Translate this page into:
Effects of poly (ADP-ribosyl) polymerase (PARP) inhibitor on cisplatin resistance & proliferation of the ovarian cancer C13* cells
Reprint requests: Dr Yongjie Tian, Professor, Department of Obstetrics & Gynecology, Provincial Hospital Affiliated to Shandong University, Shandong, 250021, PR China e-mail: tianyongjie@sdu.edu.cn
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Drug resistance is the primary cause of failure in the treatment of cancers. It has been suggested that the enhancement of DNA repair capability may be responsible for the drug resistance of the tumour cells, and poly(ADP-ribosyl)ation plays an important role in DNA repair. This study investigated the effect of PARP inhibitor 3-aminobenzamide (3-AB) on the cisplatin resistance and proliferation of the cisplatin-resistant ovarian cancer C13* cells in vitro.
Methods:
C13* cells were treated with various concentrations of 3-AB in vitro. MTT assay was used to determine the effect of 3-AB on the cisplatin sensitivity and proliferation of cells. The expression levels of PARP-1 mRNA and protein in the C13* cells were examined using reverse transcription-polymerase chain reaction (RT-PCR) and Western blot, and changes caused by 3-AB treatment were investigated. Immunofluorescence microscopy was used to detect the localization and expression of the PARP-1 proteins before and after treatment with 5 mmol/l 3-AB.
Results:
The inhibitory ratio and the cisplatin sensitivity of C13* cells significantly increased with the increase of the concentration of 3-AB (P<0.05). The RT-PCR analysis revealed that the expression of PARP-1 mRNA was decreased when platinum (Pt) and 3-AB were combined. The expression levels of PARP-1 protein were decreased by 23.15 ± 2.53, 59.11 ± 2.23 and 73.24 ± 3.88 per cent, respectively, in C13* cells with the increase of the concentration of 3-AB (P<0.05). The immunofluorescence microscopy results indicated that the expression level of PARP-1 protein was significantly decreased after treatment with 3-AB (P<0.05).
Interpretation & conclusions:
3-AB inhibited the proliferation activity of C13* cells, and increased the cellular sensitivity to cisplatin. Our findings show that the PARP inhibitor 3-AB can downregulate the expression of PARP-1 at transcriptional and translational levels in C13* cells.
Keywords
3-aminobenzamide
cisplatin
drug resistance
ovarian cancer
poly(ADP-ribosyl)ation
Ovarian cancer is the second most common gynaecological malignancy and the leading cause of death of patients with gynaecological cancers1. Most of these cases lack early symptoms and are diagnosed at an advanced stage (approximately 70%), when the tumour has metastasized. The traditional treatment of advanced ovarian cancer is based on cytoreductive surgery, followed by platinum-based chemotherapy2. Unfortunately, despite the high response rate to the initial chemotherapy, there remains a significant risk for recurrence and resistance to the chemotherapy, and the long-term survival is relatively poor with only 10-15 per cent of advanced-disease patients surviving at 10 years3. Consequently, seeking promising novel agents for chemotherapy and elucidating drug resistance mechanisms are crucial for individualizing the treatment and prognosis of ovarian cancer patients.
Chemotherapy resistance is a significant problem and is the major obstacle in ovarian cancer treatment. The enhancement of DNA damage repair capability could be a critical mechanism for chemotherapy45. Poly(ADP-ribosyl)ation is a post-translational modification found in mammalian cells6. In humans, the conserved PARP [poly(ADP-ribose) polymerase] signature motif has been identified in 17 homologous genes, including parp-1, parp-2, parp-3, vault-parp, and tankyrases. PARP-1 is an essential member of the family of PARPs, which plays an important role in DNA repair, genomic stability, energy metabolism, transcriptional regulation, inflammation and cell death. DNA repair is a complex and multifaceted process, which is critical to cell survival7.
In our earlier study8, the expression levels of PARP-1 protein in cisplatin-resistant ovarian cancer C13* cells were found to be higher than cisplatin-sensitive ovarian cancer OV2008 cells and PARP-1 RNA interference can significantly downregulated PARP-1 expression and effectively reversed resistance of C13* cells to cisplastin. However, the RNA interference still has some limitations for clinical application. Therefore, in the present study PARP inhibitor 3-aminobenzamide (3-AB) was used to reduce the expression of PARP. The cisplatin-resistant ovarian cancer C13* cells were treated with various concentrations of 3-AB, and the effect of decreased expression of the PARP-1 protein in C13* cells was studied on proliferation activity and cisplatin sensitivity of cells.
