Translate this page into:
Effect of alcoholic extract of Entada pursaetha DC on monosodium iodoacetate-induced osteoarthritis pain in rats
Reprint requests: Dr Surendra Kumar Tandan, Division of Pharmacology & Toxicology, Indian Veterinary Research Institute Izatnagar, Bareilly 243 122, Uttar Pradesh, India e-mail:sktandan@ivri.res.in
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Osteoarthritis (OA) is a degenerative disease characterized by joint pain and progressive loss of articular cartilage. Entada pursaetha has been traditionally used in the treatment of inflammatory disease, liver ailment, etc. In this study we investigated suppressive effect of ethanolic extract of E. pursaetha (EPE) on monosodium iodoacetate (MIA)-induced osteoarthritis pain and disease progression by histopathological changes in joints in a rat model.
Methods:
OA was induced in right knee of rat by intra-articular injection of 3 mg of MIA and characterized by pathological progression of disease and pain of affected joint. Spontaneous movements, mechanical, thermal and cold sensitivity were monitored at days 0 (before drug and MIA injection), 7, 14 and 21 of MIA administration. EPE (30, 100 and 300 mg/kg), vehicle or etoricoxib (10 mg/kg; reference drug) were administered daily for 21 days by oral route.
Results:
EPE at various doses significantly reduced mechanical, heat, cold hyperalgesia and increased the horizontal and vertical movements in intra-articular MIA injected rats. EPE prevented the damage to cartilage structure and reduced the cellular abnormalities. Articular cartilage of rats treated with EPE at 300 mg/kg group was almost normal with well-developed smooth surface and chondrocytes were distributed individually or arranged in column.
Interpretation & conclusions:
The present findings showed that the EPE was not only able to mitigate pain and hyperalgesia but also inhibited MIA-induced cartilage degeneration in vivo. EPE may have the potential to become therapeutic modality in the treatment of osteoarthritis. However, further studies need to be done to confirm these findings in other models and clinical trials.
Keywords
Entada pursaetha
hyperalgesia
monosodium iodoacetate
osteoarthritis
pain
Osteoarthritis (OA) is a chronic joint disease characterized by progressive cartilage degeneration, synovial inflammation, subchondral bone sclerosis, and osteophyte formation1. A complex network of cytokines and proteolytic enzymes leads to degradation of the extracellular matrix (ECM) proteins of cartilage such as type II collagen (CII), proteoglycans and hyaluronic acid2. Although studies have confirmed the efficacy of non-steroidal anti-inflammatory drugs (NSAIDs) and anti-cycloxygenase-2 (COX-2) as symptomatic treatments for OA, these drugs have not proven to positively affect the natural course of OA in humans3. In this context, diacerein has been shown as an attractive candidate4. Monosodium iodoacetate (MIA)-induced OA model used in this study mimics severe and acute OA pain which is similar with human OA 5. The MIA-induced OA model is highly reproducible with predictable pain behaviour responses.
Entada pursaetha, a large gigantic woody climbing shrub (liana) among legumes is widely distributed in tropical Africa, India, China, Philippines, Guam and Northern Australia6. In India, it is found in damp forest of Bengal, Bihar and Odisha, in forest region of Eastern and Western Ghat and hilly forest tract of northern district of Bengal and Deccan7. Mainly seeds, leaves and stem or stem bark of E. pursaetha are used for different medicinal purposes.
Antibacterial, antiviral, analgesic, anti-inflammatory and hypoglycaemic activities of plant extracts have been studied earlier8. Free radical scavenging activity of leaf extract9 and anti-inflammatory and hepatoprotective activity of seed extract of E. pursaetha have also been reported1011. But no study has been done on its anti-arthritic or anti-osteoarthritis activity. In the present study, we investigated whether ethanolic extract of E. pursaetha (EPE) stem would suppress OA pain and its progression by examining behavioural pain parameters and histopathological changes elicited in MIA-induced experimental OA rat model.
Material & Methods
This study was conducted in the division of Pharmacology and Toxicology, Indian Veterinary Research Institute (IVRI), Bareilly, Uttar Pradesh, India. Male Wistar rats (Livestock Resource Section, IVRI weighing 140-175 g at the time of surgery for the induction of model were used. They were housed at a maximum of four per cage on a 12-hour day/night cycle at a temperature of 22±1°C. Water and food were provided ad libitum. All animal experimental procedures were duly approved by the Animals Ethics Committee of IVRI.
