Translate this page into:
Cytochrome P450 (CYP2C9*2,*3) & vitamin-K epoxide reductase complex (VKORC1 -1639G<A) gene polymorphisms & their effect on acenocoumarol dose in patients with mechanical heart valve replacement
Reprint requests: Dr Shubha R. Phadke, Department of Medical Genetics, Sanjay Gandhi Postgraduate Institute of Medical Sciences (SGPGIMS), Lucknow 226 014, India e-mail: shubharaophadke@gmail.com
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Studies have demonstrated the effect of CYP2C9 (cytochrome P450) and VKORC1 (vitamin K epoxide reductase complex) gene polymorphisms on the dose of acenocoumarol. The data from India about these gene polymorphisms and their effects on acenocoumarol dose are scarce. The aim of this study was to determine the occurrence of CYP2C9*2,*3 and VKORC 1 -1639G>A gene polymorphisms and to study their effects on the dose of acenocoumarol required to maintain a target International Normalized Ratio (INR) in patients with mechanical heart valve replacement.
Methods:
Patients from the anticoagulation clinic of a tertiary care hospital in north India were studied. The anticoagulation profile, INR (International Normalized Ratio) values and administered acenocoumarol dose were obtained from the clinical records of patients. Determination of the CYP2C9*2,*3 and VKORC1 -1639G>A genotypes was done by PCR-RFLP (restriction fragment length polymorphism).
Results:
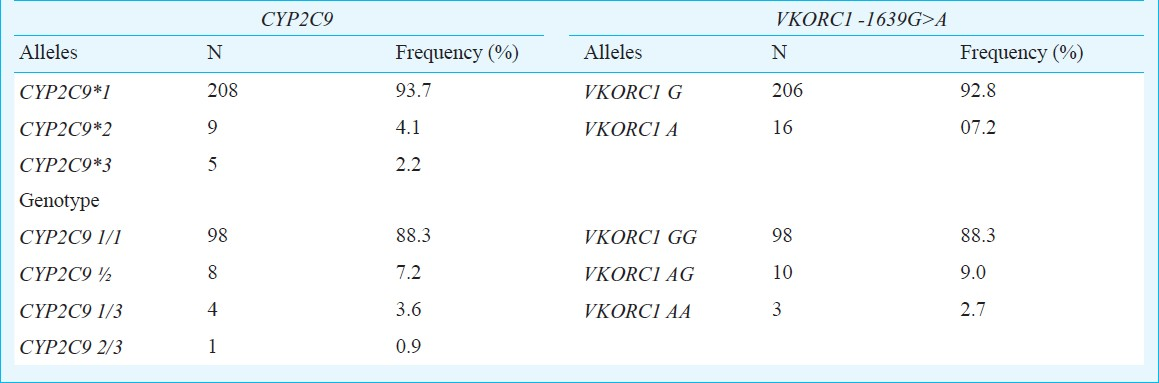
A total of 111 patients were studied. The genotype frequencies of CYP2C9 *1/*1,*1/*2,*1/*3 were as 0.883, 0.072, 0.036 and that of VKORC1 -1639G>A for GG, AG, and AA genotypes were 0.883, 0.090, and 0.027, respectively. The percentage of patients carrying any of the variant alleles of CYP2C9 and VKORC1 in heterozygous or homozygous form was 34% among those receiving a low dose of ≤20 mg/wk while it was 13.8 per cent in those receiving >20 mg/wk (P=0.014). A tendency of lower dose requirements was seen among carriers of the studied polymorphisms. There was considerable variability in the dose requirements of patients with and without variant alleles.
Interpretation & conclusions:
The study findings point towards the role of CYP2C9 and VKORC1 gene polymorphisms in determining the inter-individual dose variability of acenocoumarol in the Indian patients with mechanical heart valve replacement.