Material & Methods
Cell culture: Cisplatin-resistant ovarian cancer C13* cells, (kindly provided by Dr Du Meirong, Department of Gynecology, Fudan University), were cultured in RPMI1640 medium (Hyclone, Logan, UT, USA) supplemented with 10 per cent foetal bovine serum (FBS; Hyclone, Logan, UT, USA) and 1 per cent penicillin/streptomycin (Hyclone, Logan, UT, USA) at 37° C in a 5 per cent CO2 atmosphere.
Effect of 3-AB on the proliferation of C13* cells: C13* cells (4 × 104/ml) were plated in each well of a 96-well plate containing 100 μl growth medium. On the following day, cells were treated with increasing concentration of 3-AB (0, 0.5, 1, 2.5, 5, 7.5 and 10 mmol/l) and cultured for 24 h, each group contained three samples. After 24 h, cells were added with 20 μl 3-4,5-demethylthylthiazol -2yl] -2,5 diphenyltetrazolium broide MTT (5 mg/ml; Sigma, St Louis, MO, USA) and incubated for 4 h. Cells were collected and centrifuged at 3000 g for 2 min, and the supernatant was discarded. The formazan crystals were dissolved in 150 μl Dimethyl sulfoxide (DMSO), and shaken for 15 min. The absorbance value (A) at 490 nm was read using an automatic multiwell spectrophotometer (Bio-Tek, USA). The inhibitory ratio was calculated as follows: Inhibitory ratio (%) = 1-(mean experimental absorbance mean control absorbance)× 100%.
Effect of 3-AB on the cell survival of C13* cells: C13* cells (3 × 104/ml) were plated in each well of a 96-well plate containing 100 μl growth medium, and treated with 5 mmol/l 3-AB, each group contained six samples and plated six board. One board was taken out every day, the absorbency value was determined using the MTT assay and cell growth curve was drawn.
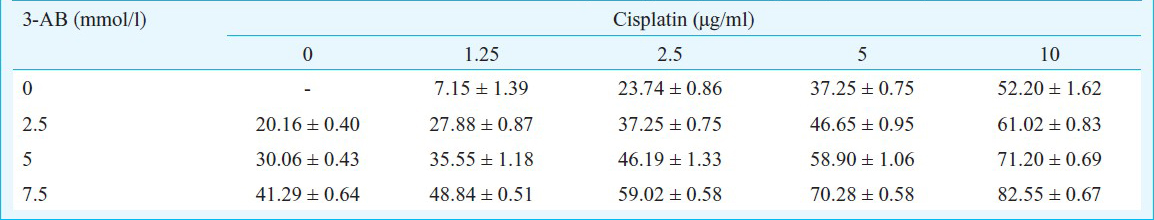
Cellular sensitivity to cisplatin after treatment with 3-AB: C13* cells (4 × 104/ml) were plated in each well of a 96-well plate containing 100 μl growth medium. Cells were incubated for 16 h to allow adhesion, and then treated with combination of 3-AB (0, 2.5, 5 or 7.5 mmol/l) plus cisplatin (0, 1.25, 2.5, 5, or 10 μl /ml) (Qilu Parmaceutical Co. Ltd, Jinan, China). Each group contained three samples. After cells were cultured for 24 h, the inhibitory ratio was determined using the MTT assay.
Reverse transcription-polymerase chain reaction (RT-PCR): Total RNA was extracted using Trizol (Takara, Japan) according to the manufacturer's protocol. The first strand cDNA was generated by reverse transcription reagent kit (Takara, Japan). After a sufficient amount of cDNA was obtained, DNA was amplified by performing PCR with primer of PARP-1; forward, 5’-AAGGCGAATGCCAGCGTTAC-3’; reverse, 5’-GGCACTCTTGGAGACCATGTCA-3’ (Takara, Japan). Amplification was carried out for 30 cycles with 30 sec of incubation at 94°C, 30 sec at 55°C and 1 min at 72°C. The products were analyzed on a 2 per cent agarose gel and examined by staining with ethidium bromide. Their amount was normalized to the value of β-actin as the standard value.