Preparation of alcoholic extract of E. pursaetha: The mature stem of E. pursaetha was brought from the jungles of Bhawanipatna, district - Kalahandi, Odisha, India, and authenticated by Dr B.N. Pandey, Department of Botany, Bareilly College, Bareilly (India). A voucher specimen (023/09) was deposited in the Indigenous Drug laboratory of division of Pharmacology & Toxicology. The powdered stem was refluxed twice with 85 per cent ethanol at 95°C for 8 h. Ethanol was removed under vacuum and solid extract of E. pursaetha stem was obtained (henceforth referred as EPE). The yield of the extract was 8.4 per cent with reference to dry starting material. EPE was used by suspending in maximum of 0.2 per cent polysorbate 80. Three different doses of EPE 30, 100 and 300 mg/kg body weight were used, based on the earlier work carried out in our laboratory (unpublished data).
Estimation of phytoconstituents of EPE - EPE was made to the concentration of 100 mg/ml in ethanol and this was used as stock solution for the quantitative phytochemical estimation.
Estimation of total phenolic content - Total phenols were determined as described earlier9. In brief, 0.5 ml of plant extract was mixed with 5 ml Folin Ciocalteu reagent (SRL Pvt. Ltd, India) (1:10 diluted with distilled water) and aqueous 4 ml, 1 M sodium carbonate. The mixtures were allowed to stand for 15 min and the total phenols were determined by colorimetry at 765 nm. The standard curve was prepared using 100, 50, 25 and 12.5 µg/ml solution of gallic acid in methanol: water (50:50, v/v). Total phenol values were expressed in terms of gallic acid equivalent mg/g of extract.
Estimation of total tannin content - Stock ethanolic extract (0.1ml) was mixed with 0.5 ml of Folin-Denis reagent (Sigma Aldrich, USA) followed by 1 ml of Na2CO3 (0.5% w/v) solution and made up to 10 ml with distilled water. The absorbance was measured at
755 nm within 30 min of the reaction against the reagent blank. Standard curve was prepared using 500, 250, 125 and 62.5 μg/ml tannic acid solution. Total tannins in extracts were expressed as equivalent to tannic acid (mg TE/g extract)10.
Estimation of total flavonoid content - Total flavonoids were determined by aluminum chloride colorimetric method11. In brief, 0.5 ml of plant extract in methanol was separately mixed with 1.5 ml of methanol, 0.1 ml of 10 per cent aluminum chloride, 0.1 ml of 1 M potassium acetate and 2.8 ml of distilled water. After keeping at room temperature for 30 min, the absorbance of the reaction mixture was measured at 415 nm with a double beam UV/Visible spectrophotometer. The calibration curve was prepared by preparing quercetin solutions at concentrations 100, 50, 25 and 12.5 µg/ml in methanol. Total flavonoids in extracts were expressed as equivalent to quercetin (mg QE/g extract).
Estimation of total saponin content - Total saponins were determined by vanillin and sulphuric acid method12 in which 0.5 ml of plant extract was mixed with 0.5 ml of 8 per cent vanillin in ethanol and then 5 ml of 72 per cent v/v sulphuric acid in deionized water was added and mixed well in ice-water bath. The mixture was kept in hot water bath at 60°C for 10 min and cooled in ice cold water bath. Absorbance was taken at 460 nm. The standard curve for saponins was prepared by using saponins for quillaja bark (sapogenin) from Sigma at concentrations 1000, 500, 250 and 125 µg/ml in distilled water.
Induction of osteoarthritis: On day 0, rats were anaesthetized with ketamine hydrochloride (50 mg/kg body weight) and xylazine (10 mg/kg body weight) and the right knee was shaved and disinfected with 70 per cent ethanol followed by povidone-iodide. A single injection of 50 μl sterile normal saline containing 3 mg monosodium iodoacetate was injected into right knee joint through infrapatellar ligament using a 300 μl syringe fitted with a 29 G needle, as described earlier13. A pilot study was undertaken to induce osteoarthritis with MIA. On day 21 post MIA administration, the knee joint was examined histopathologically and cartilage was stained with special stain safranine-O for accessing whether osteoarthritis was produced or not14. After establishing MIA-induced osteoarthritis in pilot study, six experimental groups I, II, III, IV, V and VI, each consisting of six rats were taken. Animals of groups I and II were naive control and vehicle control, respectively. On day 0, rats of all groups except naïve control were administered with a single dose of MIA in their right knee joint cavity while naïve control was injected with 50 μl of normal saline only. From day 0 to 21, rats in groups III, IV, and V were administered EPE daily at 30, 100, and 300 mg/kg doses, respectively through oral gavage before MIA injection. Rats of groups I and II were administered normal saline and vehicle, respectively. Rats of group VI were administered with etoricoxib at10 mg/kg from day 0 to 21, daily orally. Pain assessment was done on days 0 (before administration of MIA), 7, 14, and 21 of experiment by measuring different parameters such as mechanical hyperalgesia, thermal hyperalgesia and cold hypersensitivity, spontaneous motor activity. Knee diameter was taken on day 0 and 21. On day 21, after 3 h of EPE administration rats of all groups were sacrificed by cervical dislocation and right knee was collected for histopathological examination.
