Translate this page into:
Differential induction of Toll-like receptors & type 1 interferons by Sabin attenuated & wild type 1 polioviruses in human neuronal cells
Reprint requests: Dr Madhu C. Mohanty, Enterovirus Research Center (ICMR), Haffkine Institute Campus, Acharya Donde Marg, Parel, Mumbai 400 012, India e-mail: mohantymc@icmr.org.in
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Polioviruses are the causative agent of paralytic poliomyelitis. Attenuated polioviruses (Sabin oral poliovirus vaccine strains) do not replicate efficiently in neurons as compared to the wild type polioviruses and therefore do not cause disease. This study was aimed to investigate the differential host immune response to wild type 1 poliovirus (wild PV) and Sabin attenuated type 1 poliovirus (Sabin PV) in cultured human neuronal cells.
Methods:
By using flow cytometry and real time PCR methods we examined host innate immune responses and compared the role of toll like receptors (TLRs) and cytoplasmic RNA helicases in cultured human neuronal cells (SK-N-SH) infected with Sabin PV and wild PV.
Results:
Human neuronal cells expressed very low levels of TLRs constitutively. Sabin PV infection induced significantly higher expression of TLR3, TLR7 and melanoma differentiation-associated protein-5 (MDA-5) m-RNA in neuronal cells at the beginning of infection (up to 4 h) as compared to wild PV. Further, Sabin PV also induced the expression of interferon α/β at early time point of infection. The induced expression of IFN α/β gene by Sabin PV in neuronal cells could be suppressed by inhibiting TLR7.
Interpretation & conclusions:
Neuronal cell innate immune response to Sabin and wild polioviruses differ significantly for TLR3, TLR7, MDA5 and type 1 interferons. Effects of TLR7 activation and interferon production and Sabin virus replication in neuronal cells need to be actively investigated in future studies.
Keywords
Innate immune response
interferons
poliomyelitis
RLRs
Sabin attenuated virus
TLRs
Poliovirus (PV) a member of the genus Enterovirus, Family Picornaviridae is the causative agent of paralytic poliomyelitis1. Man is the only natural host of poliovirus and less than 1 per cent infections of poliovirus result in invasion of the central nervous system. Nerve cell damage and inflammatory reactions are localized mainly in the motor neurons in the anterior horn of the spinal cord and neurons in the brainstem, resulting in acute flaccid paralysis2. Importantly, pathological changes are not observed in non-neural tissues. This tissue tropism may be determined by the interaction between the host and viral factor3.
Sabin developed the live attenuated oral poliovirus vaccine strains of the three poliovirus types by multiple passages through monkey kidney cell cultures maintained at sub optimal temperatures (33-34°C), plaque purification and selection of strains of progressively lowered neurovirulence in monkeys4. Although poliovirus attenuation has been studied extensively at the molecular level, yet the basis of this paradoxical change in infectivity of Sabin strains to neuronal cells is not completely understood. Single point mutations at nucleotide position 480 in type 1, 481 in type 2, and 472 in type 3 Sabin vaccine viruses were identified to have strong attenuating effect in monkey and transgenic mouse models5. The Sabin vaccine strains do not replicate as efficiently as the wild type polioviruses in human central nervous system (CNS) and in monkeys inoculated intraspinally and intracerebrally6. However, both Sabin attenuated and wild type polioviruses grow equally well in non-neural cells in vitro.
Polioviruses use CD155, a member of the immunoglobulin super family, as the sole cell surface receptor for cell entry. CD155 is, therefore, also known as human poliovirus receptor (PVR). Transgenic mice expressing human PVR serve as an animal model for the study of poliovirus pathogenesis7. In transgenic mice PVR was found to be necessary for poliovirus infection but not as the sole determinant of tissue tropism8. Resistance of non-neuronal cells expressing PVR transcripts to poliovirus infection led to the conclusion that unknown host factors played significant role in productive poliovirus infection3. Poliovirus replication in extra-neural tissues in PVR-transgenic/Ifnar knockout mice suggested that type 1 interferon (IFN) response plays an important role in poliovirus infection3. In experimentally infected non-human primates, poliovirus produces paralytic disease similar to man9. Though polioviruses produce pathological changes only in motor neurons, a variety of cell types of human and monkey origins can be infected in vitro. Cultured monkey kidney cells do not produce type 1 IFN, hence become susceptible to productive poliovirus infection. In vivo, however, rapid IFN response is normally maintained in extra-neural tissues310.
Innate immune response may play crucial role in the outcome of enteroviral infections. Recent reports show that Enteroviruses (EV) can stimulate production of type 1 IFN through endosomal Toll-like receptors (TLR) like TLR7 and TLR81112. TLR3 and TLR4 play a role in pathogenesis of coxsackievirus B4 and EV711314. Cytoplasmic RNA helicases like melanoma differentiation associated protein 5 (MDA5) and retinoic acid inducible gene 1 (RIG I) which are known to activate production of type 1 IFN are degraded in poliovirus infected cells1516.
We hypothesized that innate immune mechanisms of neuronal cells recognize and respond differently to wild and vaccine poliovirus infections. Here we report on expression and regulation of antiviral pattern recognition molecules and induction of antiviral mediators in cultured human neuronal cells infected with a poliovirus type 1 wild type isolate (wild PV) and Sabin PV attenuated vaccine virus (Sabin PV). Flow cytometry and quantitative real time PCR were used to detect and quantify cellular responses to poliovirus infection.
Material & Methods
This study was conducted in the Enterovirus Research Centre, Mumbai, India.
Cells and viruses: SK-N-SH (human neuroblastoma) cells were obtained from National Centre for Cell Science (NCCS, Pune, India), RD (Human Rhabdomyosarcoma) cells were obtained from CDC, Atlanta. The cells were maintained in Eagles MEM (minimum essential medium, Sigma, USA) with 2 mM L-glutamine and Earle's BSS (balanced salt solution) adjusted to contain 1.5 g/l sodium bicarbonate with 10 per cent foetal calf serum (FCS); 0.1 mM non-essential amino acids and 1.0 mM sodium pyruvate were added in case of SK-N-SH cells.
