Translate this page into:
Effect of growth factors (BMP-4/7 & bFGF) on proliferation & osteogenic differentiation of bone marrow stromal cells
Reprint requests: Dr Zhenggang Bi, Department of Orthopedics, First Affiliated Hospital of Harbin Medical University, Harbin 150001, P.R. China e-amil: zhenggangbi@126.com
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
BMP (bone morphogenetic protein)-4/7 and bFGF (basic fibroblast growth factor) significantly promote the osteogenic activity and the proliferation of rabbit BMSCs (bone marrow stromal cells), respectively. However, their synergistic effects on the proliferation and the differentiation of BMSCs remain unclear. In the present study, the effects of bFGF and BMP-4/7 were investigated on the proliferation and the differentiation of rat BMSCs in vitro.
Methods:
BMSCs were isolated from New Zealand white rabbits and cultured to the third passage. The samples were divided into five groups according to the material implanted: (A) 80 ng/ml BMP-4/7; (B) 80 ng/ml bFGF; (C) 30 ng/ml BMP-4/7 and 30 ng/ml bFGF; (D) 50 ng/ml BMP-4/7 and 50 ng/ml bFGF; and (E) 80 ng/ml BMP-4/7 and 80 ng/ml bFGF. Cell proliferation was analyzed using methyl thiazolyl tetrazolium (MTT) assay. Alkaline phosphatase activity and osteocalcin (OC) dynamics were also measured.
Results:
BMP-4/7 alone significantly (P<0.05) promoted the proliferation of BMSCs. At the same time, it also promoted or inhibited the osteogenic differentiation of BMSCs. The synergistic effects of BMP-4/7 and bFGF significantly promoted both the proliferation and the osteogenic differentiation of BMSCs. The treatment of the synergistic effects was dose and time dependent.
Interpretation & conclusions:
A rational combination of BMP-4/7 and bFGF can promote the proliferation and the osteogenic differentiation of BMSCs. In addition, the synergistic functions are effective.
Keywords
Basic fibroblast growth factor
bone mesenchymal stromal cells
bone morphogenetic protein-4/7
bone tissue engineering
Bone or fracture healing is a proliferative physiological process in which the body facilitates the repair of a bone or a fracture. The repair involves complicated processes such as cell and tissue differentiation and proliferation1. This multi-step cascade is orchestrated by cytokines and morphogens. Cytokines influence cell migration, proliferation, differentiation, and matrix synthesis23. Bone morphogenetic proteins (BMPs) are a group of growth factors also known as cytokines or metabologens4. BMPs are considered to constitute a group of pivotal morphogenetic signals orchestrating tissue architecture throughout the body5. At least 20 structurally and functionally related BMPs are known, most of which play roles in embryogenesis and morphogenesis of various tissues and organs. BMP-2/BMP-7 and BMP-4/BMP-7 have been suggested to exist and function in vivo. These are more potent inducers of bone formation than their respective homodimers, which are widely used in bone tissue engineering678.
Fibroblast growth factors (FGFs) are heparin-binding proteins that belong to a family of growth factors involved in angiogenesis, wound healing, and embryonic development. Previous studies have shown that the interactions of FGFs with cell-surface-associated heparin sulphate proteoglycans are essential for FGF signal transduction. FGFs are key players in the proliferation and the differentiation of a wide variety of cells and tissues910. Moreover, FGFs can also strongly promote the formation of cartilage and bone tissues1112.
Colony-forming unit fibroblast is isolated from bone marrow by their selective adherence to tissue culture plastics. The biological effects of various cytokines on bone marrow stromal stem cell (BMSCs) have been extensively studied. Previous studies specifically focused on the effects of BMP-2, 4, 7 and bFGF on the proliferation and the differentiation of BMSCs13141516.
A recent study17 has demonstrated that the fusion of BMP-4/7 and bFGF significantly promotes the osteogenic activity and the proliferation of rabbit BMSCs, respectively, but their synergistic effects remain unclear. In this study, the proliferation and the osteogenic activities of BMSCs in vitro were detected by single or combined use of different doses of BMP-4/7 and bFGF.