Behavioural assessment of nociception: Pain assessment was done on days 0 (before EPE/etoricoxib/vehicle or normal saline administration), 7, 14, and 21. Rats were habituated to the testing situation for at least one week before the start of experiment and 5 to 10 min before each testing until the exploration activity ceases.
Secondary mechanical hyperalgesia: Secondary mechanical hyperalgesia was assessed by the Randall- Selitto test15 using an analgesimeter/Randall Selitto paw pressure (Ugo Basile, Varese, Italy) device. The rats were handled carefully and partly restrained. The hind paw was placed on platform under a cone-shaped pusher and then linearly increasing mechanical force was applied. The paw withdrawal threshold (PWT) was defined as the pressure in g at which the rat pulled out its paw. The cut-off pressure was set to 250 g.
Heat hyperalgesia: Sensitivity to heat was determined by measuring paw flick latency to infrared heat source (Tail Flick unit, Ugo Basil, Varese, Italy). Planter side of paw was placed above the heat source and paw withdrawal latency was noted in seconds. A maximum cut-off time of 20 sec was used to minimize paw damage. Measurements were taken 2-3 times with at least 5 min gaps between the tests.
Cold hypersensitivity: Paw withdrawal latency was recorded in sec by submerging ipsilateral and contralateral paw in cold water (4±1°C). Measurements were taken 2-3 times with at least 5 min gaps between the tests.
Spontaneous motor activity: Horizontal and vertical spontaneous motor activity of rats of each group was accessed by automatic activity cage (Ugo Basil, Varese, Italy) before recording pain parameters. An infrared beam cage, which consists of an animal cage of clear perspex, complete with two sets of emitter/sensor arrays for horizontal and vertical activity, respectively. The numbers of interruptions in beam path animal make while moving or standing on hind limbs are counted during five minutes.
Histopathological analysis: The right knee joints were dissected, fixed, and decalcified in 10 per cent formic acid for 72 h. The tissue sections (10 μm) were subjected to hematoxylin and eosin (H&E) staining for routine histological examination.
Statistical analysis: Statistical analysis of data was done by GraphPad InStat software version 4.00 (San Diego, California, USA) using one way ANOVA with Tukey multiple comparison. All groups were compared with each other for significance.
Results
The concentration of saponin was highest (57.3 mg/g extract) followed closely by total phenols (47.6 mg/g extract). Total concentration of tannins and flavonoids was almost similar (13.72 and 13.1 mg/g extract, respectively).
Mechanical hyperalgesia: Paw withdrawal threshold (PWT) values of right paw of different groups on days 0, 7, 14 and 21 are summarized in Table I. Significant mechanical hyperalgesia was observed in ipsilateral hind paw in MIA-injected animals at all observed time points (days 7, 14 and 21) as compared to naïve group of rats. Treatment of MIA-injected rats with EPE 30, 100 and 300 mg/kg and etoricoxib 10 mg/kg induced a significant increase in PWT compared to MIA-injected vehicle - treated animals on days 7, 14 and 21.
