Translate this page into:
Entomological study of chikungunya infections in the State of Kelantan, Malaysia
Reprint requests: Dr. Rozilawati binti Harun, Medical Entomology Unit, Infectious Diseases Research Center, Institute for Medical Research, Jalan Pahang 50588, Kuala Lumpur, Malaysia e-mail: watynom@yahoo.com
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Chikungunya infection has become a public health threat in Malaysia since the 2008 nationwide outbreaks. Aedes albopictus Skuse has been identified as the chikungunya vector in Johor State during the outbreaks. In 2009, several outbreaks had been reported in the State of Kelantan. Entomological studies were conducted in Kelantan in four districts, namely Jeli, Tumpat, Pasir Mas and Tanah Merah to identify the vector responsible for the virus transmission.
Methods:
CHIKV cases records were obtained from State Health Department, Kelantan and localities involved were identified. Larva survey was conducted to collect the immature mosquito stages. Modified aspirators were used to collect the adult mosquitoes. All samples on dry ice were transferred to laboratory and the presence of the virus was detected using reverse transcriptase PCR.
Results:
A total of 1,245 mosquito larvae were collected during larval survey and 2,019 adult mosquitoes were collected using aspirator. From these collections, 640 mosquito pools were tested for the presence of CHIKV by RT-PCR but none found positive. Ae. albopictus was the most abundant mosquito collected, followed by Culex sp., Armigeres sp. and Anopheles sp. A total of 2, 814 artificial containers were inspected during the study.
Interpretation & conclusions:
Since none of the mosquito samples was found to be positive for chikungunya virus, the vector(s) of chikungunya virus in these localities could not be identified.
Keywords
Chikungunya
entomological investigation
Kelantan
Since its first report in 19531, chikungunya has caused numerous massive outbreaks worldwide. Asia was reported to have the first outbreak in Bangkok in 19582 and later in Cambodia, Vietnam, Laos, Myanmar, Malaysia, the Philippines and Indonesia3. In Malaysia the disease was first reported in Klang, Selangor between 1998 and February 19994 and reemerged in Bagan Panchor, Perak in 20065. In April 2008, another outbreak occurred in Johor State which then spread to other States and federal territories in Malaysia6.
Chikungunya virus (CHIKV) has been known as enzootic in many countries in Asia and Africa, transmitted by various wild Aedes mosquitoes7 and has been isolated from different mosquito species8. Aedes aegypti9 and Aedes albopictus are usually considered as potential vectors of CHIKV since they have been proven susceptible to this virus in many laboratory studies. Ae. albopictus Skuse has been detected with chikungunya virus in Ipoh, Perak State in 200610. In 2009, several outbreaks had been reported in the State of Kelantan11, the border State between Malaysia and Thailand. In order to identify the vector(s) responsible for the outbreaks in Kelantan State, we conducted several entomological investigations in Kelantan between June to December 2009.
Material & Methods
Study sites: Based on reported cases by State Health Department, Kelantan, a survey was conducted between June to December 2009 in localities with cases of chikungunya in four districts, namely Jeli, Tumpat, Pasir Mas and Tanah Merah.
Mosquito collection: Larva survey was conducted to collect the immature mosquitoes based on the recommended method12. All indoor and outdoor containers that were potential breeding sites were inspected, whereas adult collection was conducted using sweep net and modified aspirator. Collection started between 0800-1200 h and 1500-1800 h between June to December 2009 one day after cases being notified by the District Health Department.
Mosquito processing: All mosquito samples were pooled on dry ice according to species, sex and type of breeding containers in sterile 2.0 ml plastic tubes, with maximum of 30 individuals per pool and transported to Medical Entomology Unit, Institute for Medical Research laboratory in dry ice.