Wild poliovirus type 1 (wild PV) used in these studies was isolated from a case of acute flaccid paralysis in our laboratory according to the standard methodology recommended by WHO17. Sabin oral poliovirus vaccine strain (Sabin PV) was obtained from National Institute for Biological Standards and Control (NIBSC), Potters Barr, UK. Both viruses were passaged once in SK-N-SH and RD cells to prepare working stocks. Genomic RNA sequencing was used to confirm the viruses as wild type 1 and Sabin PV.
Single step growth curve: The extent of virus replication was determined by single-step growth as follows. Confluent SK-N- SH cells were infected with 10 MOI (Multiplicity of Infection) of virus. After absorption for 45 min, the monolayers were washed with three changes of PBS, incubated in MEM containing 2 per cent FBS and frozen at -20°C at 1, 2, 4, 6, 8, 16, 24 h post-infection (hpi). The cultures were freeze thawed three times and the culture supernatant harvested was centrifuged to remove cell debris. Supernatants were titrated (10 fold serial dilution) to determine virus concentration.
Virus replication in cultured cells: Monolayers of SK-N-SH and RD cells in six-well plates (Nunclon, USA) were infected with wild or Sabin PV at 10 MOI for different time periods at 37°C. Infected cells were washed with PBS at specified time intervals post-infection and collected by cold PBS treatment and centrifugation. The cells were counted and used in real time PCR assay for quantification of TLRs, RLRs (RIG-1 like receptors), type 1 interferons and interferon regulatory factor (IRF) gene expression.
Quantitative real time RT-PCR analysis: Total RNA was isolated from the infected and mock treated cells using the RNAqueous Kit according to the manufacturer's instructions (Ambion Inc., USA). First-strand cDNA synthesis was performed using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems Inc, USA). For quantitative real time PCRanalysis, TaqMan Universal Master mix (Applied Biosystems Inc, USA) and ABI 7500 Real-Time PCR System was used3. Primers and fluorescein amidile (FAM)-labelled probes were obtained from Applied Biosystems (TaqMan Assay on Demand). The following probes and primers were used: TLR2 (Hs00152932_ml), TLR3 (Hs01551078_ml), TLR4 (Hs00152939_ml), TLR7 (Hs01933259_Sl), TLR8 (Hs00607866_ml), TLR9 (Hs0092832_ml), Interferon induced with helicase C domain 1 (IFIH-1) (Hs00223420_m1), Retinoic acid receptor responder protein 3 (RARRES3) (Hs00184937_m1), IRF-3 (Hs01547283_m1), IRF-7 (Hs00185375_m1), IFNA1 (Hs00256882_s1), IFNB1 (Hs02621190_s1). The amount of mRNA was determined by comparison with standard templates of cloned cDNA of known copy number. The expression levels were then normalized to the level of GAPDH (glyceraldehyde 3 phosphate dehydrogenase).
Flow cytometry: Uninfected SK-N-SH cells were analyzed for expression of neuronal growth factor receptor (NGFR) by surface staining. The expression of TLR proteins on neuronal cells as well as muscle cells were analyzed by intracellular flowcytometry. For intracellular staining, uninfected and infected cells were washed once with PBS and fixed in 4 per cent paraformaldehyde for 30 min at room temperature. Cells were then washed and blocked with 3 per cent bovine serum albumin (BSA) for 30 min. After washing with PBS, the cells were permeabilized with PERM wash (BD, USA) for 20 min and incubated with antihuman TLR antibodies coupled to FITC (fluorescein isothiocyanate), for 30 min at 4°C in the presence of permeabilization and wash buffer. Appropriate isotype controls were included in each experiment. After incubation, cells were washed twice with permeabilization and wash buffer and resuspended in wash buffer for analysis. Cells were analyzed using FACSaria (Beckton Dickinson), DIVA and Flowjo software (Tree Star, USA).
Serotype specific poliovirus type 1 monoclonal antibody was procured from Light Diagnostics, Millipore, Temecula, CA (cat no. 3331). Anti-mouse Alexaflour 647 was from Molecular Probes. FITC conjugated anti-human TLR antibodies and monoclonal antibodies to TLRs were purchased from Imgenex, India. The PE conjugated NGFR was procured from BD Biosciences (San Jose, USA).
Statistical analysis: Each experiment was performed at least three times. Differences in gene expression was compared by using the Student‘t’ test. Sigma plot software (version 10.0.1, Systat software Inc, USA) was used for all statistical analysis.
Results
Human neuronal cells express low levels of TLR3 and TLR7 constitutively: Neuronal origin of the SK-N-SH cells used in this study was confirmed by flow cytometry staining with phycoerythrin (PE) conjugated NGFR. As shown in Fig. 1, 15.8 per cent of SK-N-SH cells expressed NGFR.
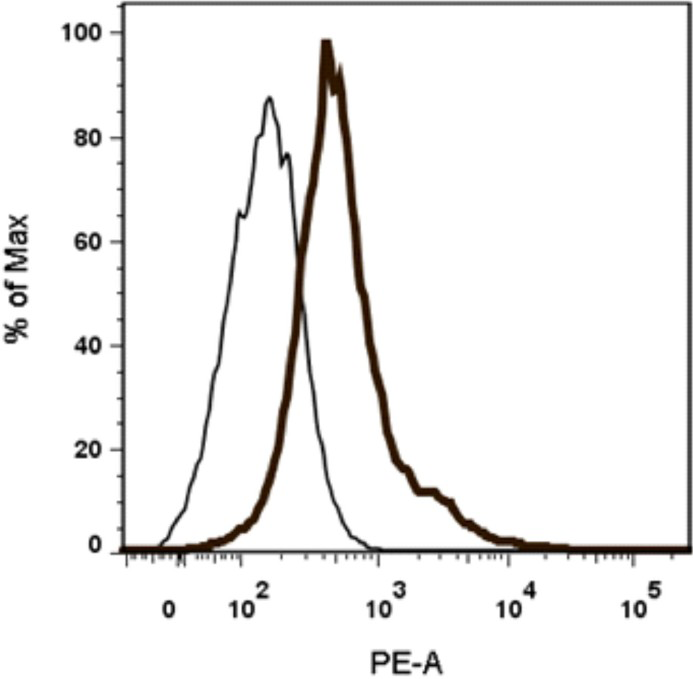
- Expression of neuronal growth factor receptor (NGFR) in SK-N-SH cells. SK-N-SH cells were stained with anti-human NGFR antibody coupled to phycoerythrin (PE) along with isotype matched control. Thin and thick line histograms represent the cells stained with isotype matched control and NGFR-PE, respectively.
