Translate this page into:
Effect of disease-modifying antirheumatic drug therapy on immune response to trivalent influenza vaccine in rheumatoid arthritis
Reprint requests: Dr. Vir Singh Negi, Department of Clinical Immunology, Jawaharlal Institute of Postgraduate Medical Education & Research (JIPMER), Puducherry 605 006, Tamil Nadu, India e-mail: vsnegi22@yahoo.co.in
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Patients with autoimmune rheumatic diseases may be at an increased risk of infection due to disease and use of disease-modifying antirheumatic drug (DMARD) therapy. The present study was done to evaluate the immune response to influenza vaccination in patients with rheumatoid arthritis (RA).
Methods:
Fifty one RA patients on stable methotrexate (MTX) therapy (≥15 mg/wk), 51 newly diagnosed DMARD-naïve RA patients and 45 healthy controls received a single dose of inactivated seasonal trivalent influenza vaccine. Blood samples were collected just prior to and four weeks after vaccination. Pre- and post-vaccination antibody titres against the three virus strains were measured by hemagglutination inhibition assay. The impact of age, gender, DMARD treatment and pre-vaccination seroprotection on response to the vaccine was assessed by binary logistic regression analysis for each of the virus strains.
Results:
Pre-vaccination antibody titres were found to be high in the three study groups for all influenza strains, except for Yamagata strain, the titres for which were low in healthy controls. Trivalent influenza vaccination was found to be safe and stimulated a good antibody response in all study groups. On regression analysis, there was no association of age, gender or MTX therapy with vaccine response, except for Yamagata strain where healthy controls had higher positive immune response (P=0.008; odds ratio – 3.37, 95% confidence interval: 1.36-8.32).
Interpretation & conclusions:
Our results indicated that influenza vaccination was safe in RA patients with no detrimental effect on disease activity. MTX therapy at a dose ≥15 mg/wk did not affect the vaccine response. Presence of high pre-vaccination seroprotective antibody levels in the study population indicates the need for re-examination of recommended annual influenza vaccination in such subgroups of population.
Keywords
Antibody titre
disease-modifying antirheumatic drug
influenza
methotrexate
rheumatoid arthritis
vaccination
Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by synovial inflammation and joint destruction, which if left untreated can result in severe disability and premature mortality1. RA affects nearly 0.5-1 per cent of the general population worldwide2. The standardized prevalence of RA in India is reported to be 0.34 per cent [95% confidence interval (CI): 0.08 - 0.79]3.
Recommendations for the treatment of RA include methotrexate (MTX) which is the anchor disease-modifying antirheumatic drug (DMARD). Other synthetic DMARDs are used if MTX is contraindicated or not tolerated. These are also prescribed in combination with MTX for optimal disease control in patients. Low-dose steroids are used initially as a bridge to effective DMARD therapy. The use of biologic DMARDs is indicated when the former treatment options are not sufficiently effective or as initiating therapy with or without MTX if poor prognostic indicators are present and the disease activity is high4.
Patients with RA have increased morbidity and mortality compared to general population, for which infections are a major cause56. Infections have been reported as the second most common cause of death (23%) in RA from India7. The most common sites of infections in RA are joints, respiratory tract, skin and soft tissues8. Increased risk of infection in RA could be due to intrinsic immunological alterations, disease-related factors such as immobility and organ damage, use of immunosuppressive medications including corticosteroids, synthetic DMARDs and biological therapy and associated comorbid conditions such as diabetes.
Seasonal influenza is an acute viral infection and yearly epidemics of influenza can seriously affect vulnerable groups. The pandemic of H1N1 influenza virus was first reported from Mexico in April 20099, which later spread to 214 countries across the world. India was also severely affected with thousands of deaths reported since 20091011. Yearly vaccination with influenza vaccine is recommended in patients with autoimmune inflammatory rheumatic diseases. Its safety in these patients is reported to be comparable to healthy controls41213. However, there is uncertainty about the immunogenicity of vaccines in view of treatment with various combinations of synthetic DMARDs and with biological therapy1415. A potential risk of disease flare following vaccination has also been reported16.
The aim of the present study was thus to evaluate the immunogenicity of influenza vaccine in drug-naïve and RA patients on stable DMARD therapy as compared to healthy controls.