Material & Methods
BMSCs isolation and expansion: Thirteen three-month old New Zealand white rabbits weighing 2 kg each were provided by the laboratory center of the First Affiliated Hospital of Harbin Medical University (Harbin, P.R. China). Primary rabbit BMSCs were isolated from the white rabbits using the whole marrow isolation method13. The BMSCs were expanded under standard conditions in α-Intensity Modulation Direct Modulation (α-IMEM) complete growth medium (Gibco, USA). After the cells reached confluence, adherent BMSCs were trypsinized and subcultured to the third passage for further experiment. This study was conducted with approval from the Ethics Committee of the First Affiliated Hospital of Harbin Medical University. Written informed consent was obtained from all participants. This study was conducted at the laboratory center of the First Affiliated Hospital of Harbin Medical University from January 2001 to December 2010.
The rabbit BMSCs were divided into five groups according to the material implanted: group A, 80 ng/ml BMP-4/7; group B, 80 ng/ml bFGF; group C, 30 ng/ml BMP-4/7 and 30 ng/ml bFGF; group D, 50 ng/ml BMP-4/7 and 50 ng/ml bFGF; and group E, 80 ng/ml BMP-4/7 and 80 ng/ml bFGF. The control group was cultured in a low-sucrose α-IMEM medium supplemented with 20 per cent foetal bovine serum. After the cells reached 60 per cent confluence, bFGF was added into the medium.
Cell morphology observation and calcium nodules staining: The cell morphology, cell growth state and the process of secretion to form the calcium nodules were observed under inverted microscope every day. Cells at day 5 were dyed with Von Kossa staining method18. Growth state of cells at day 5 after passage was observed and the effects of different concentrations of growth factor on the cell growth were compared.
MTT (methyl thiazolyl tetrazolium) assay: The confluent cells were trypsinized and washed as described above, plated onto 96-well plates, and cultured in a basal medium. The third-passage cells were seeded in a 96-well plate with a density of 1×104/well to examine their proliferation ability. The medium was changed after 24 h by adding the culture medium containing the corresponding growth factors. After incubation in a humidified atmosphere at 37°C and 5 per cent CO2 for 12, 24, 48, 72, 96, and 120 h, the cells at each time point were counted to draw the growth curve. The MTT working solution was prepared and 50 μl was added per well. The solution was incubated at 37 °C for 4 h before removing the supernatant and replacing it with 150 μl of DMSO. After a few minutes of oscillation at room temperature, absorbance was detected. The proliferation of the cells was determined according to the optical density at a wavelength of 603 nm using a microtiter plate reader.
Alkaline phosphatase (ALP) activity: On days 3, 4, and 5, the cells were harvested and resuspended in 100 μl PBS. The cells were lysed by sonication after three times of freezing-thawing. The quantification analysis of the total protein was performed using the Brandford assay19. ALP activity was determined by the measurement of p-nitrophenyl phosphate using a commercial assay kit (Boster Reagent Co., Ltd, China). Absorbance of the reaction mixture was measured using a microtiter plate reader (KHB labsystems Wellscan K3, Finland) at 520 nm. Six replicates from each group were analyzed.
OC dynamics: An osteocalcin (OC) kit was purchased from East Asia Immune Technology Institute (Beijing, China). The cells of different groups were seeded onto 6-well plates at a density of 5×104/well and cultured at 37°C in a humidified incubator containing 5 per cent CO2. The supernatant was collected after 3, 4, and 5 days following the addition of the corresponding growth factors. After freeze-drying, OC dynamics was detected using the Osteocalcin kit in accordance with manufacturer instructions. Briefly, the 100 μl medium was collected and mixed with 100 μl OC antibody labelled as 125I at 4 °C for 24 h. After the separating agent was added and mixed, the mixture was stored at room temperature, centrifuged at 4 °C and the supernatant was discarded. The radiation dose in the pellet was detected through the OC antibody labelled as 125I, and the relative OC values were 125I calculated.