Constitutive expression of TLR3, TLR7 and TLR8 was studied in SK-N-SH cells and RD cells by intracellular flow cytometry. It was concluded that TLR 3 and TLR7 were expressed constitutively at low levels in the neuronal origin SK-N-SH cells as compared to RD cells (Fig. 2A and B). SK-N-SH cells did not express TLR8.
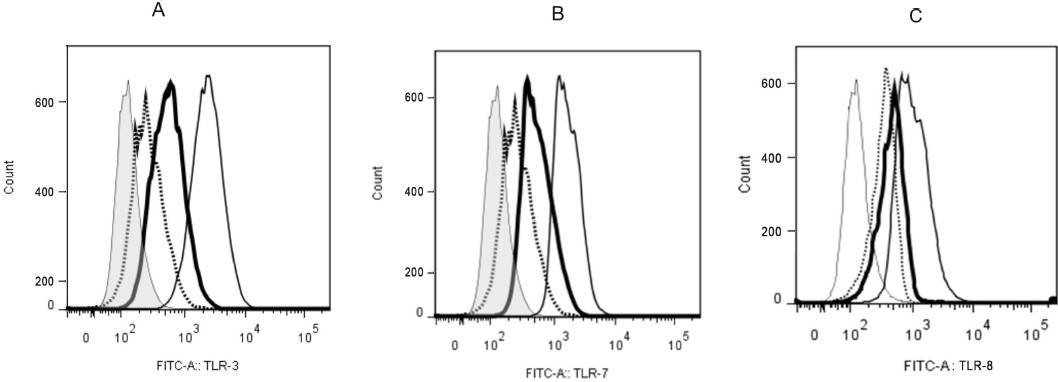
- Constitutive expression of TLR3 and TLR7 in SK-N-SH cells. SK-N-SH and RD cells were processed for intracellular flow cytometry analysis. Cells were incubated with (A) anti-human TLR3 FITC or (B) anti-human TLR7 FITC or (C) anti-human TLR8 FITC along with isotype controls. Thin and normal line histograms represent RD cells stained with isotype matched control and anti-human TLR3 or anti-human TLR7 or anti-human TLR8 FITC mAbs, respectively. Dotted and heavy lines represent SK-N-SH cells stained with isotype control and anti-human TLR3 or anti-human TLR7or anti-human TLR8 FITCmAbs, respectively.
Human neuronal cells are more susceptible to wild PV infection than Sabin PV: In order to assess and compare the infectivity pattern of the wild PV and Sabin PV, SK-N-SH cells were infected with 10 MOI of viruses from both the strains and single step growth curve was generated (Fig. 3). Both the poliovirus strains replicated more or less equally up to 8 hpi. However, the yield of mature infectious virions at the end of 16 hpi was lower by approximately 1 log in Sabin infected cells compare with the wild PV.
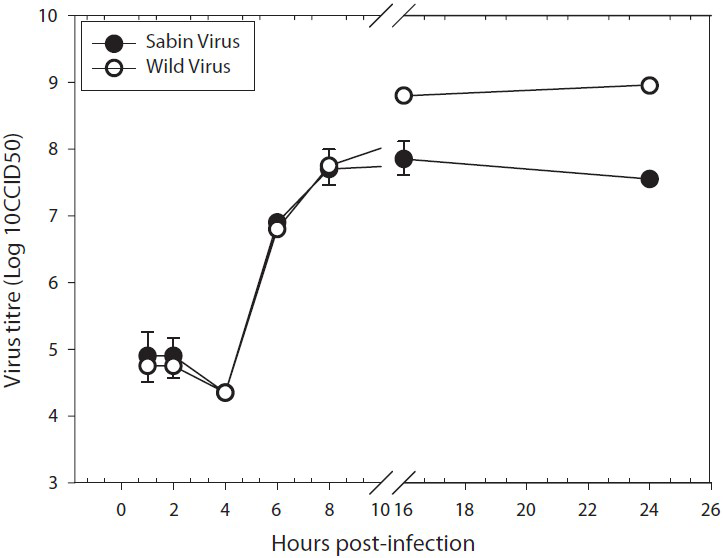
- One step growth curve of Sabin type and wild type polioviruses in human neuronal cell lines. Single-step growth was measured under the infection and culture conditions described in Material & Methods. The curves were plotted based on the mean ± SE of virus titre in three independent experiments for each strain. The virus titre differs significantly at 16 h (P<0.02) and 24 h (P<0.02).
Sabin PV infection upregulates the expression of TLR3, TLR7 and MDA5 in human neuronal cells: SK-N-SH cells were infected with 10 MOI of Sabin PV and wild PV separately. Total RNA harvested at 4 and 8 h post-infection (hpi) was used to determine TLR3, TLR7, MDA5 and RIGI specific mRNA expression by quantitative real time PCR (Fig. 4A-D).