Material & Methods
This was a prospective study conducted at the department of Clinical Immunology, Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Puducherry, a tertiary care institute in south India. Adult consecutive patients with RA who attended the outpatient department between February and May 2014 were included in the study. Patients with serious infection, malignancy, pregnancy, concurrent leflunomide therapy or prednisolone therapy ≥10 mg/day, those who received biological therapy within the past six months and a history of previous influenza vaccination were excluded from the study. Healthcare personnel of JIPMER without any known illness and who received the trivalent influenza vaccine between February and May 2014 were chosen as controls. A two-sided confidence level of 95 per cent with power of 80 per cent was considered for sample size calculation. Assuming the antibody geometric mean titres (GMT) to be 128, 237 and 340, respectively17, in DMARD, DMARD-naïve and healthy controls with a standard deviation (SD) of 185 and adding for a dropout of 10 per cent, the minimum sample size to detect any difference in GMT was 51 in each group.
Consecutive patients were enrolled till the desired sample size was reached. Fifty one patients fulfilling the 2010 American College of Rheumatology - European League Against Rheumatism (ACR EULAR) criteria for RA1 on treatment with MTX ≥15 mg/wk for three months or more were enrolled as DMARD group. Fifty one newly diagnosed patients who were MTX naïve were selected as DMARD-naïve group. However, during the study period, only 45 healthcare personnel without any known illness and having received the trivalent influenza vaccine between February and May 2014 were available and all were selected as healthy controls. During the study period, the DMARD-naïve group continued to receive non-steroidal anti-inflammatory drugs and intra-articular or low-dose oral steroids (prednisolone 7.5 mg/day or less), as clinically indicated. Concurrent sulphasalazine, hydroxychloroquine and/or oral prednisolone ≤7.5 mg/day were continued in DMARD group.
Each group received a single intramuscular (deltoid region) dose of 0.5 ml inactivated seasonal trivalent influenza vaccine (Agrippal S1, 2013/14 season, Novartis Vaccines, India) containing 15 μg HA of two different A strains: A/California/7/2009 (H1N1) pdm 09 (A/California/7/2009, NYMCX-181) and A/Victoria/361/2011 (H3N2) (A/Texas/50/2012, NYMCX-223) and one B strain: B/Massachusetts/2/2012 (B/Massachusetts/2/2012 wild type) according to World Health Organization recommendations for the year 2013-201418. Blood samples (5 ml) were collected from all groups just prior to and four weeks after vaccination, serum was separated and stored at -80°C. Pre- and post-vaccination antibody titres were determined by hemagglutination inhibition (HI) assay19 using all the three antigens (Influenza reagent resource, USA) with two-fold serum dilutions. The reciprocal of the highest dilution that caused complete inhibition of hemagglutination was taken as antibody titre. Antibody titres ≥40 were considered seroprotective. Seroprotection rate was defined as percentage of patients with antibody titres ≥40 in a group. Positive immune response was defined as 4-folds or more rise in titre if pre-vaccination titre was ≥10, or post-vaccination titre ≥40 if pre-vaccination titre was <10. An antibody titre of <10 determined on HI assay, was assigned a value of 5 for the purpose of statistical analysis20.
Pre- and post-vaccination seroprotection rates along with fold rise in titres in all groups for three virus strains were tabulated. Geometric mean titres (GMTs) and GMT fold rise in titres were calculated for all the three strains. Predictors of positive immune response including effect of DMARD therapy on the immune response were evaluated. Disease activity and functional status pre- and post-vaccination were assessed using the Disease Activity Score28 (DAS28)21 and Indian Health assessment questionnaire22, respectively. Adverse events post-vaccination were recorded. Efficacy of the vaccine was assessed by calculating the percentage of patients who met the European Union Committee of Human Medicinal Products (CHMP) licensing criteria23. The study was approved by the Institutional Ethics committee. Informed written consent was obtained from all individuals before enrolment.