Keywords
Acenocoumarol
CYP2C9
dose requirements
INR
oral anticoagulants
VKORC1
Acenocoumarol is a commonly prescribed oral anticoagulant for the prevention and treatment of thromboembolic events. It belongs to the coumarin group of anticoagulants. Considerable inter-individual variability in the dose requirements of coumarin group of anticoagulants is seen with the consequent risks of bleeding or thrombosis in case of over or under anticoagulation respectively1. Genetic and environmental factors are contemplated to play role in determining optimum dose for an individual.
Studies have demonstrated the effect of CYP2C9 (cytochrome P450) and VKORC1 (vitamin K epoxide reductase complex) gene polymorphisms on the dosages of oral coumarin anticoagulants (OCAs namely warfarin, acenocoumarol)2. Cytochrome P450 2C9 enzyme is involved in the elimination of acenocoumarol from the body. Allelic variants of CYP2C9 gene, CYP2C9*2 (Arg144Cys) and CYP2C9*3 (Ile359Leu), have less catalytic activity than the wild type CYP2C9*1 (Arg144/Ile359). The presence of these variants in an individual is thus expected to lower the requirements of the drug. Vitamin K epoxide reductase complex subunit 1 (VKORC1) is the target enzyme of OCAs. The inhibition of this enzyme by the OCAs reduces the regeneration of vitamin K from vitamin K epoxide reductase3. Several polymorphisms have been found in the coding and the non-coding regions of the VKORC1 gene. VKORC1 -1639G>A is a polymorphism in the promoter region of VKORC1 gene. The presence of the polymorphism reduces the binding of the transcription factor and thereby reduces the gene expression. The reduced level of the target enzyme reduces the dose requirements of the OCAs.
The association of these polymorphisms with coumarin group of drugs has been well studied among various ethnic groups. However, the data from India are scarce and only a couple of studies correlating the dose of acenocoumarol with VKORC1-1639G>A polymorphism have been published from India45. The aim of the current study was therefore, to determine the presence of CYP2C9*2,*3 and VKORC1 -1639G>A gene polymorphisms in Indian patients and to study their effects on the dose of acenocoumarol required to maintain a target INR (International Normalized Ratio) in patients with mechanical heart valve replacement.
Material & Methods
Patients with rheumatic heart disease who had undergone heart valve replacement were selected from the Anticoagulation Clinic, held by the Department of Cardiovascular Thoracic Surgery, Sanjay Gandhi Postgraduate Institute of Medical Sciences (SGPGIMS), a tertiary care hospital in Lucknow, north India. The inclusion criteria were a patient (i) of either sex (ii) of any age (iii) requiring long term anticoagulation with acenocoumarol after undergoing heart valve replacement and (iv) on a regular follow up in the anticoagulation clinic. The exclusion criteria were a patient (i) who had underlying renal or hepatic insufficiency (ii) history of smoking and/or alcohol intake (iii) on a concomitant medication which could interact with acenocoumarol thus affecting its dose requirements. The patient was excluded if he or she was on any of the following medications: antibiotics (ciprofloxacin, co-trimoxazole, erythromycin, fluconazole, isoniazid, metronidazole, voriconazole, rifampicin), cardiovascular drugs (amiodarone, propranolol, diltiazem) non steroidal anti-inflammatory drugs (including COX2 inhibitors), lipid lowering agents, antiepileptics (carbamazepine, phenytoin), selective serotonin reuptake inhibitor (sertraline and omeprazole)6. Demographic and clinical data such as the age, sex, weight, height, indication for acenocoumarol, concomitant medications, bleeding episodes, thromboembolic phenomenon were recorded from the clinical records.
Ethical clearance for the study protocol was obtained from the Institutional Ethics Committee. A prior written informed consent was taken from all the patients.
The anticoagulation profile of each patient was obtained from their respective clinical records and included the INR value, dose of acenocoumarol being taken and dose advised at each visit. Two variables the stable therapeutic dose and the percentage of time spent outside the target INR were calculated for each patient. The stable therapeutic dose was defined as mean dose the patient was getting when his/her INR was in a stable therapeutic range7. Stable therapeutic INR was defined as at least 2 consecutive INR measurements between the target range of 2-3 measured at least 2 wk apart. The percentage of time spent outside the target INR was calculated as the ratio of the total number of weeks when the INR remained above or below the target INR to the total number of weeks of follow up. For the reported allele frequencies of CYP2C9*2 and *3 as 15 and 3 per cent, respectively with an α error of 0.05 and power of 0.80, it was calculated that a sample size of 110 patients would be sufficient for this study1.