Virus detection by reverse transcriptase polymerase chain reaction: The mosquitoes were ground in the tubes with 1 ml of maintenance medium (Eagle's minimum essential medium, MEM)9, using a sterile homogenizer and the RNA was extracted using QIAamp Viral RNA Mini Kit (Qiagen, Germany) according to manufacturer's protocol. For positive control, equal volume of cultured cells infected with chikungunya virus was used while for negative control, uninfected cultured cells were used. The RT PCR assay was conducted using the Titan One Tube RT-PCR kit (Roche, Germany), adapted from the methods by Hasebe et al13. Amplified product was analyzed by gel electrophoresis and all positive samples was confirmed by sequencing the amplicons.
Result & Discussion
Through the disease epidemiology study done simultaneously in the localities, a total of 70 patients were confirmed infected with chikungunya virus. This indicated that the transmission was still active in the localities. Using modified aspirator and sweep net, a total of 3,264 mosquitoes (1245 larvae, 2019 adult) were collected (Table I) which comprised 57.9 per cent Aedes albopictus, 37.46 per cent Culex sp, 4.6 per cent Armigeres sp and 0.03 per cent of Anopheles sp in four different districts in Kelantan during the study. Aedes albopictus was found to be the predominant species collected. However, Ae. aegypti was not found during the survey, which might indicate that Ae. albopictus was the main breeder in artificial breeding containers available in the localities.
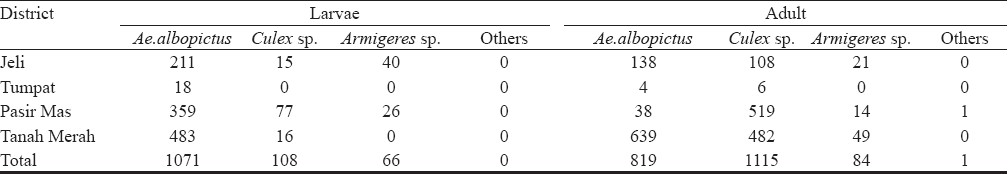
Although Ae. aegypti has been considered to be the principal vector, Ae. albopictus was repeatedly shown to be a competent vector of CHIKV during recent outbreaks in Indian Ocean, Italy, Gabon, and even in Malaysia1014–16. This virus was also detected from field collected Ae. albopictus in Madagascar during 2006 outbreak17. This is believed to be associated with CHIKV with a mutation in envelope protein gene (E1-A226V) which enabled the CHIKV to adapt to Ae. albopictus15. A total of 640 mosquito pools were tested during the study. Virus detection by RT-PCR showed that none of the pools were positive, however, the positive controls confirmed that the PCR tests worked well. Therefore, our study was not able to clarify the role of Ae. albopictus, Ae. aegypti or other mosquitoes species as vector transmitting chikungunya virus at the studied localities. The possibility of other mosquitoes in transmitting the virus needs to be taken into consideration since chikungunya virus has reportedly been transmitted by a variety of species mostly belonging to the genus Aedes18–20. Several Aedes species (Ae. furcifer, Ae. vittatus, Ae. fulgens, Ae. luteocephalus, Ae. dalzieli, Ae. vigilax, Ae. camptorhynchites) have been known to transmit the virus in Africa, while Culex annulirostris, Mansonia uniformis, and Anopheles mosquitoes have also occasionally been incriminated1820. In contrast, transmission in Asia has been documented where Ae. aegypti and Ae. albopictus were identified vectors21. Our results showed that Ae. albopictus was the predominant species in all localities studied, which could have possibly played the role as the main vector responsible for the CHIKV transmission. In addition, the possibility of catching infected mosquitoes in the field could have been enhanced, if the collections were done before the implementation of control in the outbreak areas.
A total of 2, 814 artificial containers were inspected in the localities surveyed during the study. It was found that 97 containers were positive with Ae. albopictus. The most abundant containers available at the localities were plastic containers (41%), followed by 12 per cent in pail, 11 per cent in tyre and 8 per cent in water containers. None were positive with Ae. aegypti. Several indices were calculated to estimate the Ae. aegypti/Ae. albopictus population density including the house index, container index, and Breteau index (Table II).