Western blot: Control and treated cells were lysed, and protein concentration was measured by using the BCA protein assay kit (Source?). Cell lysates (60 μg/lane) were separated by SDS-PAGE (10% polyacrylamide gel), transferred to an nitrocellulose (NC) membrane. After blocking with 5 per cent non-fat milk in Tris-buffered saline, the blots were incubated with human anti-rabbit parp-1 antibody (1:1000; Cell Signaling Technology, Beverly, MA, USA) at 4°C overnight. Mouse anti-beta-actin monoclonal antibody (1:1000; Cell Signaling Technology, Beverly, MA, USA) served as a control for protein loading in each lane. Membranes were incubated with horse radish peroxidase (HRP)-conjugated secondary antibodies (1:8000; Santa Cruz, CA, USA), for 1 h at room temperature. The protein bands were visualized by ECL detection system, and the density of the bands was quatified by Alpha Imager 2200 (Alpha Innotech, San Leandro CA, USA).
Immunofluorescence: Control and treated cells were washed with PBS, fixed in 4 per cent paraformaldehyde, and permeabilized with 0.2 per cent Triton-X 100 for 20 min in a humidified tissue culture incubator at 37° C, then processed using standard immunofluorescence staining procedures9. Cells were incubated with a human anti-rabbit parp-1 antibody (1:200; Cell Signaling Technology, Beverly, MA, USA) at 4°C overnight. One well was incubated with PBS instead of the primary antibody and thus was used as the negative control. Fluorescein isothiocyanate (FITC)-conjugated secondary antibodies (1:300; Santa Cruz, CA, USA), for 30 min at 37°C. The nuclei were stained with 4’6-diamidino-2-phenylindole (DAPI) solution for 2 min. The cells were observed using fluorescence microscopy (Thermo Hybaid, USA) and photographed with a CCD camera (Olympus, Japan).
Statistical analysis: Each experiment was repeated three times. All statistical analyses were performed using SPSS 18.0 software (SPSS, Inc., Chicago, IL, USA). Differences among various treatment groups were determined by analysis of variance followed by Dunnett's t test.
Results
The effects of 3-AB on the proliferation of C13* cells: The inhibitory ratios of the C13* cells treated with 3-AB at 0.5, 1, 2.5, 5, 7.5 or 10 mmol/l were 5 ± 1.4, 13 ± 1.2, 21 ± 1.3, 30 ± 1.5, 41 ± 1.8, and 59 ± 1.0 per cent, respectively. Hence, the inhibitory ratio was associated with the concentration of 3-AB (Fig. 1; P< 0.05).
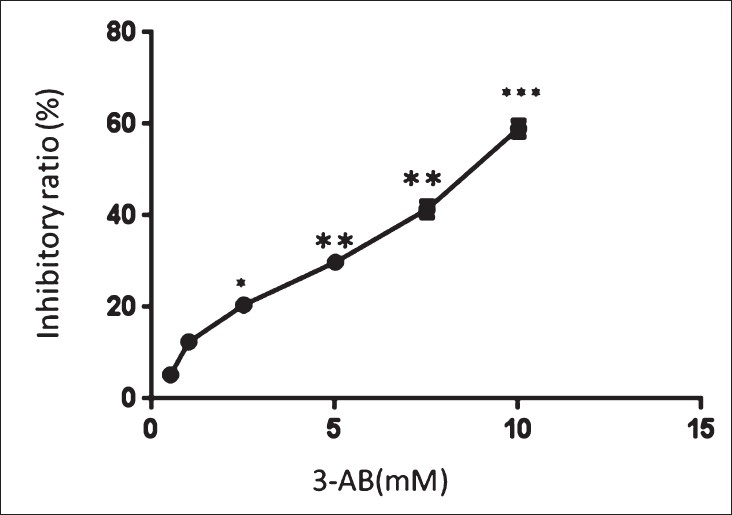
- Influence of 3-AB on the cellular proliferation (inhibitory ratio) detected by MTT. The inhibitory ratios of the C13* cells were associated with the concentration of 3-AB (0, 0.5, 1, 2.5, 5, 7.5 and 10 mmol/l).