Statistical analysis: MTT, ALP activity, and OC dynamics in each group were analyzed using ANOVA test. Statistical significance of multiple comparisons was evaluated using LSD-t test. All statistics were performed with SPSS13.0 software (SPSS Inc., USA).
Results
Effect of different concentrations of growth factors on the growth state of cells: The growth state of BMSCs showed significant differences among the various groups 5 days after the third passage. A few monocytes were adhered to the cell wall in the control group with elongated spindles (Fig. a). When 80 ng/ml BMP-4/7 was added into the medium, the proliferation of the cells showed a spiral-shaped arrangement (Fig. b). Some of the cells were more intensive while the others were confluent (Fig. c) when 80 ng/ml bFGF was added. The cells formed a colony when the medium was added with 30 ng/ml BMP-4/7 and 30 ng/ml bFGF (Fig. d). When the concentration of BMP-4/7/ bFGF was increased to 50 ng/ml (Fig. e), calcium nodules could be clearly seen among the dense cells. Moreover, calcium nodules that already formed were seen as black nodules stained by Von Kossa-VG and surrounded by the adherent osteoblasts attached (Fig. f).
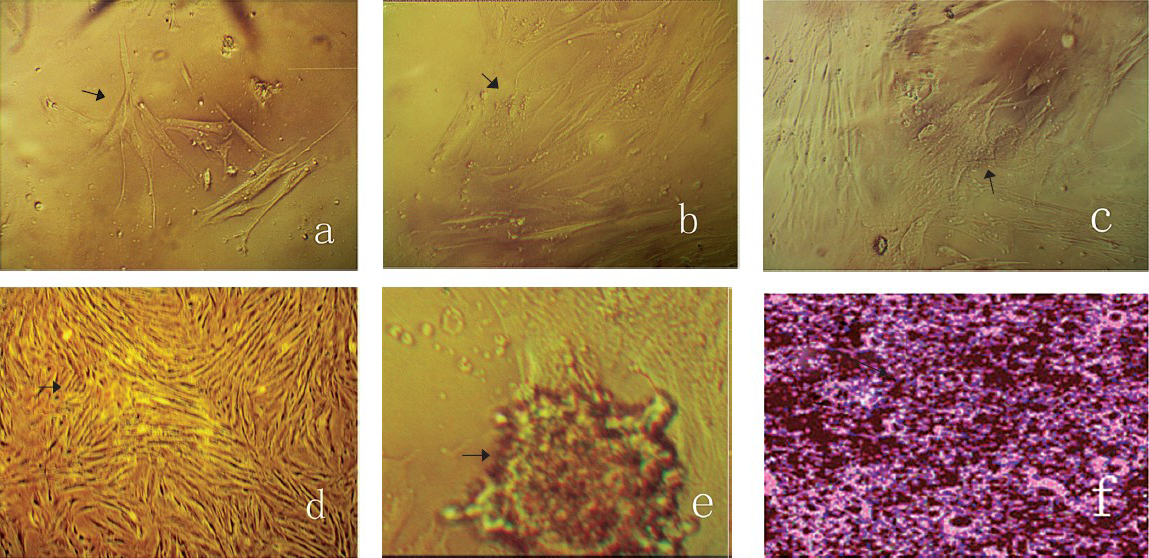
- The growth state of BMSCs cultured in medium with the additional supplementary of different growth factors after five days culture (arrows). a: Control; b: 80 ng/ml BMP-4/7; c: 80 ng/ml bFGF; d: 30 ng/ml BMP-4/7+30 ng/ml bfGF; e: 50 ng/ml BMP-4/7+50 ng/ml bFGF; f: 80 ng/ml BMP-4/7+80 ng/ml bFGF
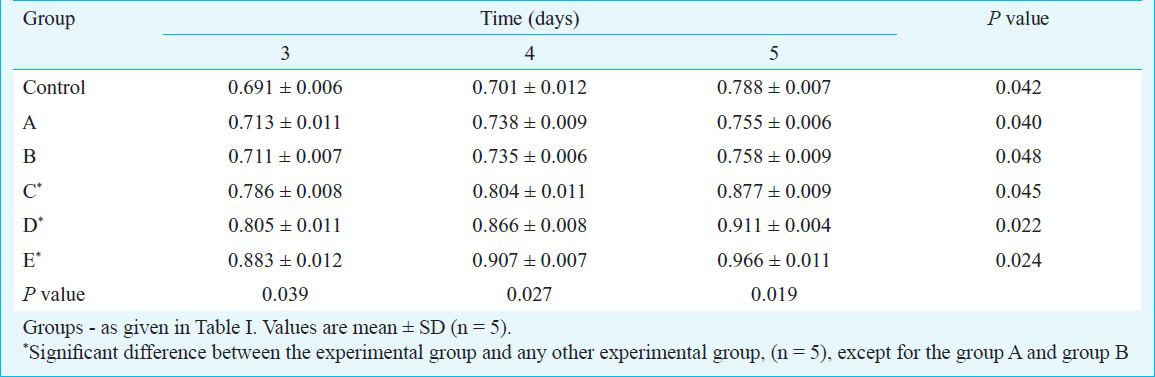
