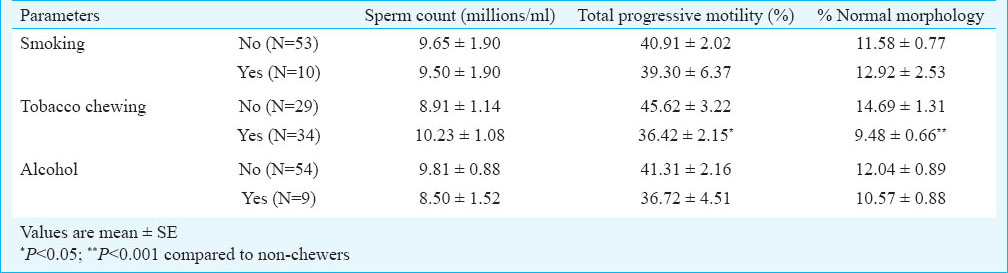
The occurrence of asthenozoospermia (AS) and azoospermia (A) was significantly (P<0.05) higher among the exposed subjects as compared to the non-exposed subjects. Also the percentage of subjects with normal semen variables was more among non-exposed population as compared to the exposed group (Table III). Semen variables amongst tobacco smokers, chewers and alcoholics with respect to subjects having no such habits were also determined. The percentage of subjects with normal semen variables of count, motility and morphology was lower among the tobacco smokers, chewers and alcoholics as compared to the subjects having no such habit. The frequency of oligoasthenoteratozoospermia was found to be significantly (P<0.05) higher among the chewers as compared to subjects with no such habit (Table IV).
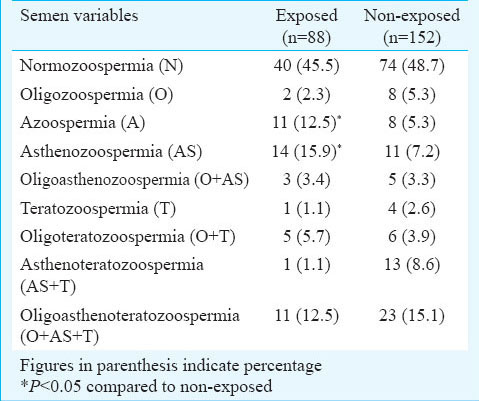
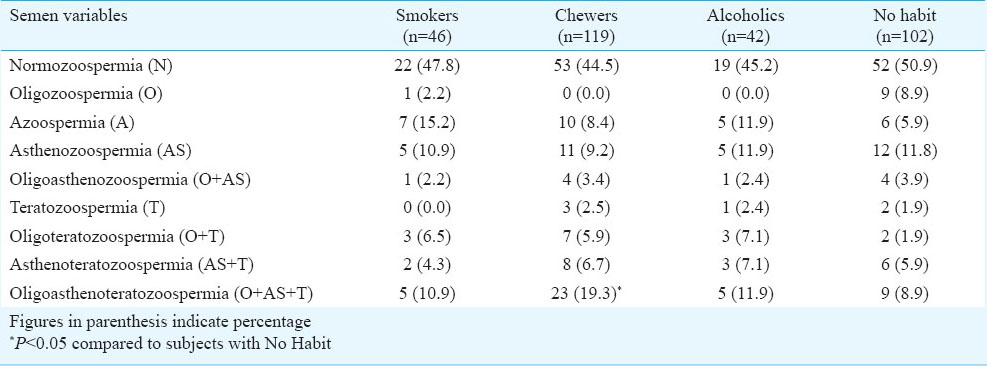
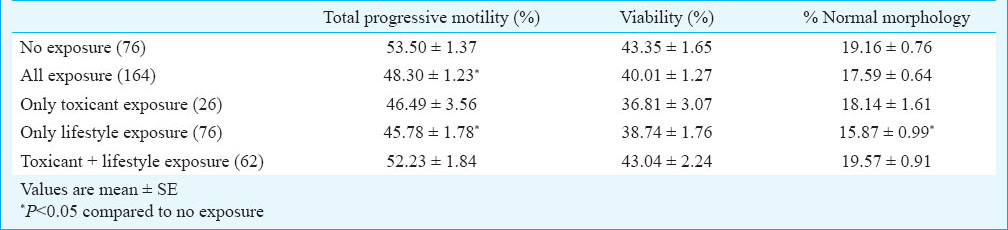
The data on sperm quality parameters were also assessed with respect to overall lifestyle and toxicant exposure (Table V). Total progressive motility was significantly lower among the exposed population (lifestyle habit and/or toxicant exposure) as compared to non-exposed subjects. The percentage of total progressive motility and normal morphology was significantly (P<0.05) lower among the only lifestyle exposed subjects, compared to non-exposed subjects. Further, the role of these lifestyle factors on sperm quality parameters were assessed among the oligozoospermic subjects (Table VI). Total progressive motility (P<0.05) and percentage of normal morphology (P<0.001) were significantly lower among chewers in comparison to non-chewers among the oligozoospermic subjects.