Statistical analysis: Continuous variables were summarized as mean (SD) and categorical variables were summarized as proportions. GMTs were calculated using log-transformed antibody levels. Chi-square test was used to test the difference in proportions of positive immune response between three groups. Fold rise in titres between individual groups were compared using ANOVA. Difference between pre- and post-vaccination GMT titres between the groups for each virus strain was compared using repeated measures ANOVA. Due to baseline difference in age and gender between the groups, a binary logistic regression model adjusting for age, gender and pre-vaccination antibody titres was used to analyze the possible predictors of immune response. Data were analyzed using SPSS software version 19.0 (IBM Corp. Armonk, New York, USA).
Results
Mean disease activity (DAS28 score) was significantly higher in the DMARD-naïve group compared to the DMARD group (P< 0.001). Rheumatoid factor positivity was higher in DMARD group (74.51 vs. 56.86%, P< 0.05) while anti-cyclic citrullinated peptide positivity was comparable between the two disease groups (Table I).
Immunogenicity of trivalent influenza vaccine
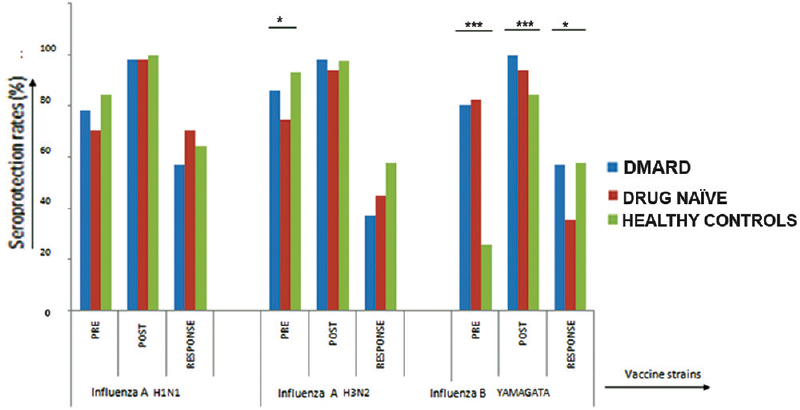
Pre- and post-vaccination seroprotection rates in all study groups: Baseline seroprotection rates prior to vaccination were high in the three groups for all the three virus strains, except for Yamagata strain which was low in healthy controls (25.49%). There was a significant difference in proportion of patients with pre-vaccination seroprotection in H3N2 (86.27 vs. 74.51 vs. 93.33%; P< 0.05) and Yamagata strains (80.39 vs. 82.35 vs. 25.49%; P< 0.001) among the DMARD, DMARD-naïve and healthy control groups, respectively. In all groups, post-vaccination seroprotection rates were >90 per cent for all the three strains except for Yamagata strain (84.4%). There was a significant difference in post-vaccination seroprotection for Yamagata strain in all the groups (100 vs. 94.11 vs. 84.44%; P=0.001) (Figure).
-
P*<0.05, **<0.01, ***<0.001. Pre- and post-vaccination seroprotection rates and positive immune response to trivalent influenza vaccination in the three groups. Pre, prevaccination seroprotection; Post, postvaccination seroprotection; Response, positive immune response indicating four fold rise in antibody titres.
Positive immune response in all study groups: The maximum immune response (70.58%) was seen for H1N1 strain and the least immune response for Yamagata strain (35.29%) in DMARD-naïve patients. Significant difference in immune response among the three groups was seen only for the Yamagata strain (56.85 vs. 35.29 vs. 57.78%; P< 0.05). The DMARD group had lower rates of positive immune response compared to healthy controls only for H3N2 strain [37.25 vs. 57.78% for H3N2 (P< 0.05, odds ratio (OR) – 0.43, 95% CI: 0.19-0.98)] (Figure).