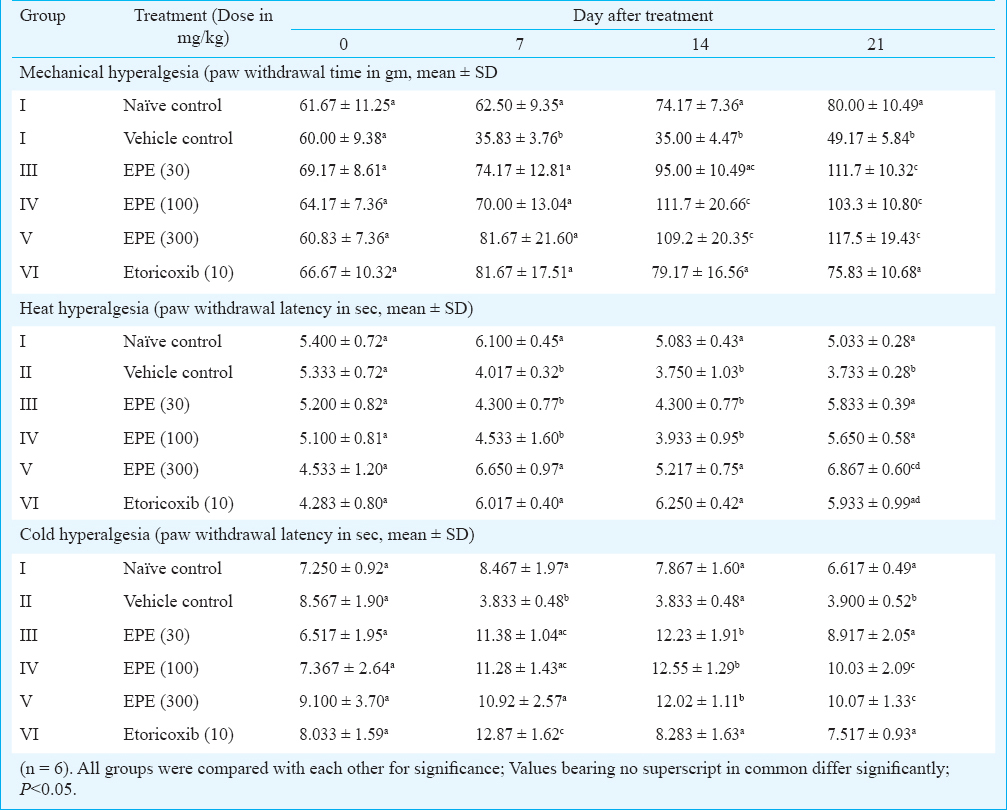
Heat hyperalgesia: Paw withdrawal latency (PWL) in seconds to noxious heat of different groups on days 0, 7, 14 and 21 time points is summarized in Table I. On day 0, right paw withdrawal latency to noxious heat of different groups did not differ significantly. On days 7, 14 and 21, significant (P<0.05) heat hyperalgesia was observed in vehicle-treated control following MIA injection in right joint as compared to naïve control. EPE 300 mg/kg and etoricoxib 10 mg/kg significantly increased paw withdrawal latency as compared to vehicle control rats on days 7 and 14. All doses of EPE employed in this study and etoricoxib 10 mg/kg significantly increased paw withdrawal on day 21 of observation as compared to vehicle-treated rats.
Cold hyperalgesia: Table I shows paw withdrawal latency (PWL) in seconds to noxious cold of different groups on days 0, 7, 14 and 21. PWL of right paw to noxious cold (4°C ±1.0) was similar in all groups at day 0 before MIA administration in right knee joint. On days 7 and 21, significant hyperalgesia to cold developed in vehicle-treated control group of rats as compared to naïve control but not on day 14 of MIA injection. Significant hyperalgesia was reduced on these days following EPE administration at 30, 100 and 300 mg/kg doses and also etoricoxib.
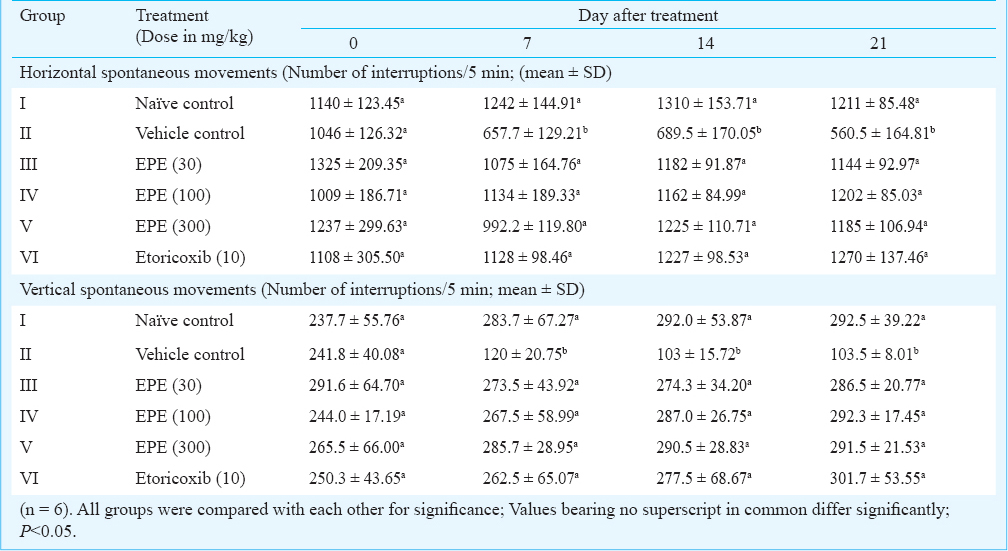
Spontaneous movements: Horizontal spontaneous movements (HSM) of all groups did not differ significantly on day 0. A significant decrease in movements was found in vehicle control on days 7, 14 and 21 whereas values were normal in other groups as compared with naïve control data (Table II). Vertical spontaneous movements (VSM) of all groups of rats did not differ significantly on day 0, but a significant decrease in VSM was found in vehicle control on days 0, 7, 14 and 21 whereas in other treatment groups, number of movements did not differ significantly compared with naïve control on these time points. This increase in movements in treatment groups corresponded to decrease in pain (hyperalgesia) (Table II).