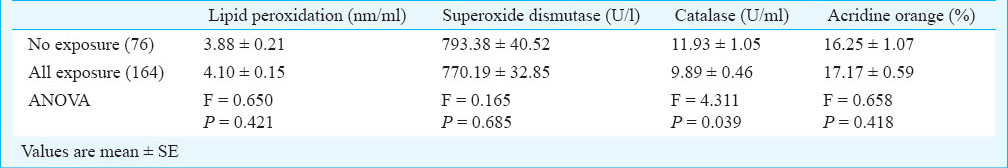
The data on oxidative stress, seminal antioxidant activity and DNA damage parameters were also analyzed with respect to the lifestyle and/or toxicant exposure Table VII. The activity of catalase decreased significantly (P<0.05) among the exposed subjects. LPO and SOD levels remained almost similar among the exposed and non-exposed subjects. Further, results revealed that the percentage of DNA damage as assessed by acridine orange staining test was higher among the subjects having lifestyle and/or toxicant exposure as compared to non-exposed subjects, but these changes were non significant.
Discussion
In the present study, the subjects ever exposed to toxic chemicals were found to have increased risk of subfertility. This may be due to the direct or indirect adverse effect of toxicants on spermatogenesis and hormonal regulation. Toxicants damage directly the testicular tissues, which can result in various adverse effects, namely reduced sperm count, the production of defective spermatozoa, and impaired androgen production17. The present study demonstrated that the presence of azoospermia and asthenozoospermia was significantly higher among the exposed subjects as compared to non-exposed subjects. Mehta et al18 had also suggested the higher prevalence of azoospermia in Jodhpur and Kurnool, hypothesized to be caused due to fluoride levels in drinking water and pesticide exposure respectively.
Lifestyle factors play an important role in the aetiology of various diseases and have also been implicated to cause reproductive impairment. In the present study, the total progressive motility and normal sperm morphology were significantly lowered among the only lifestyle exposed subjects. The relationship between tobacco chewing and male infertility remains unclear and data specifically addressing this issue are scanty. Oligoasthenoteratozoospermia was found to be significantly higher among the chewers as compared to subjects having no lifestyle habit. Said et al19 have reported an increasing trend of oligoasthenoteratozoospermia from mild to moderate to severe with respect to addiction of tobacco chewing. However, Dikshit et al20 found no such effect of tobacco chewing on male reproductive function. In the present study, significant decrease was observed in fast progressive motility and % normal morphology among chewers as compared to non-chewers. Among oligozoospermic subjects, total progressive motility and normal sperm morphology decreased significantly among chewers as compared to non-chewers. Banerjee et al21, reported adverse effect of tobacco chewing on motility and total sperm count of tobacco chewers as compared to control subjects.
In the present study, among the oligozoospermic subjects, there was a non-significant lowering of sperm count and total progressive motility between smokers and non-smokers. This shows that these men may be susceptible to the effect of tobacco smoke and supports the earlier findings2223. The effects of alcohol consumption on male reproductive function have remained under discussion24. A reduction in sperm concentration and percentage of normal morphology has been reported among chronic alcohol consumers2526. In the present study, deterioration in sperm count, total progressive motility and normal sperm morphology was observed among alcohol consumers who had oligozoospermia. However, these alterations did not reach statistical significance. One of the reasons might be the small number of alcohol consumers in the study population.
It has been suggested that insufficient antioxidants and increased oxidative stress may attribute to the risk of declining semen quality27. Studies on the peroxidation of phospholipids in mammalian sperm have demonstrated that peroxidation reaction causes membrane damage, which leads to loss of motility and membrane integrity28. Our results demonstrated that lipid peroxide levels were higher in the seminal plasma of subjects who had lifestyle habits and/or were exposed to environmental factors, which might be due to increased oxidative stress, exerted by these factors. In the present study, SOD levels were slightly lower among the subjects exposed to environmental factors and/or having lifestyle habits. Khosrowbeygi et al29 observed that both catalase activity and total anti-oxidant capacity (TAC) were significantly correlated with sperm motility and morphology.