Genotyping: For genomic DNA extraction, blood (2 ml) was collected from each patient in EDTA. DNA was extracted from blood by using Invitrogen DNA extraction kit Pure Link Genomic DNA kit (Invitrogen Corporation, Carlsbad CA, USA). PCR was used to amplify segments of the CYP2C9 and VKORC1 genes from 100 ng genomic DNA in a total reaction volume of 25 μl. A total of 2.5 mM MgCl2, 200 μM each of dATP, dCTP, dGTP, dTTP, 0.025 units/μl Taq DNA Polymerase, 75 mM Tris-HCl (pH 8.8 at 25°C), 20 mM (NH4)2SO4, 0.16 μM of each forward and reverse primers and 1 μl of template DNA were added per reaction. Polymerase chain reaction was performed using the following conditions: 94°C for 5 min, followed by 34 cycles at 94°C for 45 sec, 54.3°C for 1 min and 72°C for 1 min 30 sec followed by final extension at 72°C for 8 min in a thermal cycler (PTC 200 Thermal Cyclers, BioRad Inc)89. The Forward and reverse primer sequences for CYP2C9*2, CYP2C9*3, VKORC1 were as follows: (*2F= 5’TACAAATACAATGAAAATATCATG-3’,*2R=5’CTAACAACCAGGACTCATAATG3’; for*3F=5’AGGAAGAGATTGAACGTGTGA-3’,*3R=5’GGCAGGCTGGTGGGGAGAAGGTCAA-3’forVF=-5’ATCCCTCTGGGAAGTC AGC-3’ and VR=-5’CACCTTCAACCTCTCCATCC-3’, respectively89. AVAII, STY I, and NCI1 were used for DNA digestion for CYP2C9*2, CYP2C9*3, VKORC1, respectively. The PCR products were electrophoresed on 2 per cent agarose gel at 120 V for 60 min stained with 0.5 μg/ml ethidium bromide in Tris borate EDTA (TBE) buffer pH (8.3) and visualized by ultraviolet irradiation. The representative gel pictures are shown in Fig. 1. The amplicon sizes for the CYP2C9*2,*3 and VKORC1 were 690,130, and 636, respectively. The wild and the minor alleles respectively were identified by 521,169 and 690 bp for CYP2C9*2, 130 and 104, 26 for CYP2C9*3 and 472, 114, 50 and 522,114 for VKORC1.