Effect of different concentrations of growth factors on the cell growth numbers: After the additional supplement with different cytokines for 12, 24, 48, 72, 96, and 120 h, the number of BMSC cells increased compared with that of the control group. The number of cells in groups C, D, and E was significantly higher than that in groups A and B (P<0.05). The growth state of the cells in groups C, D, and E was upregulated by the growth factors in a dose-dependent manner (Table I).

Effect of different concentrations of growth factors on cell proliferation: MTT results showed that the OD values of each group increased with extended treatment time (P<0.05). At the same time (P<0.05), the OD values of the experimental groups were higher than those of the control group. No significant difference was found between groups A and B. However, the OD values of groups C, D, and E were higher than those of groups A and B. The former was positively related to the cell proliferation of BMSCs in a dose-dependent manner (P<0.05) (Table II).
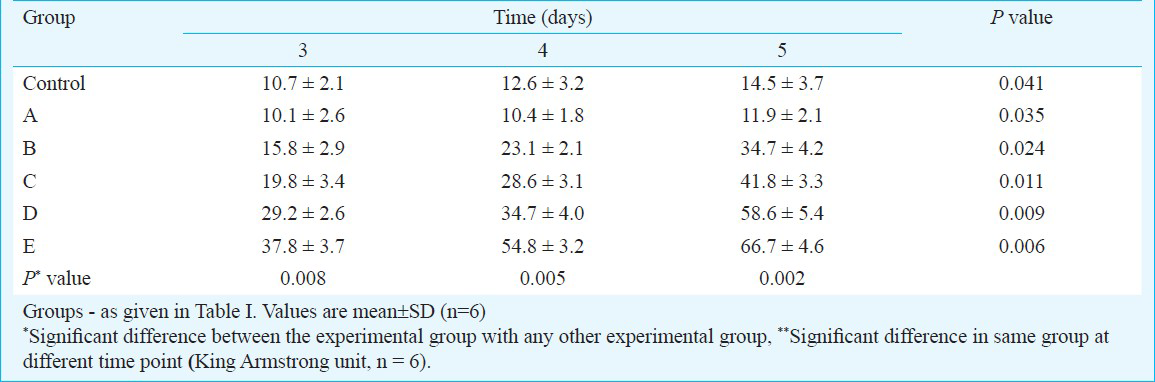
Effect of different concentrations of growth factors on ALP activity: The ALP level in each group increased with extended treatment time (P<0.05). At the same time, the ALP level of BMSCs in group A was lower than that in the control group, suggesting that osteogenic activity was inhibited (P<0.05). However, the ALP levels of BMSCs in groups B, C, D, and E were higher than those of BMSCs in the control group. The osteogenic activity in the combination groups using the growth factors was higher than that of the single-cell factor group (P<0.05) and showed significant positive association with the growth factors in a dose-dependent manner (P<0.01) (Table III).