The free radical scavenging system, SOD and catalase are present in all oxygen metabolizing cells and their function is to provide a defense against the potentially damaging activities of superoxide and H2O230. Depletion in the enzymatic activity observed in the present study may be due to decreased synthesis of enzyme or oxidative inactivation of enzyme protein. Decrease in the SOD activity indicated an incomplete removal of the free superoxide radical generated by various lifestyle habits and/or toxicant exposure. Further, reduction in catalase activity also reflected the inability to eliminate H2O2 produced by lifestyle and/or toxicant exposure. All these could contribute to the imbalance of ROS. The integrity of sperm chromatin structure in turn is influenced by certain endogenous and exogenous factors, such as peroxidative damage of DNA, which may arise from infectious or toxic agents31. Our results on deterioration in sperm DNA integrity suggested that lifestyle and/or toxicant exposure could lead to improper packaging of sperm DNA as revealed by AO test, though the results were non significant.
In summary, the present study suggested the role of lifestyle factors and reproductive toxicants in deterioration of semen quality as well as inducing oxidative and DNA damage in sperm. Free radical generation induced by various lifestyle factors and reproductive toxicants might be associated with the impairment of semen quality, which was also revealed in terms of the variations in the antioxidant enzyme system and increased sperm DNA damage. The data might be useful in advocating for adopting the healthy lifestyle in order to reduce the male reproductive health problems.
Acknowledgment
Authors thank the Director, NIOH, for support and the study subjects for their co-operation. The financial support received from the Indian Council of Medical Research, New Delhi, India is acknowledged.
References
- Cigarette smoke condensate induces aryl hydrocarbon receptor-dependent changes in gene expression in spermatocytes. Reprod Toxicol. 2012;34:665-76.
- [Google Scholar]
- Alcohol, tobacco and other addictive substances and reproductive health. Cas Lek Cesk. 2011;150:339-43.
- [Google Scholar]
- Inflammatory mediators exert toxic effects of oxidative stress on human spermatozoa. J Androl. 2007;28:325-33.
- [Google Scholar]
- Cellular basis of defective sperm function and its association with the genesis of reactive oxygen species by human spermatozoa. J Reprod Fertil. 1987;81:459-69.
- [Google Scholar]
- Free radicals, antioxidants and human spermatozoa: clinical implications. Hum Reprod. 1998;13:1422-4.
- [Google Scholar]
- Development of an image-analysis system to monitor the retention of residual cytoplasm by human spermatozoa - correlation with biochemical markers of the cytoplasmic space, oxidative stress, and sperm function. J Androl. 1996;17:276-87.
- [Google Scholar]
- Mechanism, measurement and prevention of oxidative stress in male reproductive physiology. Indian J Exp Biol. 2005;43:963-74.
- [Google Scholar]
- Protective effect of antioxidants on the impairment of sperm motility by activated polymorphonuclear leukocytes. Fertil Steril. 1996;65:411-9.
- [Google Scholar]
- Sperm DNA damage and its clinical relevance in assessing reproductive outcome. Asian J Androl. 2004;6:139-48.
- [Google Scholar]
- WHO, Laboratory manual for the examination of human semen and serum-cervical mucus interaction. (4th ed). Cambridge, UK: Cambridge University Press; 2003.
- [Google Scholar]
- Involvement of the superoxide anion radical in the auto-oxidation of pyragallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469-74.
- [Google Scholar]
- A test for the practical evaluation of male fertility by acridine orange (AO) fluorescence. Fertil Steril. 1984;42:87-91.
- [Google Scholar]
- Contribution of environmental factors to the risk of male infertility. Hum Reprod. 2001;16:1768-76.
- [Google Scholar]
- Prevalences of oligozoospermia and azoospermia in male partners of infertile couples from different parts of India. Asian J Androl. 2006;8:89-93.
- [Google Scholar]
- Relationship between semen quality and tobacco chewing in men undergoing infertility evaluation. Fertil Steril. 2005;84:649-53.
- [Google Scholar]
- Effect of tobacco consumption on semen quality of a population of hypofertile males. Fertil Steril. 1987;48:334-6.
- [Google Scholar]
- Semen quality of infertile couples - comparison between smokers and non-smokers. Andrologia. 1987;19:42-6.
- [Google Scholar]
- Effects of alcohol and cigarette consumption on human seminal quality. Fertil Steril. 2004;82:374-7.
- [Google Scholar]
- Semen quality and frequency of smoking and alcohol consumption: an explorative study. Int J Fertil Menopausal Stud. 1995;40:135-8.
- [Google Scholar]
- Association between semen quality, oxidative stress and seminal antioxidant activity. Clin Biochem. 2011;44:319-24.
- [Google Scholar]
- Lipid peroxidation in human spermatozoa and maintenance of progressive sperm motility. Andrologia. 1999;31:17-22.
- [Google Scholar]
- Correlation between sperm quality parameters and seminal plasma antioxidants Status. Iran J Reprod Med. 2004;2:58-64.
- [Google Scholar]
- DNA integrity in human spermatozoa: relationships with semen quality. J Androl. 2000;21:33-44.
- [Google Scholar]