Knee diameter: Knee diameters were taken on days 0 and 21 of all the groups. Data of knee diameter on these time points did not differ significantly on day 0 and further on day 21 indicating no inflammatory oedema at day 21 of the experiment (data not shown).
Histopathology: Cartilage of naive control rats showed intact superficial, mid and deep zones (from top to bottom of image). The chondrocytes were arranged in columns (Figs 1A and B). In MIA–treated vehicle control rats, in articular joint, articular cartilages of tibia as well as femur were completely absent in most the rats and replaced by fibrocartilage (Figs 1C and 1D). Bony tissue underneath fibrocartilage was found to be irregular in thickness and showed discontinuity at places. Proliferation of synovium was also observed and in some animals, it was so severe that complete synovial cavity was filled by fibrous tissue.
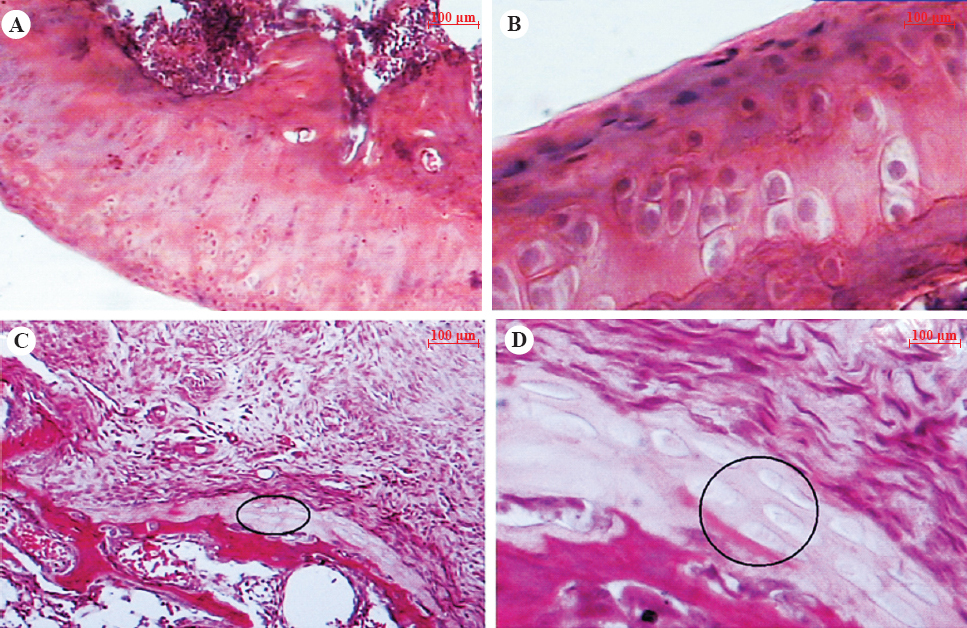
- Hematoxylin and eosin stained femoral articular cartilage of naïve control rats (A, 10x; B, 40x) showing the normal histology having smooth surface with intact superficial mid and deep zones, whereas vehicle-treated MIA rats cartilage (C, 10x; D, 40x) showing severely damaged cartilage with widespread cell necrosis, replaced with fibrocartilage (circle), irregularly arranged and clustering of chondrocytes.