Tumpat was excluded from the analysis because of the late involvement and only six houses were inspected during the study. As there is no vaccine available for this virus, the only option to control this disease is vector control. The information of potential vector(s) in the outbreak localities can be used to plan an effective chikungunya vector control programme.
The authors thank the Director General of Health, Malaysia, for permission to publish this paper to the Director of Institute for Medical Research, Kuala Lumpur, staff of Medical Entomology Unit and Epidemiology Unit IMR and staff of State Health Department, Kelantan for their help throughout the study. This project was supported by a National Institutes of Health Research Grant (code no: JPP 09-013) 2009-2010.
References
- An epidemic of virus disease in Southern Province, Tanganyika Territory, in 1952-53. I Clinical features. Trans R Soc Trop Med Hyg. 1955;49:28-32.
- [Google Scholar]
- Genome microevolution of chikungunya viruses causing the Indian Ocean outbreak. PLoS Med. 2006;3:e263.
- [Google Scholar]
- Chikungunya infection-an emerging disease in Malaysia. Southeast Asian J Trop Med Public Health. 2001;32:447-51.
- [Google Scholar]
- Chikungunya virus of Asian and c0 entral/ e0 ast African genotypes in Malaysia. J Clin Virol. 2009;46:180-3.
- [Google Scholar]
- Specific detection of chikungunya virus using a RT-PCR/ nested PCR combination. Infect Dis Vet Public Health. 2002;49:49-54.
- [Google Scholar]
- Vectors of chikungunya virus in Senegal: current data and transmission cycles. Am J Trop Med Hyg. 1999;60:281-6.
- [Google Scholar]
- Rapid detection of chikungunya virus in laboratory infected Aedes aegypti by Reverse-Transcriptase-Polymerase Chain Reaction (RT-PCR) Trop Biomed. 2005;22:149-54.
- [Google Scholar]
- Outbreak of chikungunya due to virus of Central/East African genotype in Malaysia. Med J Malaysia. 2007;62:323-8.
- [Google Scholar]
- Ministry of Health, Malaysia. Press Statement by the Director-General of Health, Federal Territory Putrajaya, Malaysia. 2009
- [Google Scholar]
- Mosquitoes and mosquito-borne diseases: biology, surveillance, control, personal and public protection measures. Kuala Lumpur: Academy of Sciences Malaysia; 2000. p. :167-83.
- [Google Scholar]
- Combined detection and genotyping of c0 hikungunya virus by a specific reverse transcription-polymerase chain reaction. J Med Virol. 2002;67:370-4.
- [Google Scholar]
- A CHIKV study group. Infection with chikungunya virus in Italy: an outbreak in a temperate region. Lancet. 2007;370:1840-6.
- [Google Scholar]
- A single mutation in chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog. 2007;3(12):e201.
- [Google Scholar]
- Concurrent chikungunya and dengue virus infections during simultaneous outbreaks, Gabon, 2007. Emerg Infect Dis. 2009;15:591-3.
- [Google Scholar]
- Outbreak of dengue and Chikungunya fevers, Toamasina, Madagascar, 2006. Emerg Infect Dis. 2008;14:1135-7.
- [Google Scholar]
- Aedes furcifer and other mosquitoes as vectors of chikungunya virus at Mica, northeastern Transvaal, South Africa. J Am Mosq Control Assoc. 1990;6:415-20.
- [Google Scholar]
- Chikungunya virus disease. The arbovirus: Epidemiology and ecology. 1988;II:137-57.
- [Google Scholar]
- Laboratory vector studies on six mosquito and one tick species with chikungunya virus. Trans R Soc Trop Med Hyg. 1981;75:15-9.
- [Google Scholar]
- Entomologic investigations of a chikungunya virus epidemic in the Union of the Comoros, 2005. Am J Trop Med Hyg. 2008;78:77-82.
- [Google Scholar]