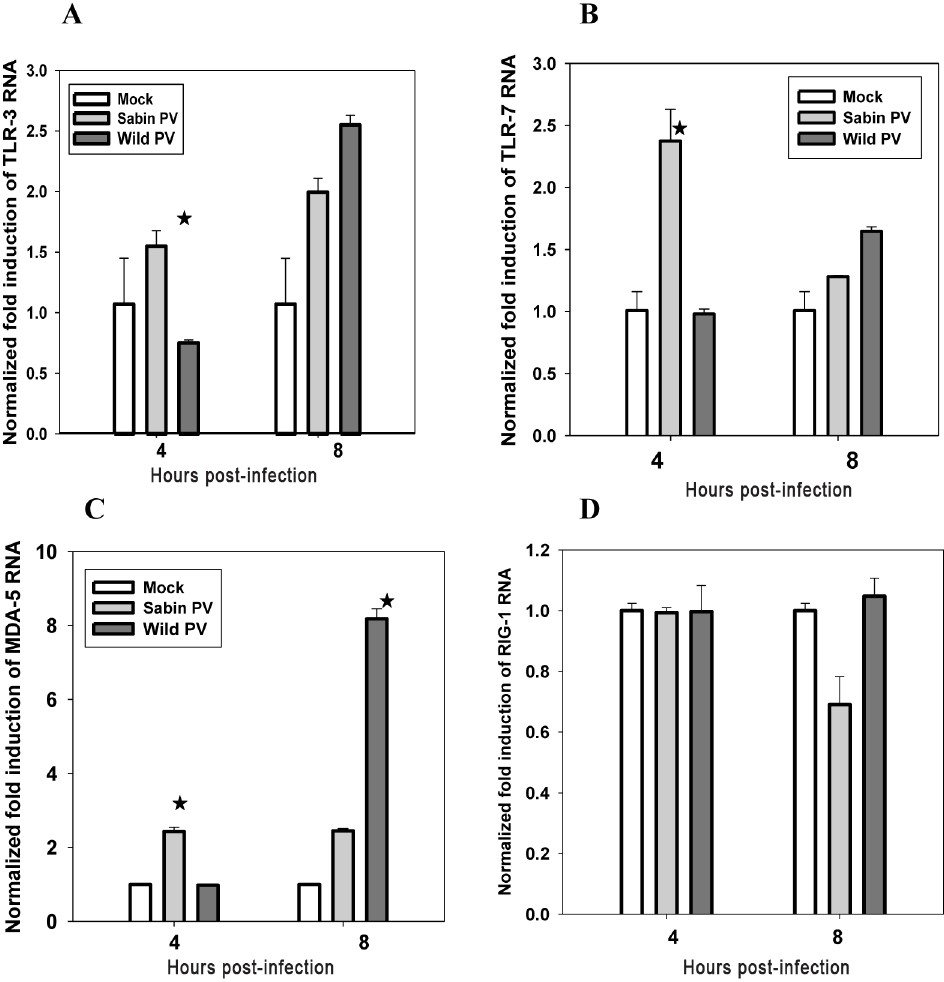
- Increased m-RNA expression of (A) TLR3, (B) TLR7, (C) MDA-5, and (D) RIGI following Sabin PV infection of neuronal cells at early stages analyzed by real time PCR method. Data are presented as the fold induction of each gene of interest relative to uninfected control. Data are (mean ± SE) representative of three independent experiments. The asterisks indicate P<0.001 (A, B and C) between expression by Sabin and wild PV.
At 4hpi, TLR3 specific mRNA expression was 1.5 fold (elevated) in Sabin1 and 0.7 fold (decreased) in wild PV infected cells as compared with mock-infected cells. At 8 hpi, TLR3 expression was 2 and 2.5 fold elevated in Sabin PV and wild PV infected cells, respectively. Expression of TLR3 in Sabin PV infected cells increased marginally but was substantially elevated in wild PV infected cells between 4 and 8 hpi (Fig. 4A).
TLR7 expression was induced rapidly (2.5 fold) at 4 hpi in Sabin PV infected cells but either remained unchanged or decreased in wild PV infected cells. TLR7 response decreased to the level of uninfected cells in Sabin PV infected cells at 8 hpi but was found to have increased in wild PV infected cells (Fig. 4B).
Thus it was concluded that neuronal cells rapidly induced TLR3 and TLR7 expression in response to Sabin PV infection. In wild PV infected cells the response was delayed to late stages of virus infection.
MDA5 mRNA was induced in Sabin PV infected cells at 4hpi and remained elevated throughout the infection cycle. In wild PV infected cells MDA5 mRNA expression was delayed until after 4 hpi and reached very high levels at 8 hpi (Fig. 4C).
We did not observe any significant difference in RIGI expression in uninfected and Sabin PV or Wild PV infected neuronal cells up to 8 hpi (Fig. 4D).
Induction of type I IFN in neuronal cells infected with Sabin PV at early stages of infection is faster than wild PV infection: Total RNA harvested from Sabin PV and wild PV infected SK-N-SH cells at 4 and 8 hpi were tested for expression levels of IFNα and IFNβ by quantitative real time-PCR (Fig. 5A-B). Cells infected with Sabin PV showed a sharp increase (3 fold) in IFNα and IFNβ mRNA levels within 4 hpi. In contrast, only marginal increase (1.2 fold) was found in Wild PV infected cells. At 8 hpi, IFN levels had decreased in Sabin PV infected cells but remained elevated in wild PV infected cells. The difference in expression of IFN genes in Sabin PV and Wild PV infected cells was significant (P<0.01) at 4hpi. Thus neuronal cells respond by rapid and large-scale expression of type 1 IFN genes early during Sabin PV infection but the same response is delayed in wild PV infection.
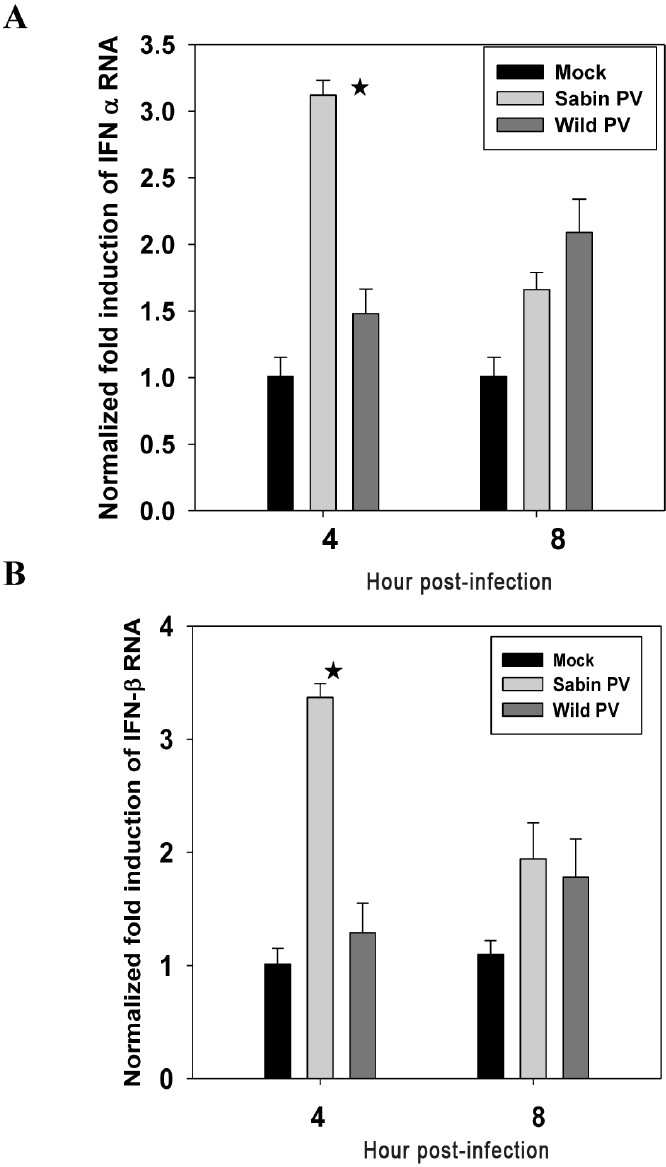
- Induction of (A) IFNα and (B) IFNβ expression following wild PV and Sabin PV infection of neuronal cells at early stages of infection analyzed by real time PCR method. Data are presented as the fold induction of each gene of interest relative to uninfected control. Data are (mean ± SE) representative of three independent experiments. The asterisks indicate P<0.01 between expression by Sabin PV and wild PV at 4 hpi.