Histological changes observed in MIA-treated group of rats with lowest dose of EPE (30 mg/kg body weight) were identical to MIA- treated vehicle control group. Ameliorative effect was not seen in this group. Ameliorative effect was seen in Group IV where osteoarthritic rats were treated with EPE at a dose of 100 mg/kg body weight continuously for 21 days. A clear transition from fibrocartilage to cartilage formation was seen in this group (Figs 2A and B). In most of the animals complete articular cartilage was formed though it was hypocellular. EPE treatment (300 mg/kg body weight) distinctly prevented the damage to cartilage structure and reduced the cellular abnormalities. Articular cartilage of both femur and tibia in rats of group V were almost found to be normal with well-developed smooth surface, articular cartilage containing chondrocytes distributed individually or arranged in column (Figs 2C and D).
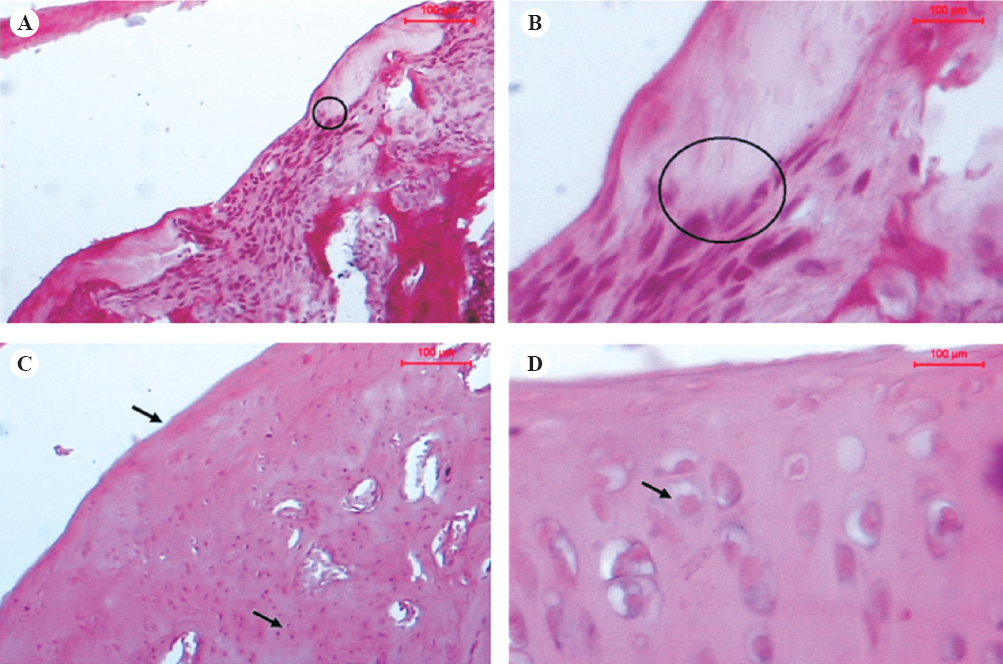
- Hematoxylin and eosin stained femoral articular cartilage of 100 mg/kg EPE treated rats showing transition of fibrocartilage (circle) to acellular cartilage (A, 10x; B, 40x) and 300 mg/kg EPE treated rats (C, 10x; D, 40x) showing reduced joint cartilage damage and cartilage surface with well developed superficial, mid and deep zones (arrows).
In rats treated with etoricoxib (group VI), articular surface (both femur and tibia) showed transition phase from fibrocartilage to cartilage formation. Degree of damage of articular cartilage was mild to moderate type. In some animals, fibrocartilage was found to be converted into cartilage but at some areas patches of fibrocartilage were present. Newly formed cartilage was acellular. Synovial membrane proliferation was present in articular cartilage. Protection was also seen in etoricoxib-treated group of rats.
Discussion
The present results showed development of secondary mechanical and heat hyperalgesia at all time points i.e. 7, 14 and 21 days post MIA administration while cold hyperalgesia developed at the end of study i.e. 21 days post MIA treatment. Little is known regarding the exact mechanisms responsible for MIA-induced joint pain but it may be related to early inflammatory reactions in the joint. The acute inflammatory response in the MIA model lasts approximately three days during which time trophic factors are likely to be released into the joint that upregulate pro-nociceptive receptor expression leading to a reduction in mechanosensory threshold16. According to Beyreuther and co worker17 during the first weak, pain is induced mainly by inflammation in the iodoacetate model, but afterward inflammation plays a minor role in pain and it is more likely caused by biomechanical forces affecting articular cartilage and subchondral bone. Therefore, in the present study, three time points 7, 14 and 21 days were taken for pain determination to see the effect of EPE and etoricoxib on both inflammatory phase and neuropathic phase.