Effect of 3-AB on the cell survival of C13* cells: After treated with 5 mmol/l 3-AB, the proliferation capacity of C13* cells was inhibited, and cell growth slowed down. The difference was significant at 2, 3, 4, 5, and 6 days compared to C13* group (Fig. 2, P< 0.05).
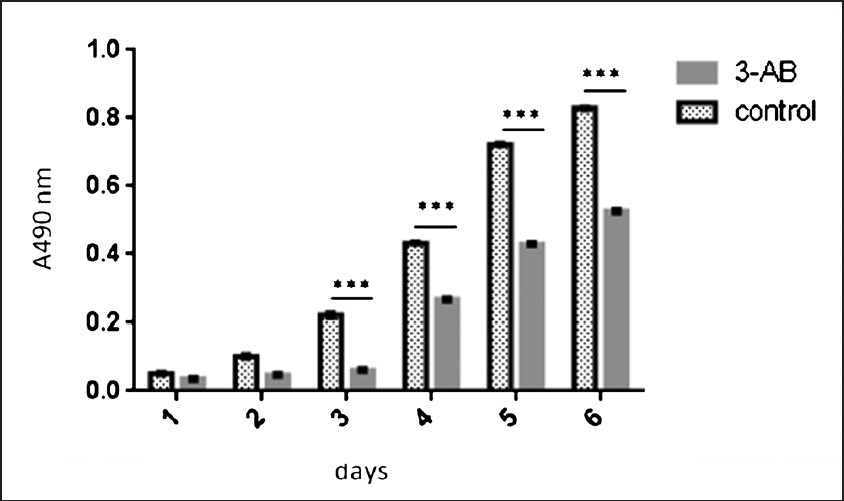
- Effect of 3-AB on the cell survival of C13* cells detected by MTT. The difference of cell growth was significant at 3, 4, 5 and 6 days compared to control group.
Cellular sensitivity to cisplatin after treatment with 3-AB: When the Pt concentration was fixed (2.5 μg/ml), the inhibitory ratio of C13* cells exhibited a significant increase in response to higher 3-AB (5 mmol/l) concentrations (P< 0.05). Similarly, when the 3-AB concentration was fixed, the inhibitory ratio of C13* cells was also increased by higher concentrations of Pt (P<0.05). These results indicated that the PARP inhibitor 3-AB inhibited the proliferation activity of C13* cells, and increased the cellular sensitivity to cisplatin (Table).
RT-PCR analysis: The RT-PCR analysis revealed that after cells were treated with 2.5 μg/ml Pt, 5 mmol/l 3-AB, or 2.5 μg/ml Pt plus 5 mmol/l 3-AB, the expression levels of PARP-1 mRNA in the C13* cells were 0.6188 ± 0.03, 0.4348 ± 0.01, and 0.2219 ± 0.02, respectively, compared to the blank control (0.6844 ± 0.06). Our data indicated that the expression of PARP-1 mRNA was unaffected by Pt treatment, but was significantly decreased when Pt and 3-AB were combined (P< 0.05).
Western blot analysis: The Western blot analsyis revealed that after C13* cells were treated with 3-AB (0, 2.5, 5 or 7.5 mmol/l) for 24 h, the expression levels of PARP-1 in C13* cells were 1.69 ± 0.08, 1.30 ± 0.04, 0.69 ± 0.04 and 0.45 ± 0.07, respectively. Compared to the blank control (0 mmol/l 3-AB), the PARP-1 expression levels of 3-AB treated cells were significantly decreased by 23.15 ± 2.53 per cent for the 2.5 mmol/l 3-AB group, 59.11 ± 2.23 per cent for the 5 mmol/l 3-AB group, and 73.24 ± 3.88 per cent for the 7.5 mmol/l 3-AB group (P< 0.05). These results suggested that 3-AB significantly downregulated the PARP-1 expression at the translational level, and further implicated that the overexpression of PARP-1 protein in the ovarian cancer C13* cells was related to the cellular sensitivity to cisplatin.
Immunofluorescence: PARP-1 proteins were largely located in the nuclei, and a small fraction of the proteins appeared in the kytoplasm (Fig. 3). After 3-AB treatment, the expression level of PARP-1 in the C13* cells was decreased. These data demonstrated that 3-AB significantly downregulated the expression of PARP-1.
