Translate this page into:
Diagnosis of TB from smear & culture negative sputum specimens by IS 6110 based PCR
+For correspondence: ctshiv@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Sir,
Early diagnosis of tuberculosis (TB) is one of the primary challenges in curtailing the spread of TB and is an important step for TB control programme worldwide1. Its diagnosis by smear microscopy remains the main stay in developing countries even though it suffers from low specificity and variable sensitivity2. Classically, there is a correlation between the presence of acid fast bacilli (AFB) in sputum specimens and the method of tuberculosis diagnosis. The sensitivity of both smear and culture positivity mainly depends on the number of viable or dormant mycobacteria in the sputum. The bacillary load in the collected sputum depends on the severity of the disease and the process of sputum collection which is influenced by the time of collection34. In this study we compared sputum specimens collected at different timings (i.e. spot and early morning) by smear, culture and IS 6110 based polymerase chain reaction (PCR) methods for the diagnosis of tuberculosis.
Two consecutive sputum specimens (up to 5 ml each), one spot and the other early morning, were collected from 49 clinically suspected tuberculosis individuals attending designated microscopic centres (DMC) of Gulbarga district, south India during February 2005 to March 2006. Sputum smears were stained for AFB by Ziehl-Neelsen method at the DMC5. The remaining specimen was preserved in equal volumes in a solution of 1 per cent cetylpyridinium chloride and 2 per cent sodium chloride (CPC-NaCl) and transported to National JALMA Institute for Leprosy and Other Mycobacterial Diseases, Agra. These specimens were processed for culture on Lowenstein-Jensen's medium and mycobacterial growth was identified by standard biochemical tests67. The sputum specimens negative for smear and culture were subjected to PCR using IS 6110 specific primers8.
Overall, 42.86 per cent (21/49) spot specimens were positive by smear compared to 65.32 per cent (32/49) being smear positive in the early morning specimens. The specimens positive for smear collected at spot were also positive for specimens collected early in the morning. Among the smear negative spot specimens, nearly 40 per cent (11/28) were found to be smear positive in the early morning specimens. The isolation rate of M. tuberculosis is exactly double (61.22%; 30/49) in specimens collected in the morning than at spot (30.61%; 15/49) and contamination rate remained same in both. Smear and culture positivity has almost 2.5 times higher when sputum specimens were collected in the morning (55.10%; 27/49) compared to only 22.45% (11/49) in spot specimens whereas, smear and culture negativity in both spot and early morning specimens was found to be 75 per cent and 76.4 per cent respectively. On further analysis of smear and culture negative specimens by IS 6110 based PCR, it yielded 85.72 per cent and 84.62 per cent positivity for spot and early morning specimens, respectively (Fig.).
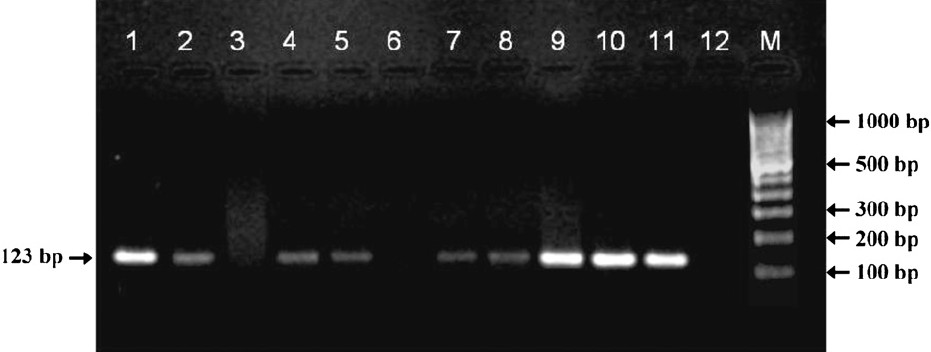
- Agarose gel electrophoresis of IS 6110 based Polymerase chain reaction for detection of M. tuberculosis from sputum specimens. Lane 1= positive control from M. tuberculosis H37Rv; Lanes 2, 4, 5, 7, 8, 9, 10, 11= PCR positive; Lanes 3 and 6= PCR negative; Lane 12=negative control; M= molecular weight marker.
Earlier guidelines of WHO and RNTCP suggested to test three sputum specimens, with one of these collected in the early morning. Recently, it is reduced to two specimens, one of which should be the morning specimen910. The only source of specimen for the diagnosis of pulmonary tuberculosis is sputum. The number of AFB present in the collected sputum and the method applied decide the detection rate. Clinically, there is a correlation between the presence of AFB in clinical specimens and the isolation of M. tuberculosis by culture. The cultures of M. tuberculosis are ultimately required to understand the aetiological agent and its resistance to various drugs and also important for diagnosis of smear negative TB. The volume, quality and also the time of collection of sputum specimens are important for the increase in the TB case detection rate from suspected TB cases by both smear and culture methods especially in TB prevalent countries341011.