Our results demonstrated that etoricoxib mitigated secondary mechanical and heat hyperalgesia at all time points of observation and also mitigated cold hyperalgesia developed on day 21. Though data regarding use of etoricoxib in MIA- induced osteoarthritic pain model are not available, another coxib, celecoxib has been found to reduce mechanical secondary hyperalgesia in this model in early inflammatory phase and in the last phase of study, except for 18th and 23rd days of experiment18. E. pursaetha is a novel plant and no information is available regarding its mechanism of action in OA. EPE might have reduced production of inflammatory mediators responsible for peripheral and central pain sensitization and hyperalgesia in MIA model of OA.
The reduction of spontaneous activity by adjuvant (RSAA) as an objective and quantifiable behavioural model of inflammatory pain that can predict the analgesic activity of a variety of agents following single-dose administration of complete Freund's adjuvant had been noted19. Thus, RSAA model operationally defines analgesia as a drug-induced increase in spontaneous behaviour (vertical rearing in a novel environment) and is valuable as an objective measure of analgesic efficacy that is not dependent on an evoked stimulus response20. Such observations have prompted us to investigate the effect of EPE on spontaneous motor activity in MIA-induced osteoarthritis pain in this study Due to reduction of hyperalgesia by etoricoxib and EPE, horizontal and vertical spontaneous movements of rats were brought to normal in treatment group as compared to MIA-treated group suggesting lack of depressant effect of EPE on CNS and the anti-hyperalgesic effects of EPE were observed in the absence of any locomotor impairment. The ethanolic extract of E. pursaetha administered orally did not cause acute (24 h) and subacute (28 days) toxicities in the albino rats up to 2000 mg/kg in an earlier study suggesting that it is quite safe for long term use21.
In the present study, histological examination showed that intra-articular injection of MIA in rat knee joint resulted in cartilage matrix degradation and chondrocyte disorganisation. EPE at a dose of 300 mg/kg mitigated MIA effect and at 100 mg/kg showed protective effect. Etoricoxib also exhibited protective effect.
The pathogenesis and source of pain in OA are uncertain. Although articular cartilage is primarily affected by the disease, it does not contain nerve fibres. Additional anatomic structures that have been proposed as potential sources of pain include the synovial capsule/membrane, menisci and subchondral bone22. Since subchondral bone is richly innervated, it is important to understand how the occurrence and progression of bone lesions relate to the onset of joint pain. In one study13 evidence of subchondral bone involvement was apparent as early as seven days post-MIA. The changes are consistent with the initiation of bone remodelling and may have been induced by increased load in the subchondral bone due to the advanced loss of cartilage. Though we did not study histopathology of joint at seven days post MIA injection, it is hypothesized that pain and secondary hyperalgesia observed at this time point could be due to histopathological changes suggested by Guzman and coworkers20. Inhibition of osteoclastic activity when initiated early leads to improved efficacy23. Osteoclastic activity was decreased at day 21 with 300 mg/kg dose of EPE accounting for relief of pain at this time.
Results obtained in the present study revealed that total phenolic compounds in the ethanol extract of the stem of E. pursaetha were considerably high. Polyphenolic compounds are known to have antioxidant activity and it is likely that the activity of the extract is due to these compounds2425. Oxidative stress is known to be associated with the development of OA and the beneficial therapeutic effects of antioxidants such as vitamin E and vitamin C have been demonstrated in animal studies of OA 26. Total flavanoid, tannin and saponin contents were found to have anti-osteoarthritic effect in different studies 272829 and our study findings suggested that EPE treatment led to a significant reduction in pain and structural changes in MIA- induced OA in rats.
In conclusion, the results demonstrated that EPE was not only able to mitigate pain and hyperalgesia but also inhibited MIA-induced cartilage degeneration in vivo. The disease modifying activity of EPE in osteoarthritis may provide a lead for a new treatment modality for osteoarthritis.
Acknowledgment
The authors thank Director and Joint Director (Academic), Indian Veterinary Research Institute, Izatnagar, Bareilly, for financial support.
References
- Characterization of articular cartilage and subchondral bone changes in the rat anterior cruciate ligament transection and meniscectomized models of osteoarthritis. Bone. 2006;38:234-43.
- [Google Scholar]
- Proteinases in the joint: clinical relevance of proteinases in joint destruction. Arthritis Res Ther. 2007;9:221-30.
- [Google Scholar]
- Changing the outcome of osteoarthritis: Still a challenge for cyclooxygenase 2 inhibitors. Arthritis Rheum. 2012;64:37-9.