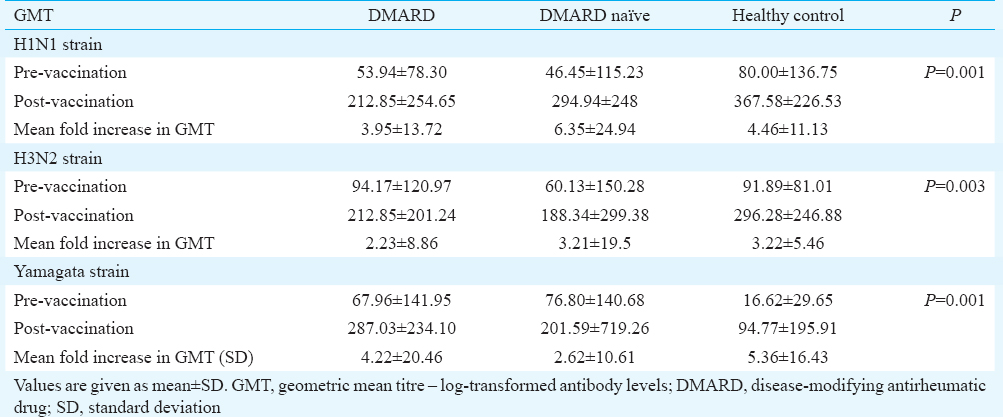
Pre- and post-vaccination geometric mean titre (GMT): The difference between pre- and post-vaccination GMT between the three groups for all the three virus strains was significant (Table II).
Predictors of vaccine response: Regression analysis to determine the predictors of positive immune response to vaccination showed that age, sex and DMARD treatment did not affect the immune response for both H1N1 and H3N2 strains. Healthy controls had greater odds of positive immune response (P=0.008, OR=3.37; 95% CI: 1.36-8.32) compared to DMARD group for Yamagata strain. No difference was observed between DMARD and DMARD-naïve groups. Pre-vaccination seroprotection was significantly associated with positive immune response for all the three strains (Table III).

Effect of vaccine on disease activity: The mean disease activity was reduced in the DMARD group during post-vaccination period. However, the reduction in DAS28 score (ΔDAS28 - 0.42) was not clinically significant. DAS28 reduction in DMARD-naïve group, however, was clinically significant (ΔDAS28 - 1.41). Disease activity worsened post-vaccination in five (9.80%) patients and remained the same in two (3.92%) patients in DMARD group, while it increased in three patients (5.88%) in DMARD-naïve group.
Adverse effects: No major serious adverse events were recorded after vaccination. Local injection site reactions were seen in four (7.8%) patients in DMARD group, four (7.84%) in DMARD-naïve group and two (4.44%) in healthy controls. One patient in DMARD group developed fever following vaccination that lasted for one day and resolved spontaneously. Five (9.80%) patients in DMARD group, four (7.8%) in DMARD-naïve group and five (11.1%) healthy controls had mild flu-like symptoms following vaccination. None of the patients in any group had severe adverse effects requiring hospitalization.
Discussion
In this single-centre study, the immunogenicity of trivalent influenza vaccination was evaluated in drug-naïve RA patients and those on stable MTX therapy and compared it with healthy controls. The pre-vaccination antibody titres were found to be high in all the three groups. This indicated that subclinical influenza infections were common in this population and the prevalent antibody titres might be protective in a proportion of those exposed. High antibody titres had a significant association with positive immune response. In such individuals, the vaccine given may be acting as a booster dose. Trivalent influenza vaccination was safe and generated a good antibody response in all groups. There was no impact of the age, gender or MTX therapy on the response to vaccination, except for Yamagata strain where healthy controls had a higher positive immune response. There was no effect of vaccination on disease activity.
MTX administration has been reported to be associated with impaired immune response in studies on H1N1 vaccination172425. Gabay et al17 found antibody titres being nearly 50 per cent lower in MTX-treated patients. Our study showed high pre-vaccination seroprotective titres for all three strains in all three study groups. This may be a compounding factor while trying to assess the effect of drug on vaccine response. In addition, an augmented immune response in individuals with pre-vaccination seroprotective titres as shown in our study might be a reason why MTX had no impact on immune response to vaccination.
The only study in which MTX group had the best serological response to trivalent influenza vaccine was by Kapetanovic et al26. This was, however, in comparison to TNF (tumour necrosis factor) blockers with and without MTX. Patients also received simultaneous pneumococcal vaccine, and the effect of simultaneous exposure to both polysaccharide and polypeptide antigens on immune response may be responsible for the different results. The reason for inconsistency in different studies is probably due to difference in baseline immunity due to past exposure or immunization status, monovalent (adjuvunated or adjuvant free) or trivalent vaccine used and comparison with healthy controls.