Blockade of Sabin PV induced type I IFN response by antibodies to TLR7 in human neuronal cells at early stages of infection: Since this study was focused on early phase response to poliovirus infection, TLR7 was chosen for further study based on the observation that, unlike TLR3 and MDA5 (remain elevated up to 6-8 hpi) Sabin PV induced TLR7 was significantly high at 4 hpi only and reduced to basal level at 8 hpi. SK-N-SH cells incubated with anti-TLR7 monoclonal antibodies (1 μg/ml) for 24 h were infected with 10 MOI of Sabin PV and wild PV separately. IFNα and IFNβ mRNA expression was quantified at 4 hpi (Fig. 6A-B). A significant (P<0.05) reduction in IFN genes expression was observed in anti-TLR7 antibody treated Sabin PV infected cells as compared with the untreated Sabin PV infected cells. Only marginal decrease was observed in anti-TLR antibody treated and untreated cells infected with wild PV or uninfected (mock) cells. Thus blocking of TLR7 induction resulted in inhibition of IFN gene expression during Sabin PV infection. Treatment with TLR7 did not affect IFNα expression in wild PV infected cells.
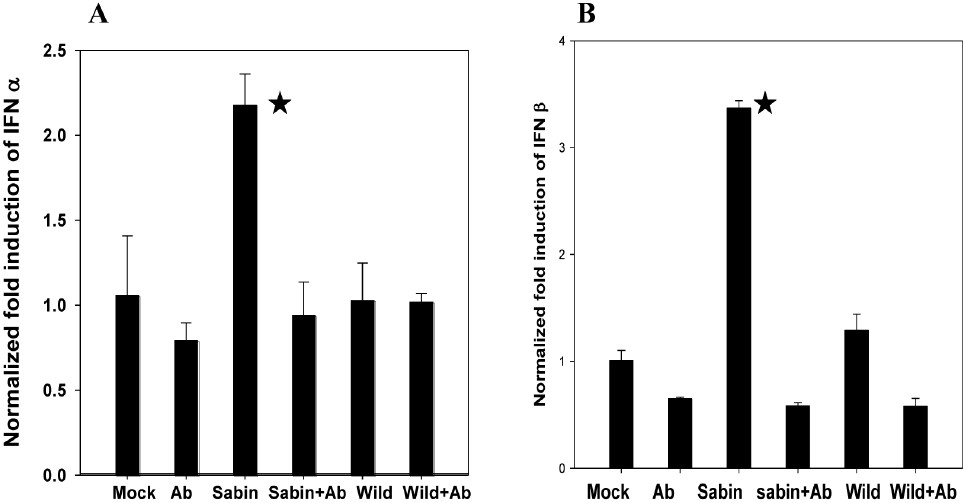
- Effect of antibodies to TLR7 on IFN α/β expression by Sabin PV infected neuronal cells analyzed by real time PCR method. Data are presented as the fold induction of each gene of interest relative to uninfected control. Data are (mean ± SE) representative of three independent experiments. The asterisks indicate (A) P<0.01, (B) P<0.001 between expression by Sabin PV in cells with or without addition of antibody to TLR7 (Sabin PV only vs Sabin PV+ a-TLR7).
Induction of IRF-7 in Sabin PV infected neuronal cells at early stages of infection: Downstream signaling pathways for interferon response were investigated by studying induction of IRF-3 and IRF-7 at early stages of poliovirus infected neuronal cells. No significant increase in IRF-3 levels was observed in response to either wild or attenuated poliovirus infection (Fig. 7A). IRF-7 expression was about 40-fold higher in Sabin PV infected cells and 5 to 7 fold higher in wild PV infected cells as compared with mock infected (uninfected) cells. In other words, IRF-7 expression was 8 to 10 times higher in Sabin1 infected cells as compared with wild PV infected cells.