- [Google Scholar]
- Effects of diacerein at the molecular level in the osteoarthritis disease process. Ther Adv Musculoskelet Dis. 2010;2:95-104.
- [Google Scholar]
- The monosodium iodoacetate model of osteoarthritis: a model of chronic nociceptive pain in rats? Neurosci Lett. 2004;370:236-40.
- [Google Scholar]
- Entada pursaetha. In: Chopra's Indigenous drug of India (1st ed). Kolkata: Academic Publisher; 2006. p. :334-5. (reprint)
- [Google Scholar]
- Screening of Indian plants for biological activity: Part VI. Indian J Exp Biol. 1977;15:208-19.
- [Google Scholar]
- Phenolic content and antioxidant activity of olive extracts. Food Chem. 2001;73:73-84.
- [Google Scholar]
- Spectrophotometric estimation of total tannins in some ayurvedic eye drops. Indian J Pharm Sci. 2007;69:574-6.
- [Google Scholar]
- Estimation of total flavonoids content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10:178-82.
- [Google Scholar]
- Color reaction of some sapogenins and saponins with vanillin and sulfuric acid. Planta med. 1976;29:116-22.
- [Google Scholar]
- Evaluation of pain behavior and bone destruction in two arthritic models in guinea pig and rat. Pharmacol Biochem Behav. 2007;87:349-59.
- [Google Scholar]
- Effect of iNOS inhibitor S-methylisothiourea in monosodium iodoacetate-induced osteoathritic pain: implication for osteoarthritis therapy. Pharmacol Biochem Behav. 2013;103:764-72.
- [Google Scholar]
- A method for measurement of analgesic activity of inflamed tissue. Arch Int Pharmacodyn Ther. 1957;111:209-19.
- [Google Scholar]
- Grading of monosodium iodoacetate-induced osteoarthritis reveals a concentration-dependent sensitization of nociceptors in the knee joint of the rat. Neurosci Lett. 2009;13:184-8.
- [Google Scholar]
- Antinociceptive efficacy of lacosamide in the monosodium iodoacetate rat model for osteoarthritis pain. Arthritis Res Ther. 2007;9:R14.
- [Google Scholar]
- Gait analysis and pain response of two rodent models of osteoarthritis. Pharmacol Biochem Behav. 2011;97:603-10.
- [Google Scholar]
- Inflammation-induced reduction of spontaneous activity by adjuvant: A novel model to study the effect of analgesics in rats. J Pharmacol Exp Ther. 2007;320:194-201.
- [Google Scholar]
- Mono-iodoacetate-induced histologic changes in subchondral bone and articular cartilage of rat femorotibial joints: an animal model of osteoarthritis. Toxicol Pathol. 2003;31:619-24.
- [Google Scholar]
- Evaluation of acute and sub acute toxicity of ethanol extracts of Entada pursaetha, Toddalia aculeata, and Ziziphus mauritiana. World J Life Sci Med Res. 2011;1:43-7.
- [Google Scholar]
- Pain mechanisms in osteoarthritis of the knee: effect of intra-articular anesthetic. J Rheumatol. 1966;23:1031-6.
- [Google Scholar]
- Inhibition of osteoclasts prevents cartilage loss and pain in a rat model of degenerative joint disease. Osteoarthr Cartil. 2010;18:1319-28.
- [Google Scholar]
- Food phytochemicals for cancer prevention II. In: Ho C, Osawa T, Huang MT, Rosen RT, eds. Chemistry and antioxidative effects of phenolic compounds from licorice, tea and Compositae and Labiateae herbs. Washington, DC: American Chemical Society; 1994. p. :132-43.
- [Google Scholar]
- Screening of the antioxidant potentials of six Salvia species from Turkey. Food Chem. 2006;95:200-4.
- [Google Scholar]
- The antioxidant vitamins A, C, E and selenium in the treatment of arthritis: a systematic review of randomized clinical trials. Rheumatology. 2007;46:1223-33.
- [Google Scholar]
- Oral intake of purple passion fruit peel extract reduces pain and stiffness and improves physical function in adult patients with knee osteoarthritis. Nutr Res. 2010;30:601-6.
- [Google Scholar]
- Therapeutic effect of the saponin fraction from Clematis chinensis Osbeck roots on osteoarthritis induced by monosodium iodoacetate through protecting articular cartilage. Phytother Res. 2010;24:538-46.
- [Google Scholar]