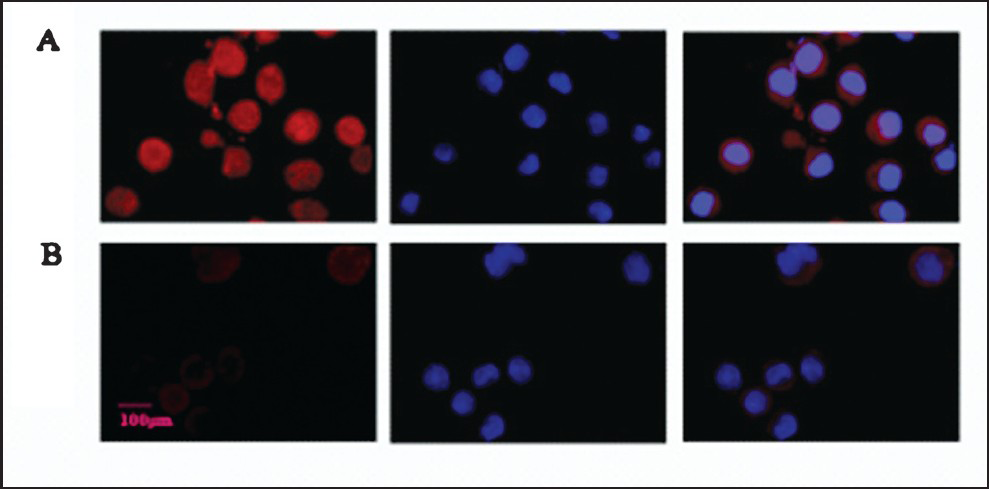
- Detection of the PARP-1 protein expression in C13* cells by Immunofluorescent microscopy. (A) PARP-1 protein expression and localization in C13* cells (red). (B) PARP-1 protein expression and localization in C13* cells after treated with 5 mmol/l 3-AB (red) for 24 h. Nuclei stained with DAPI (blue). Original magnification, × 400.
Discussion
Platinum-based chemotherapy has been used as primary treatment for recurrent ovarian epithelial cancer. However, the median time to recurrence is not more than 2 years, with a 5-years survival rate of 20-25 per cent10. Acquired resistance to chemotherapy is the primary cause of failure in the treatment of cancers. Therefore, identification of the potential molecular pathways involved in cisplatin-resistant chemotherapy has become one of the significant areas in the research of tumour treatment.
Platinum is a non-specific anti-tumour medicine, and its cytotoxicity against tumour cells has been thought to be mediated through several mechanisms11, including formatting and enhancing the platinum-DNA adduct repair capacity, decreasing platinum accumulation, and increasing drug inactivation and its ability to tolerate platinum-DNA damage12. The DNA repair capability of cells may be a rate-limiting factor for cisplatin resistance and sensitivity in cisplatin resistant ovarian cancer cells13.
Poly(ADP-ribosyl)ation is post-translational type of protein modification involved in the regulation of cell cycle, transcription, initiation of the DNA repair and apoptosis1415. The poly(ADP-ribosyl)ation process can regulate a series of molecular events through regulating enzymatic activities and macromolecular interactions between proteins, DNA and RNA. PARP-1 is responsible for sensing the signals of DNA damage16. DNA strand breaks can be caused by exposures to ionizing radiation, UV light, chemotherapy, or products of cellular metabolism17. Activation of PARP-1 can lead to the addition of poly-(ADP-ribose) branched chains onto the damaged DNA, as well as the assembling proteins involved in DNA repair18. In addition to its role in DNA repair, poly(ADP-ribosyl)ation can also protect the naked DNA from being degraded by nuclease.