In the present study, no impact of age was observed on vaccine response on regression analysis. Kapetanovic et al27 showed that vaccine response did not differ in patients above and below 60 yr of age which was in accordance with our study. Patients aged above 60 yr, however, constituted only 6.8 per cent of our study groups. None of our healthy controls were above 60 yr. Ribeiro et al24 observed that higher age, RA and MTX therapy were associated with impaired seroconversion. Gabay et al17 showed that every additional 10 yr of age results in 31 per cent decline in antibody titres. High pre-vaccination antibody titres may be one of the factors for decreased sensitivity in our study to detect the effect of age on vaccine response.
Pre-vaccination seroprotection (≥40) was very high in our study population reflecting previous clinical or subclinical exposure as none of them were previously vaccinated. Pre-existing immunity to influenza virus and cross-reactivity levels however, vary in age groups and in populations28. The high pre-vaccination antibody titres in our study were similar to the study by You et al29. Indian data regarding seroepidemiology of influenza virus infection showed a seropositivity of 26.4 and 55.3 per cent, respectively, for seasonal H1N1 and H3N2 in general population30. Past infection rather than vaccination results in cross-reactivity31. Seroprotection rates of 33 per cent (17-41%) in a population is considered as herd immunity threshold (HIT)32. Using this cut-off, the need for yearly influenza vaccine in populations with high pre-vaccination seroprotection rates like ours needs to be reconsidered.
Patients with pre-vaccination seroprotection showed higher odds of positive immune response for all the three virus strains in our study. This was probably due to a secondary immune response in these patients which was stronger than the primary immune response. Kapetanovic et al25 showed that the pre-vaccination antibody titres were inversely associated with post-vaccination immune response. The same group26 in an earlier study had also noted that RA patients with protective levels before vaccination responded less well to vaccination as a group.
Vaccination was safe and had no significant impact on disease course in our study. Disease activity (DAS28) was not affected with vaccination. This was consistent with other studies1724. However, Kapetanovic et al25 reported mild worsening of joint symptoms in 8.2 per cent of patients consisting of arthralgias, increased morning stiffness and fatigue. The safety profile of the trivalent vaccine was good with no serious adverse events. Most studies have shown similar results172425.
The limitation of our study was that we could not study the effect of combination DMARDs and biological therapy, especially TNF inhibitors and B-cell depleting therapy. The results of this study cannot be generalized to the entire population as baseline seropositivity may vary in different geographical regions. Finally, immune response is only a surrogate marker and may not completely translate into protection against the disease.
In conclusion, our study showed that influenza vaccination was safe in RA patients. There was no adverse effect of vaccination on disease activity. MTX therapy at dose ≥15 mg/wk did not affect the vaccine response. One important finding of our study is high pre-vaccination seroprotective antibody levels which suggests to re-examine the recommendation for annual influenza vaccination in our country.
Acknowledgment
This study was supported by Jawaharlal Institute of Postgraduate Medical Education and Research Intramural Research grant.
Conflicts of Interest: None.
References
- 2010 rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2010;69:1580-8.
- [Google Scholar]
- Disease burden of rheumatic diseases in India: COPCORD perspective. Indian J Rheumatol. 2015;10:70-7.
- [Google Scholar]
- 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64:625-39.
- [Google Scholar]
- Shortening of life span and causes of excess mortality in a population-based series of subjects with rheumatoid arthritis. Clin Exp Rheumatol. 1995;13:149-53.
- [Google Scholar]
- Mortality and survival in rheumatoid arthritis: A 25 year prospective study of 100 patients. Ann Rheum Dis. 1990;49:363-9.
- [Google Scholar]
- Epidemiology of rheumatoid arthritis. In: Mukherjee S, Ghosh A, eds. Monograph on rheumatoid arthritis. Kolkata: Indian College of Physicians, Academic Wing of API India, Marksman Media Service; 2012. p. :1-9.
- [Google Scholar]
- Frequency of infection in patients with rheumatoid arthritis compared with controls: A population-based study. Arthritis Rheum. 2002;46:2287-93.
- [Google Scholar]
- Emergence of pandemic 2009 influenza A H1N1, India. Indian J Med Res. 2012;135:534-7.