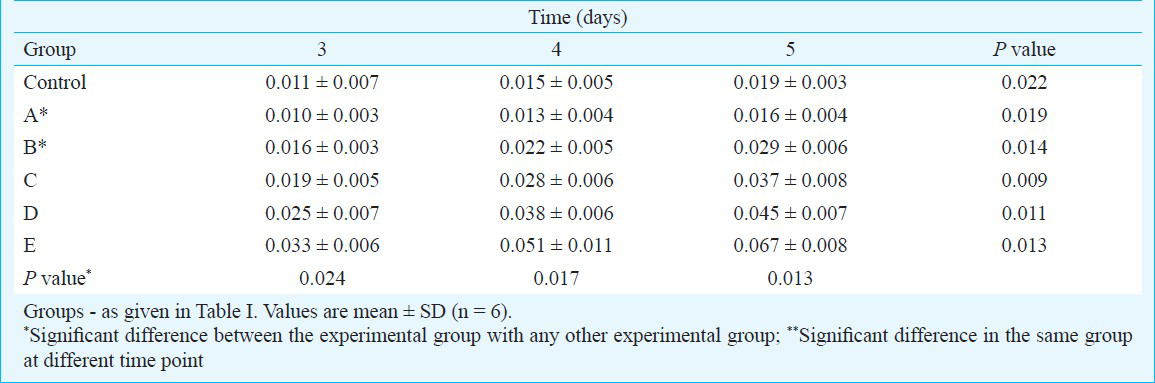
Effect of different concentrations of growth factors on OC dynamics: The results showed that OC dynamics increased with extended treatment time (P<0.05). At the same time (P<0.05), the OD levels of BMSCs in group A was lower than those in the control group, indicating that osteogenic activity was inhibited. On the other hand, the OD levels of BMSCs in groups B, C, D, and E were higher than those of BMSCs in the control group. Moreover, the osteogenic activity in the combination groups using the growth factors was higher than that in the single-growth group (P<0.05) and showed significant positive association with the growth factors in a dose-dependent manner (P<0.01) (Table IV).
Discussion
Bone tissue engineering has rapidly developed in recent years. BMSCs are considered ideal seed cells because of convenient isolation, long-term culture and passage, and no obvious immune rejection of auto-logous transplantation2021. BMSCs are mesenchymal stem cells that have the potential of transforming through ossification and can carry out cross-system differentiation under a certain environment with stimulating factors21. When BMSCs are cultured in a conditioned medium containing BMPs and bFGF, among others, these can transform into osteoblasts. It has been shown that promoting spinal fusion activity of recombinant human BMP-4 is stronger than that of recombinant human BMP-2, and the required dose is only 1/10 of the latter. In addition, the amount of osteoblasts produced is positively dose-dependent22. In a previous study, we successfully constructed a shuttle vector, including BMP-4 and BMP-7 mature peptide genes. The shuttle vector transformed into competent Escherichia coli to obtain the fusion of BMP-4 and BMP-7 mature peptides, which have significant ossification activity for BMSCs12.
The bFGF has been shown to promote the proliferation of bone marrow stromal stem cell mitosis factor, and formation of cartilage and bone1314. Varker et al23 pointed out the difference in vivo in the composition of donor cell in terms of its growth and age; furthermore, many other cell factors contribute to the bone effect15. The current research on in vitro culture BMSCs with different biological growth factors achieved meaningful results after observing their composite effect behaviours, but no unified recognition has been achieved79.