It has been suggested that PARP-1 facilitates diverse inflammatory responses through upregulating the expression of inflammation-relevant genes that encode oxidation-reduction-related enzymes, cytokines, and adhesion molecules19. Further, excessive activation of PARP-1 can induce arteriosclerosis, myocardial infarction, hypertension and cardiovascular complications of diabetes2021. However, the relationship between PARP and tumours has been rarely reported. The overexpression of PARP in breast cancer22, glioblastoma23, liver cancer24, prostatic carcinoma25 and pancreatic cancer26 can facilitate the proliferation of tumour cells. Gurung et al27 suggested that the inhibition of PARP-1 and telomerase in MEFs rendered cells is more susceptible to DNA-damaging agents. Other studies1723 demonstrated that inhibiting the activation of PARP could enhance glioblastoma and lung cancer sensitivity to radiation. The PARP-1 inhibitor ANI and the suppression of PARP-1 expression by siRNA can significantly sensitize human liver cancer cells to doxorubicin treatment24.
The inhibitory effects of 3-AB on PARP-1 expression has been well established, Valenzuela et al28 showed that a concentration of 5mM of 3-AB after 2 h-exposure effciently blocks PARP activity avoiding PARP-polymer formation in response to to DNA damage in primary mouse embryonic firoblast. Wang et al29 reported that 3-AB was able to reduce PARP-1 activity in the millimole range in the repair proficient human glioma M059K cells. Zheng et al30 indicated that 3-AB suppressed cell growth, cell invasion and enhanced the suppressive effects of cisplatin in vitro in U2OS cells. They suggested that 3-AB may be developed into an effective agent for the treatment of human osteosarcoma.
In summary, our study indicated that the PARP inhibitor 3-AB inhibited the proliferation activity of C13* cells, and increased the cellular sensitivity to cisplatin. We thus proposed that combined treatment of PARP inhibitor with cisplatin can reverse the drug resistance of ovarian cancer cells, reduce the dosage and side effects of cisplatin chemotherapy, and improve the prognosis of patients suffering from ovarian cancer. However, the PARP protein family includes 17 members, and chemical inhibitors may have nonspecific suppression effects. Systemic long-term administration of PARP inhibitors may harm the DNA repair and genomic stability in normal cells, which can lead to secondary tumours years after3132. Therefore, novel PARP-1 inhibitor with low toxicity and high specificity need to be tested in future. With the progress in the PARP research and combination therapy, targeted therapy using PARP inhibitors may play a vital role in the prevention of tumours.
Acknowledgment
Authors thank Dr Meirong Du for supplying the Cisplatin-resistant ovarian cancer C13* cells. This study was supported by the Shandong Natural Science Fund Project (number, ZR2009CM104) and Development Programs in Medicine & Health Science and Technology of Shandong Province of China (number, 2009HZ065).
Conflict of interest: There is no conflicts of interest to declare.
References
- Surgical cytoreduction for recurrent epithelial ovarian cancer. Cochrane Database Syst Rev. 2013;2:CD008765.
- [Google Scholar]
- Anticancer immune reactivity and long-term survival after treatment of metastatic ovarian cancer with dendritic cells. Oncol Lett. 2012;3:66-74.
- [Google Scholar]
- The E3 ubiquitin ligase EDD is an adverse prognostic factor for serous epithelial ovarian cancer and modulates cisplatin resistance in vitro. Br J Cancer. 2008;98:1085-93.
- [Google Scholar]
- Poly(ADP-ribose) polymerase-1 activation during DNA damage and repair. Methods Enzymol. 2006;4:493-510.
- [Google Scholar]
- How to kill tumor cells with inhibitors of poly(ADP-ribosyl)ation. Int J Cancer. 2011;128:251-65.
- [Google Scholar]
- Effect of reduced expression of PARP-1 by RNA interference on cisplatin-resistance and proliferation in the ovarian cancer cell line. JSDU. 2011;49:52-6.
- [Google Scholar]
- Therapeutic efficacy of trehalose eye drops for treatment of murine dry eye induced by an intelligently controlled environmental system. Mol Vis. 2012;18:317-29.
- [Google Scholar]
- Phase II study evaluating consolidation whole abdominal intensity-modulated radiotherapy (IMRT) in patients with advanced ovarian cancer stage FIGO III--the OVAR-IMRT-02 Study. BMC Cancer. 2011;11:41.
- [Google Scholar]
- Polymerization by DNA polymerase eta is blocked by cis-diamminedichloroplatinum(II) 1,3-d(GpTpG) cross-link: implications for cytotoxic effects in nucleotide excision repair-negative tumor cells. Carcinogenesis. 2010;31:388-93.