- [Google Scholar]
- Ministry of Health and Family Welfare, India. Information on Swine Flu. New Delhi: Ministry of Health and Family Welfare; 2015. Available from: http://www.mohfw.nic.in/swineflu.htm
- [Google Scholar]
- 9311 Cases Of Swine Flu Registered, 624 Lives Claimed. The Huffington Post. 2015. Available from: http://www.huffingtonpost.in/2015/02/18/swine-flu-india-_n_6702396.html
- [Google Scholar]
- EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis. 2011;70:414-22.
- [Google Scholar]
- Vaccinations in patients with immune-mediated inflammatory diseases. Rheumatology (Oxford). 2010;49:1815-27.
- [Google Scholar]
- Effect of methotrexate, anti-tumor necrosis factor a, and rituximab on the immune response to influenza and pneumococcal vaccines in patients with rheumatoid arthritis: A systematic review and meta-analysis. Arthritis Care Res (Hoboken). 2014;66:1016-26.
- [Google Scholar]
- Are immunosuppressive medications associated with decreased responses to routine immunizations? A systematic review. Vaccine. 2012;30:1413-24.
- [Google Scholar]
- Impact of synthetic and biologic disease-modifying antirheumatic drugs on antibody responses to the AS03-adjuvanted pandemic influenza vaccine: A prospective, open-label, parallel-cohort, single-center study. Arthritis Rheum. 2011;63:1486-96.
- [Google Scholar]
- WHO. Recommended composition of influenza virus vaccines for use in the 2013-2014 northern hemisphere influenza season. 2013. WHO; Available from: http://www.who.int/influenza/vaccines/virus/recommendations/2013_14_north/en/
- [Google Scholar]
- World Health Organization, Global Influenza Surveillance Network. Manual for the laboratory diagnosis and virological surveillance of influenza. 2011. Geneva: World Health Organization; Available from: http://www.whqlibdoc.who.int/publications/2011/9789241548090_eng.pdf
- [Google Scholar]
- Protective effect of A/H1N1 vaccination in immune-mediated disease – A prospectively controlled vaccination study. Rheumatology (Oxford). 2012;51:695-700.
- [Google Scholar]
- Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44-8.
- [Google Scholar]
- Validation of an Indian version of the Health Assessment Questionnaire in patients with rheumatoid arthritis. Rheumatology (Oxford). 2002;41:1457-9.
- [Google Scholar]
- Committee for Human Medicinal Products: Note for guidance on harmonization of requirements for influenza vaccines. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Medicine_QA/2009/11/WC500007567.pdf
- [Google Scholar]
- Reduced seroprotection after pandemic H1N1 influenza adjuvant-free vaccination in patients with rheumatoid arthritis: Implications for clinical practice. Ann Rheum Dis. 2011;70:2144-7.
- [Google Scholar]
- Impact of anti-rheumatic treatment on immunogenicity of pandemic H1N1 influenza vaccine in patients with arthritis. Arthritis Res Ther. 2014;16:R2.
- [Google Scholar]
- Influenza vaccination as model for testing immune modulation induced by anti-TNF and methotrexate therapy in rheumatoid arthritis patients. Rheumatology (Oxford). 2007;46:608-11.
- [Google Scholar]
- Influence of methotrexate, TNF blockers and prednisolone on antibody responses to pneumococcal polysaccharide vaccine in patients with rheumatoid arthritis. Rheumatology (Oxford). 2006;45:106-11.
- [Google Scholar]
- Cross-reactive antibodies to pandemic (H1N1) 2009 virus, Singapore. Emerg Infect Dis. 2010;16:874-6.
- [Google Scholar]
- A survey of levels of antibodies against influenza viruses in the population of Wuxi City. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2011;25:351-4.
- [Google Scholar]
- Seroepidemiology of pandemic influenza A (H1N1) 2009 virus infections in Pune, India. BMC Infect Dis. 2010;10:255.
- [Google Scholar]
- Incidence of 2009 pandemic influenza A H1N1 infection in England: A cross-sectional serological study. Lancet. 2010;375:1100-8.
- [Google Scholar]
- Influenza A(H1N1)pdm09 antibodies after pandemic and trivalent seasonal influenza vaccination as well as natural infection in November 2010 in Hamburg, Germany. Euro Surveill. 2012;17:pii: 20052.
- [Google Scholar]