Several studies have demonstrated that the combination of different growth factors can induce early BMSC proliferation and differentiation in vitro and synergistically promote new bone formation1524. Directed differentiation of BMSCs, in which cytokines and their receptors play a crucial role in the differentiation process, is tightly regulated by many factors. The revascularization process is a necessary factor in obtaining an ideal healing effect and is thus important in bone tissue engineering25. bFGF is a powerful mitosis factor that promotes differentiation of cartilage cells and BMSCs and transforms them into cartilage and bone tissues262728. Osteogenesis is influenced by numerous factors, which include medium composition, donor age, concentration and duration of cytokines, and in vitro and in vivo environments14.
bFGF has been demonstrated to promote the proliferation of BMSCs in a dose-dependent manner, but it does not have an entirely positive effect on the proliferation. As the concentration increased to more than 100 ng/ml, the proliferation of BMSCs was inhibited and the cell morphology was also altered. This alteration can affect the observation of the osteogenic potential of BMSCs15. Therefore, the concentration range from 30 to 80 ng/ml or less than 100 ng/ml was chosen. The results showed that the proliferation of BMSCs could be promoted both in the 80 ng/ml BMP-4/7 group and the 80ng/ml bFGF group. No significant difference between the two groups was observed. In the combination of bFGF and BMP-4/7, the promotion effect of low concentration (30 ng/ml BMP-4/7 + 30 ng/ml bFGF) showed more potency than a single high concentration (80 ng/ml) of BMP-4/7 or bFGF. As the concentration of the combination group increased, the promotion effect became more potent. The highest concentration group (80 ng/ml BMP-4/7+80 ng/ml bFGF) exhibited the strongest proliferation effects. The results revealed that the combination of BMP-4/7 and bFGF significantly promoted BMSC proliferation in vitro in the 30-80 ng/ml concentration range.
When the medium did not contain any growth factors, the ALP activity and the OC of the BMSCs were lower than those of 80 ng/ml BMP-4/7 and higher than those of 80 ng/ml bFGF. This result suggests that high concentrations of BMP-4/7 promote osteogenic differentiation of BMSCs by increasing ALP and OC activity. However, the high concentration of reduced ALP activity and OC, indicating that BMP-4/7 not only promotes cell proliferation, but also promotes significant osteogenic activity. In the combination of bFGF and BMP-4/7, the effects of low concentration (30 ng/ml) on the differentiation of BMSCs were higher than those on the single and higher BMP-4/7 concentration (80 ng/ml). Moreover, osteogenic activity and the proliferation ability of BMSCs were also enhanced. In the treatment with 50 ng/ml BMP-4/7 + 50 ng/ml bFGF, the osteogenic activity and proliferation ability of BMSCs were lower than those of the high concentration group and higher than those of the low concentration group. This observation suggests that the effects of the combination factors are positively related with treatment time. Therefore, the two factors are synergistic in promoting osteogenic differentiation.
BMP-4/7 can induce undifferentiated mesenchymal cells irreversibly into differentiated bone and cartilage. However, BMP only promotes bone formation and has no further proliferation effects on differentiated osteoblasts. bFGF promotes cell proliferation and differentiation, but it does not have ectopic induction ability during bone formation. The complementary biological functions between the two factors allow them to accelerate the process of bone induction and formation. The synergistic effect of BMP-4/7 and bFGF cannot be calculated by simple superposition because of complex interactions among different factors. The regulatory mechanisms of BMP-4/7 and bFGF at the molecular level are still not well understood and thus need further study.
Acknowledgment
This study was supported by the Natural Science Foundation of Heilongjiang Province (No: D200876) and the Science and Technology Research Project of Department of Education from Heilongjiang Province (No:11531149), P.R. China.
References
- Microdamage detection and repair in bone: fracture mechanics, histology, cell biology. Technol Health Care. 2009;17:67-75.
- [Google Scholar]
- Bone morphogenetic protein signaling suppresses tumorigenesis at gastric epithelial transition zones in mice. Cancer Res. 2007;67:8149-55.
- [Google Scholar]
- The bone morphogenetic protein pathway is inactivated in the majority of sporadic colorectal cancers. Gastroenterology. 2008;134:1332-41.
- [Google Scholar]
- Increased accumulation of superficial zone protein (SZP) in articular cartilage in response to bone morphogenetic protein-7 and growth factors. J Orthop Res. 2007;25:293-303.
- [Google Scholar]
- The inductive effect of bone morphogenetic protein-4 on chondral-lineage differentiation and in situ cartilage repair. Tissue Eng Part A. 2010;16:1621-32.