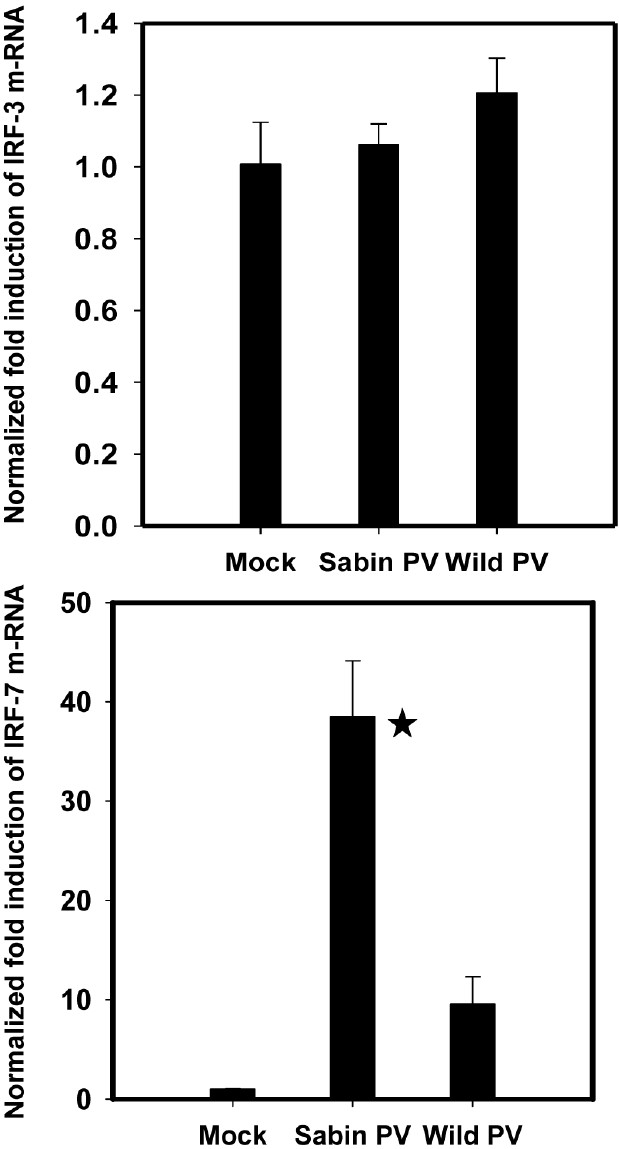
- Expression of (A) IRF-3 and (B) IRF-7 responses following wild PV and Sabin PV infection of neuronal cells at early stages of infection. Values were calculated relative to expression of GAPDH controls. Data are presented as the fold induction of each gene of interest relative to uninfected control. *P<0.01 between expression by Sabin PV and wild PV at 4 hpi.
Early activation of type I IFN by Sabin PV is specific to neuronal cells only: TLR7 and IFN gene expression was studied at 4 and 8hpi in RD cells infected with Sabin PV and wild PV by quantitative real time PCR and flow cytometry. As shown in Fig. 8A, TLR7 response was not induced at early stages of infection (4 hpi) in RD cells infected with either Sabin PV or wild PV1. Also, there was no IFN response to Sabin PV and wild PV infection at this stage of infection in RD cells (Fig. 8B). Both TLR7 and IFN expressions were significantly increased at late stages (8h) of poliovirus infection. Thus, RD cells did not activate the TLR7 and IFN signaling pathways in early stages of poliovirus infection. Differential regulation of attenuated and wild poliovirus infection as observed in neuronal cells was not found in response to RD cells.
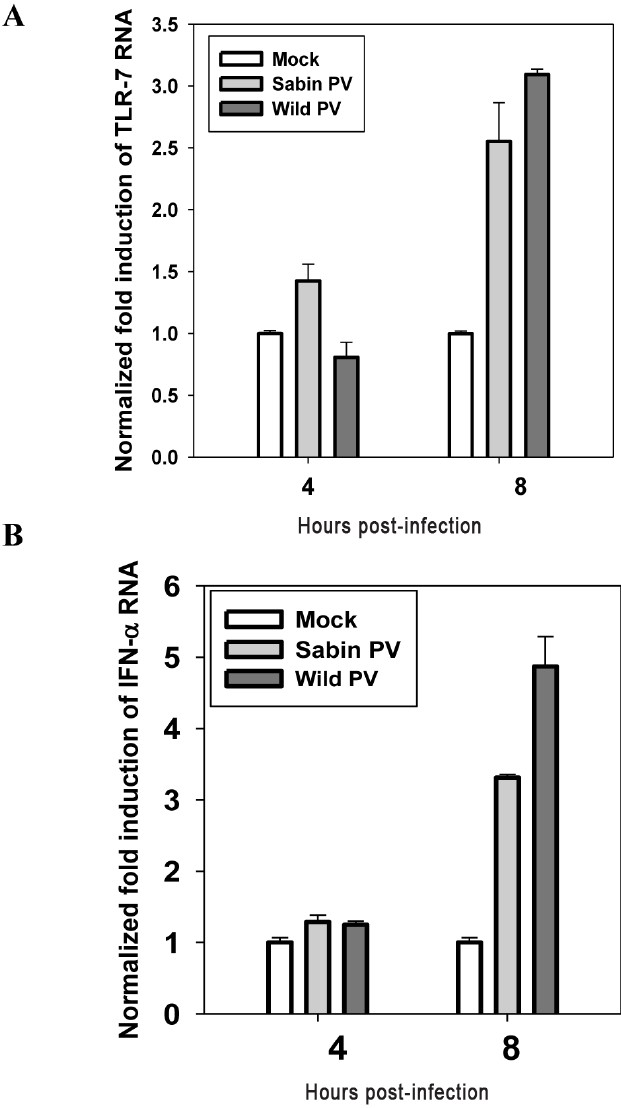
- Expression of (A) TLR7 and (B) IFN-α in RD cell line (human muscle cells) following wild PV and Sabin PV infection as analyzed by real time PCR method. Data are presented as the fold induction of each gene of interest relative to un-infected control. Data are (mean ± SE) representative of three independent experiments. Statistical analysis was completed by Students “t” test.
Discussion
Attenuating mutations in the 5’ noncoding region of poliovirus vaccine strain result in poor interaction of the RNA with translation initiation factors (eIF-2 and eIF-2b) leading to reduced translation and diminished multiplication in neural (SH-SY5Y) cells whereas both the attenuated and the wild type virus replicate equally well in HeLa cells18. The intracellular functions of the mutations have not yet been identified completely, but the data available suggest that the 5’ NTR interact with the cellular factors influencing the tropism of the virus. Cultured cells of human neuroblastoma SK-N-MC were found to be highly resistant to Sabin attenuated poliovirus type 1 and 2 strains, on the other hand related wild type neurovirulent strains produced higher yield with a protracted infectious cycle19. Understanding the intracellular events may eventually provide an explanation as to how the motor neurons become the specific target. The results of our study using neuronal cells are suggestive of differential innate immune response to Sabin and wild PV strains.