- [Google Scholar]
- Enhance cisplatin cytotoxicity by disturbing the nucleotide excision repair pathway in ovarian cancer cell lines. Cancer Res. 2003;63:1311-6.
- [Google Scholar]
- Base excision repair of reactive oxygen species-initiated 7,8-dihydro-8-oxo-2’-deoxyguanosine inhibits the cytotoxicity of platinum anticancer drugs. Mol Cancer Ther. 2009;8:2015-26.
- [Google Scholar]
- Poly(ADP-ribose) polymerase-1 activity facilitates the dissociation of nuclear proteins from platinum-modified DNA. Bioorg Med Chem. 2008;16:10121-8.
- [Google Scholar]
- Evolutionary history of the poly(ADP-ribose) polymerase gene family in eukaryotes. BMC Evol Biol. 2010;10:308.
- [Google Scholar]
- PARG is recruited to DNA damage sites through poly(ADP-ribose)- and PCNA-dependent mechanisms. Nucleic Acids Res. 2011;39:5045-56.
- [Google Scholar]
- Inhibition of poly(ADP-ribose) polymerase enhances cell death and improves tumor growth delay in irradiated lung cancer models. Clin Cancer Res. 2007;13:3033-42.
- [Google Scholar]
- Structure and identification of ADP-ribose recognition motifs of APLF and role in the DNA damage response. Proc Natl Acad Sci USA. 2010;107:9129-34.
- [Google Scholar]
- Signaling mechanism of Poly(ADP-Ribose) polymerase-1 (PARP-1) in Inflammatory Diseases. Am J Pathol. 2011;178:946-55.
- [Google Scholar]
- Poly (ADP-Ribose) polymerase inhibition attenuates atherosclerotic plaque development in ApoE-/- mice with hyperhomocysteinemia. J Atheroscler Thromb. 2009;16:641-53.
- [Google Scholar]
- Poly(ADP-ribose) polymerase-1-deficient mice are protected from angiotensin II-induced cardiac hypertrophy. Am J Physiol Heart Circ Physiol. 2006;291:1545-53.
- [Google Scholar]
- Breakthrough breast cancer treatment-PARP inhibitor, BRCA, and triple negative breast cancer. Gan To Kagaku Ryoho. 2010;37:1187-91.
- [Google Scholar]
- In vitro and in vivo radiosensitization of glioblastoma cells by the poly(ADP-ribose) polymerase inhibitor E7016. Clin Cancer Res. 2009;15:607-12.
- [Google Scholar]
- Inhibition of poly (ADP-ribose) polymerase-1 enhances doxorubicin activity against liver cancer cells. Cancer Lett. 2011;301:47-56.
- [Google Scholar]
- Prostate cancer radiosensitization through poly (ADP-Ribose) polymerase-1 hyperactivation. Cancer Res. 2010;70:8088-96.
- [Google Scholar]
- C12orf48, termed PARP-1 binding protein, enhances poly(ADP-ribose) polymerase-1 (PARP-1) activity and protects pancreatic cancer cells from DNA damage. Genes Chromosomes Cancer. 2011;50:13-24.
- [Google Scholar]
- Inhibition of poly(ADP-Ribose) polymerase-1 in telomerase deficient mouse embryonic fibroblasts increases arsenite-induced genome instability. Genome Integr. 2010;1:5.
- [Google Scholar]
- PARP-1 modifies the effectiveness of p53-mediated DNA damage response. Oncogene. 2002;21:1108-16.
- [Google Scholar]
- PARP-1 and Ku compete for repair of DNA double strand breaks by distinct NHEJ pathways. Nucleic Acids Res. 2006;34:6170-82.
- [Google Scholar]
- The poly(ADP-ribose) polymerase-1 inhibitor 3-aminobenzamide suppresses cell growth and migration, enhancing suppressive effects of cisplatin in osteosarcoma cells. Oncol Rep. 2011;25:1399-405.
- [Google Scholar]
- Exploiting the homologous recombination DNA repair network for targeted cancer therapy. World J Clin Oncol. 2011;2:73-9.
- [Google Scholar]
- PARP inhibitors in oncology: a new synthetic lethal approach to cancer therapy. Acta Clin Belg. 2011;66:2-9.
- [Google Scholar]