- [Google Scholar]
- BMP-2/4 and BMP-6/7 differentially utilize cell surface receptors to induce osteoblastic differentiation of human bone marrow-derived mesenchymal stem cells. J Biol Chem. 2008;283:20948-58.
- [Google Scholar]
- Weaving the neuronal net with target-derived fibroblast growth factors. Dev Growth Differ. 2009;51:263-70.
- [Google Scholar]
- Effect of different growth factors on human osteoblasts activities: a possible application in bone regeneration for tissue engineering. Biomol Eng. 2007;24:613-8.
- [Google Scholar]
- Construction of BMP- (4/7) fusion gene adeno-associated virus and biological effects of transfection on bone marrow stromal cells in rabbits. Orthopedic J China. 2008;11:1053-7.
- [Google Scholar]
- Bone marrow stromal cells induce BMP2/4 production in oxygen-glucose-deprived astrocytes, which promotes an astrocytic phenotype in adult subventricular progenitor cells. J Neurosci Res. 2006;83:1485-93.
- [Google Scholar]
- Osteogenic responses to different concentrations/ratios of BMP-2 and bFGF in bone formation. Ann Biomed Eng. 2010;38:77-87.
- [Google Scholar]
- Effect of culture substrates and fibroblast growth factor addition on the proliferation and differentiation of rat bone marrow stromal cells. Tissue Eng. 2004;10:995-1005.
- [Google Scholar]
- Clonal nature of fibroblast colonies formed by stromal bone marrow cells in culture. Biull Eksp Biol Med. 1987;103:356-8.
- [Google Scholar]
- An assay for the determination of biologically active bone morphogenetic proteins using cells transfected with an inhibitor of differentiation promoter-leciferase construct. Anal Biochem. 2006;349:78-86.
- [Google Scholar]
- Comparison of phenotypic characterization between differentiated osteoblasts from stem cells and calvaria osteoblasts in vitro. Int J Prev Med. 2013;4:180-6.
- [Google Scholar]
- Genetic variants associated with warfarin dose in African-American individuals: a genome-wide association study. Lancet 2013 In Press
- [Google Scholar]
- Osteogenic differentiation of recombinant adeno-associated virus 2-transduced murine mesenchmal stem cells and development of an immunocompetent mouse model for es vivo osteoporosis gene therapy. Hum Gene Ther. 2004;15:1197-206.
- [Google Scholar]
- Tissue-engineered bone repair of goat-femur defects with osteogenically induced bone marrow stromal cells. Tissue Eng. 2006;12:423-33.
- [Google Scholar]
- Non-hematopoietic bone marrow stem cells; molecular control of expansion and differentiation. Exp Cell Res. 2005;306:330-5.
- [Google Scholar]
- Multiparametric flow cytometric analysis of signal transducer and activator of transcription 5 phosphorylation in immune cell subsets in vitro and following interleukin-2 immunotherapy. Clin Cancer Res. 2006;12:5850-8.
- [Google Scholar]
- Molecular mechanisms controlling bone formation during fracture healing and distraction osteogenesis. J Dental Res. 2008;87:107-18.
- [Google Scholar]
- Vitrifying and warming of human oocytes, embryos, and blastocysts: vitrification procedures as an alternative to conventional cryopreservation. Methods Mol Biol. 2004;254:345-64.
- [Google Scholar]
- Osteogenic responses to different concentrations/ ratios of BMP-2 and bFGF in bone formation. Ann Biomed Eng. 2010;38:77-87.
- [Google Scholar]
- Co-treatment with basic fibroblast growth factor and 17beta-estradiol in the presence of dexamethasone accelerates bone formation by rat bone marrow stromal cell culture. Nihon Hotetsu Shika Gakkai Zasshi. 2008;52:366-74.
- [Google Scholar]
- Basic fibroblast growth factor (FGF-2): the high molecular weight forms come of age. J Cell Biochem. 2007;100:1100-8.
- [Google Scholar]