Contrary to the older studies suggesting that neurons do not express TLRs, it has been now reported that TLRs are constitutively expressed in the CNS of mouse and human2021. Human microglia also expresses mRNA for TLRs 1-9, whereas astrocytes express robust TLR3, low-level TLR 1, 4, 5, and 922. Our studies on expression of TLRs in SK-N-SH cells by flow cytometry have also shown presence of lower level of TLR 2, 3, 4, 7 and absence of TLR8.
The importance of interferons in poliovirus infection has been shown by injecting single dose of interferon and subsequent intracerebral infection of poliovirus RNA that completely suppressed viral replication23. Cultured monkey kidney cells acquire PV susceptibility by loosing rapid interferon response that has been normally maintained in extra neural tissue24. In a recent study using human PVR transgenic (PVRtg) mice, as well as IPS-1−/− (IFN-β promoter stimulator) and TICAM-1−/− (Toll/IL-1R homology domain-containing adaptor molecule -1) mice it was found that the TLR3/TICAM-1 pathway governs IFN induction and host protection against PV infection25. Although there are no reports available on role of TLRs in poliovirus infection of human neuronal cells, studies in other enteroviruses have shown induction of type 1 interferons by endosomal TLRs1112. In our study, cultured SK-N-SH cells infected with Sabin attenuated PV upregulated the expression of TLR3 and TLR7 at early stage of infection along with significant induction of type 1 interferons as compared to infection with wild PV. Similar studies in rabies viruses have shown that attenuated rabies viruses stimulate expression of many genes including TLR1, TLR2 and TLR3 in the brains of mice but not the wild type virus26. Our data further suggest that TLR7 is one of the critical factors required for early protection in PV infection. Although there was no previous report for protective role of TLR7 in human neurons, the TLR7 response represents a vital host-defense mechanism in a murine model of systemic West Nile virus (WNV) infection27.
Cytoplasmic helicases such as retinoic acid inducible gene-I (RIGI) and melanoma differentiation associated protein (MDA5) are important mediators for sensing intracellular viral nucleic acid. RIGI and MDA5 use the signaling adaptor mitochondrial antiviral signaling (MAVS) to activate transcription factor IRF-3 to induce production of type I IFNs. It has been found that MDA5 and its adaptor molecule MAVS are critical for type I interferon response to coxsackie virus B (CVB) infection in mice28. We observed induction of MDA5 mRNA at early stages following attenuated Sabin PV infection of neuronal cells. In contrast, wild PV did not show any change in MDA5 level up to 4 hpi but increased several fold at 8 hpi. Interestingly, in non-neuronal cells like HeLa cells wild poliovirus has been shown to cleave MDA5 at early infection stage, which could be a mechanism to antagonize production of type I interferon in response to viral infection15. Similarly another cytoplasmic sensor RIGI is also degraded in poliovirus infected HeLa cells16. We did not find any change in RIGI expression up to 4 hpi in Sabin and wild PV infected neuronal cells.
Recently it has been proposed that IRF-3 and IRF-7 both activate the initial phase of IFN α and IFN β gene expression in specific cell types29. IRF-7, which is downstream of TLR7 and TLR8 response, has been suggested as the master transcriptional regulator of type I IFN dependent immune response29. A study on WNV infection in IRF-7-/- mice has shown that IFN-α gene expression and protein production were reduced and virus titres were increased in IRF7-/- primary macrophages, fibroblasts, dendritic cells, and cortical neuron suggesting that the early protective IFN-α response against WNV occurs through an IRF-7-dependent transcriptional signal30. To investigate the downstream pathways involved in IFN mediated signaling and transcription activators, the status of IRF-3 and IRF-7 were analyzed in Sabin PV and wild PV infected cells. Since SK-N-SH cells do not express TLR8, induction of IRF-7 by Sabin PV could be the subsequent downstream activation by TLR7 to upregulate type I IFN. Role of TLR7 was re-confirmed by blocking TLR7 production using antibody treated cells.
Both the host and pathogen factors play role in PV infection of neuronal cells. Our results are consistent with the hypothesis that neuronal cells recognize infection with the Sabin attenuated poliovirus vaccine strain and the wild type virus differently. We consider this a novel hypothesis of poliovirus infection that deserves further evaluation (Fig. 9). We suggest that the neuronal cells respond to Sabin vaccine PV infection by rapidly inducing TLRs and downstream signaling molecules at the early stage of infection leading to sufficient type I IFN response. In sharp contrast, neuronal cells did not respond quickly and sufficiently to activate immune responses/antiviral cytokines in wild PV infection allowing virus replication. It will be interesting to study the effect of early innate immune responses on the replication cycle of polioviruses in neuronal cells.
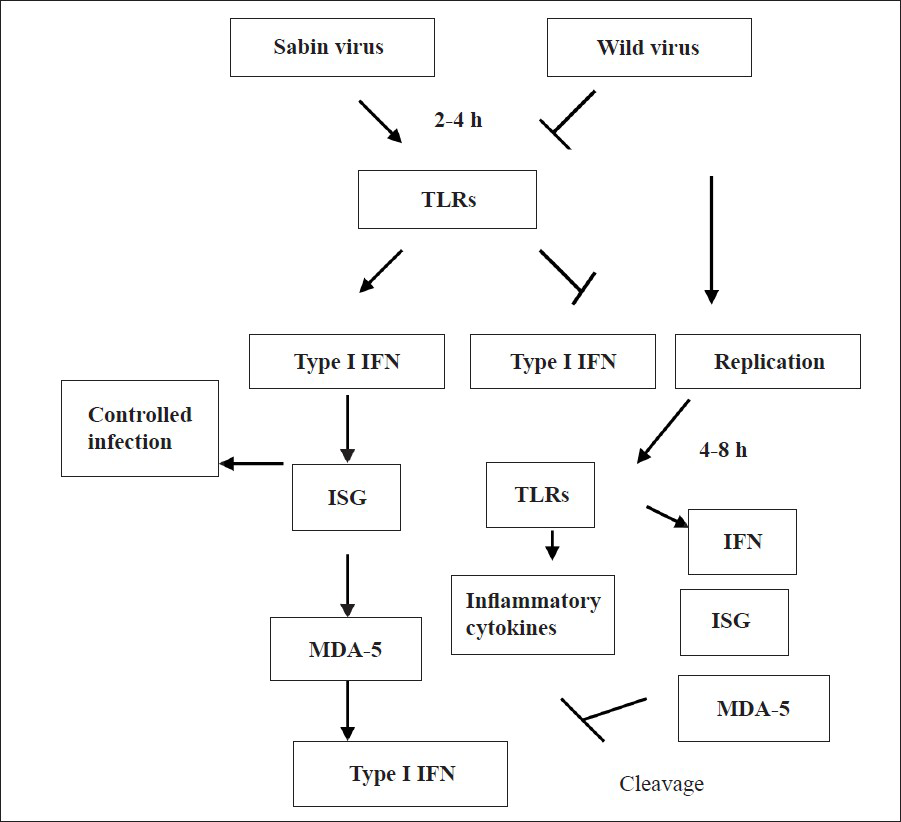
- Schematic representation of the possible hypothesis of poliovirus infection by TLR induction in SK-N-SH cells. Infection of Sabin PV to neuronal cells induces TLR expression, which subsequently induces IFN-α/β and Interferon stimulating genes (ISG) leading to controlled replication at the early stage of infection (2-4 hpi). Infection with wild virus does not induce sufficient TLRs and Interferons to restrict the replication at the early stage. However after 4-6 hpi wild viruses produce significantly high amount of TLRs and Interferons and other cytokines, which possibly induces inflammatory cytokine secretion. Cleavage of MDA5 (Melanoma differentiation associated protein 5) by wild PVmay also block the type 1 interferon production. So the expression of TLRs at the early stage of infection decides the pathway for Sabin and wild PV.