In the present study, on comparing spot and early morning sputum specimen by smear and culture methods, the results showed variations in the detection of tuberculosis from the sputum specimens collected at spot and at early morning from the same patient. Efforts made to isolate M. tuberculosis from sputum specimens preserved in CPC-NaCl reported an isolation rate ranging from 6.17 per cent to 78.3 per cent and higher isolation rates (70.22% to as high as 98%) in the smear positive specimens12–16. Our results are also in accordance with earlier reports. Hence, this study re-emphasizes the use of early morning sputum specimens to increase the possibilities of tuberculosis diagnosis by smear and culture methods. However, PCR showed almost similar positivity for spot and early morning specimens.
Overall, the detection of TB by PCR was considerably higher than that of smear and culture methods. The higher rate of diagnosis is attributed to its sensitivity as evidenced by the reported studies17–19. PCR analysis also indicated that the time of specimens’ collection does not appreciably influence results for spot and early morning specimens; and it can be applied to any of the sputum specimens irrespective of their time of collection.
Acknowledgment
Authors thank District TB Officer, Gulbarga for permitting collection of specimens for this study and also thank laboratory staff of DMR, Gulbarga and NJIL & OMD, Agra for specimen collection and processing.
References
- Novel method for processing respiratory specimens for detection of mycobacteria by using C18-Carboxypropylbetaine: Blinded study. J Clin Microbiol. 1998;36:1996-2003.
- [Google Scholar]
- Phage as a diagnostic - the use of phage in TB diagnosis. J Chem Tech Biotech. 2001;76:683-8.
- [Google Scholar]
- A minimum 5.0 ml of sputum improves the sensitivity of acid-fast smear for Mycobacterium tuberculosis. Am J Respir Crit Care Med. 2000;161:1559-62.
- [Google Scholar]
- Insufficient quality of sputum submitted for tuberculosis diagnosis and associated factors, in Klaten district, Indonesia. BMC Pulm Med. 2009;9:16.
- [Google Scholar]
- Revised National Tuberculosis Control Programme. In: Manual for laboratory technicians. New Delhi, India: Central TB Division, Directorate General of Health Services. Ministry of Health and Family Welfare; 1999. p. :12-5.
- [Google Scholar]
- Use of cetylpyridinium chloride for the decontamination of sputum specimens that are transported to the laboratory for the isolation of Mycobacterium tuberculosis. J Clin Microbiol. 1975;1:411-3.
- [Google Scholar]
- Surveillance of drug resistance in tuberculosis in two district of South India. Int J Tuberc Lung Dis. 2002;6:479-84.
- [Google Scholar]
- Polymerase chain reaction amplification of a repetitive DNA sequence specific for Mycobacterium tuberculosis. J Infect Dis. 1990;161:977-81.
- [Google Scholar]
- World Health Organization (WHO). Global Tuberculosis Control: Epidemiology, Strategy, Financing, WHO report 2009, WHO/HTM/TB/2009.411. Available from: http://www.who.int/tb/publications/global_report/2009/en/index/ html
- [Google Scholar]
- Revised National Tuberculosis Control Programme. 2009. New guidelines for diagnosis of smear positive pulmonary tuberculosis. Available from: www.tbcindia.org
- [Google Scholar]
- Comparison of two versus three smears in identifying culture-positive tuberculosis patients in a rural African setting with high HIV prevalence. Int J Tuberc Lung Dis. 2001;5:994-9.
- [Google Scholar]
- Comparison of sodium carbonate, cetyl-pyridinium chloride, and sodium borate for preservation of sputa for culture of Mycobacterium tuberculosis. J Clin Microbiol. 2003;41:4487-8.
- [Google Scholar]
- Surveillance of drug resistance to anti tuberculosis drugs in districts of Hoogli in West Bengal and Mayurbhanj in Orissa. Indian J Tuberc. 2005;52:5-10.
- [Google Scholar]
- Cetyl-pyridinium chloride is useful for the isolation of Mycobacterium tuberculosis from sputa subjected to long-term storage. J Clin Microbiol. 2005;43:442-4.
- [Google Scholar]
- From microscopy centre to culture laboratory: a viable ride for mycobacteria. Int J Tuberc Lung Dis. 2006;10:447-9.
- [Google Scholar]
- Isolation of tubercle bacilli from sputum specimens of patients in the field studies by the cetyl pyridinium chloride-sodium chloride & sodium hydroxide methods. Indian J Med Res. 1995;102:149-51.
- [Google Scholar]
- The utility of polymerase chain reaction in the diagnosis of pulmonary tuberculosis. Chest. 1995;107:631-5.
- [Google Scholar]
- Utility of Universal specimen processing methodology, combining smear microscopy, culture and PCR, for diagnosis of pulmonary tuberculosis. J Clin Microbiol. 2005;43:2703-8.
- [Google Scholar]
- Detection of Mycobacterium tuberculosis in sputum specimens using polymerase chain reaction. Am Rev Respir Dis. 1991;144:1160-3.
- [Google Scholar]