Acknowledgment
The work was supported by the Indian Council of Medical Research, New Delhi. The authors thank Dr K. Ghosh, Director, National Institute for Immunohaematology, Mumbai, for providing flow cytometry facility, and Shrimati Sushma Nadkarni, Shrimati Nikita Raikar and Shrimati Rupa Bhavsar for help in cell culture work and virus strains.
References
- Enteroviruses: polioviruses, coxsackieviruses, echoviruses and newer enteroviruses. In: Knipe DM, Howley PM, eds. Fields virology (5th ed). Philadelphia: Lippincott Williams & Wilkins; 2007. p. :838-93.
- [Google Scholar]
- Histopathologic basis of clinical findings in poliomyelitis. Am J Med. 1949;6:563-78.
- [Google Scholar]
- The alpha/beta interferon response controls tissue tropism and pathogenicity of poliovirus. J Virol. 2005;79:4460-9.
- [Google Scholar]
- History of Sabin attenuated poliovirus oral live vaccine strains. J Biol Stand. 1973;1:115-8.
- [Google Scholar]
- Poliovirus. In: Nathason N, ed. Viral pathogenesis. Philadelphia: Lippincott-Raven Publishers; 1997. p. :555-77.
- [Google Scholar]
- Human poliovirus receptor gene expression and poliovirus tissue tropism in transgenic mice. J Virol. 1992;66:296-304.
- [Google Scholar]
- Ulnar nerve inoculation of poliovirus in bonnet monkey: a new primate model to investigate neurovirulence. Vaccine. 1992;10:529-32.
- [Google Scholar]
- Establishment of a poliovirus oral infection system in human poliovirus receptor expressing transgenic mice that are deficient in alpha/beta interferon receptor. J Virol. 2007;81:7902-12.
- [Google Scholar]
- Human cardiac inflammatory responses triggered by coxsackie B viruses are mainly Toll-like receptor (TLR) 8 dependent. Cell Microbiol. 2005;7:1117-26.
- [Google Scholar]
- Cutting Edge: Antibody-mediated TLR7-dependent recognition of viral RNA. J Immunol. 2007;178:3363-7.
- [Google Scholar]
- Toll-like receptor 3 signaling on macrophages is required for survival following coxsackievirus B4 infection. PLoS One. 2009;4:e4127.
- [Google Scholar]
- Human endothelial cell activation and apoptosis induced by enterovirus 71 infections. J Med Virol. 2004;74:597-603.
- [Google Scholar]
- Manual for the virologic investigation of poliomyelitis. (WHO/EPI/GEN/97.1) Geneva: WHO; 1997.
- [Google Scholar]
- Differences in replication of attenuated and neurovirulent polioviruses in human neuroblastoma cell line SH-SY5Y. J Virol. 1989;63:2357-60.
- [Google Scholar]
- Restricted growth of attenuated poliovirus strains in cultured cells of a human neuroblastoma. J Virol. 1989;63:4034-8.
- [Google Scholar]
- Differential regulation of Toll-like receptor mRNAs in experimental murine central nervous system infections. Neurosci Lett. 2003;344:17-20.
- [Google Scholar]
- Broad expression of Toll like receptors in the human central nervous system. J Neuropathol Exp Neurol. 2002;61:1013-21.
- [Google Scholar]
- TLR signaling tailors innate immune responses in human microglia and astrocytes. J Immunol. 2005;175:4320-30.
- [Google Scholar]
- Inhibition by exogenous interferon of replication of poliovirus ribonucleic acid in chick brain. J Bacteriol. 1965;90:443-5.
- [Google Scholar]
- Role of the alpha/ beta interferon response in the acquisition of susceptibility to poliovirus by kidney cells in culture. J Virol. 2006;80:4313-25.
- [Google Scholar]
- The TLR3/TICAM-1 pathway is mandatory for innate immune responses to polio virus infection. J Immunol. 2011;187:5320-7.
- [Google Scholar]
- Attenuated rabies virus activates, while pathogenic rabies virus evades, the host innate immune responses in the central nervous system. J Virol. 2005;79:12554-65.
- [Google Scholar]
- Toll Like receptor 7- induced immne response to cutaneus West Nile virus infection. J Gen Virol. 2009;90:2660-8.
- [Google Scholar]
- MDA5 and MAVS mediate type 1 interferon responses to coxsackie B virus. J Virol. 2010;84:254-60.
- [Google Scholar]
- Type I interferon gene induction by the interferon regulatory factor family of transcription factors. Immunity. 2006;25:349-60.
- [Google Scholar]
- Interferon regulatory factor IRF-7 induces the antiviral alpha interferon response and protects against lethal West Nile virus infection. J Virol. 2008;82:8465-75.
- [Google Scholar]