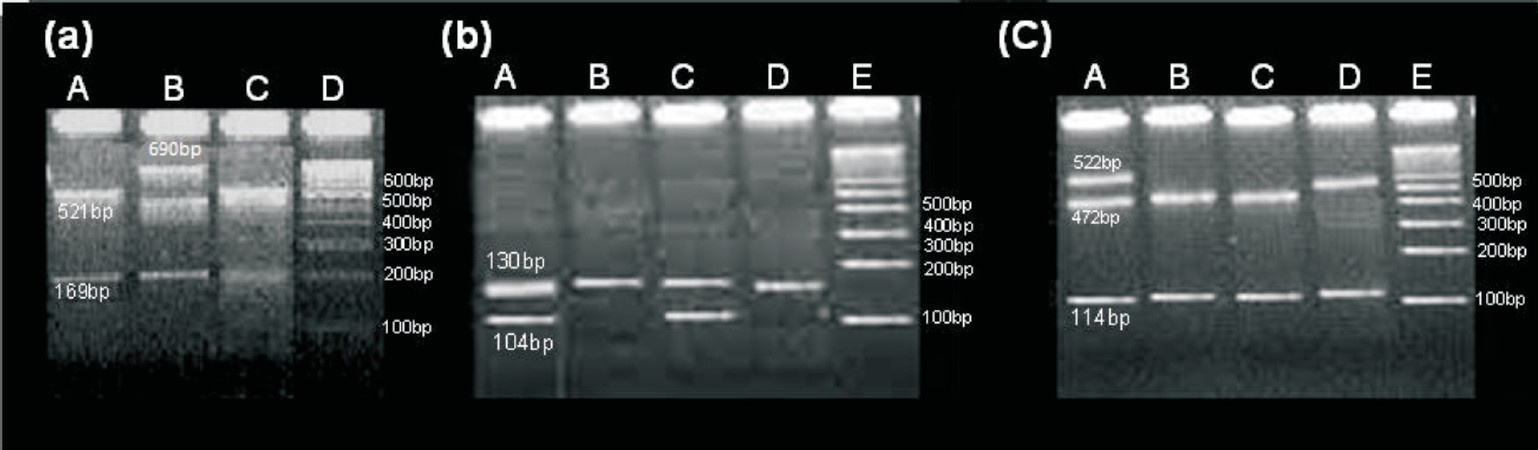
-
(a) Representative gel pictures of restriction digested DNA fragments showing wild homozygous as 521, 169 bp (lane A, C) and heterozygous as 690, 521, 169 bp (lane B) for CYP2C9*2 (b) Wild homozygous as 130 bp (lane B, D) and heterozygous as 130, 104 bp (lane A, C) for CYP2C9*3 (c) Wild homozygous as 472, 114 bp (lane B, C) and heterozygous as 522,472,114 bp (lane A) and variant homozygous as 522,114 bp (lane D) for VKORC1-1639G>A polymorphisms.
Statistical analysis: One way ANOVA was used to compare mean stable therapeutic doses between the CYP2C9 and VKORC1 genotype groups. The difference in number of patients in low and high dose groups with CYP2C9 and VKORC1 polymorphism was determined by Fisher's exact test. Student t test was used to compare the mean stable therapeutic dose requirement between patients of CYP2C9 and VKORC1 genotypes as per dominant and recessive models. Statistical analyses were done with SPSS software (version 10.0; SPSS, Chicago, IL).
Results
A total of 111 patients were enrolled in the study. Majority of the patients 110/111 (90%) belonged to the State of Uttar Pradesh (northern India). There were 77 (69.4%) males and 34 (30.6%) females with mean age of 36.4 ± SD (15-67 yr) and 32.4 ± SD (17-60 yr), respectively. In 110 patients the primary reason for anticoagulation with acenocoumarol was a heart valve replacement [75 (67.6%) mitral valve, 25 (22.5%) atrial valve, 10 (9%) double valve replacement]. One patient was receiving acenocoumarol after correction of a congenital heart malformation. The genotype and allele frequencies for the studied polymorphisms of CYP2C9 and VKORC1 genes are as shown in Table I. The frequency distribution for all the markers was in Hardy Weinberg equilibrium.
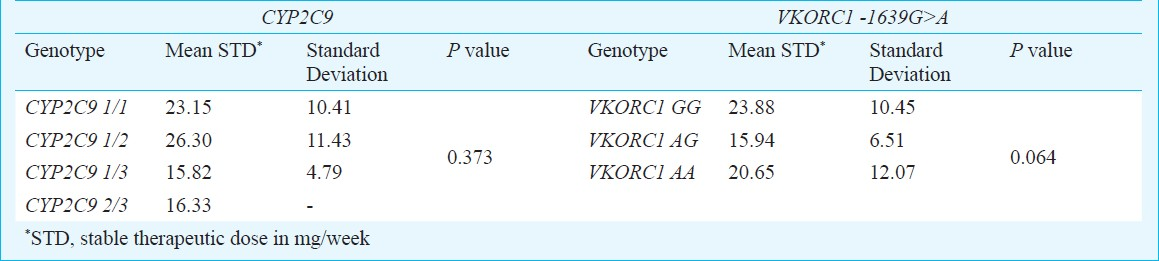
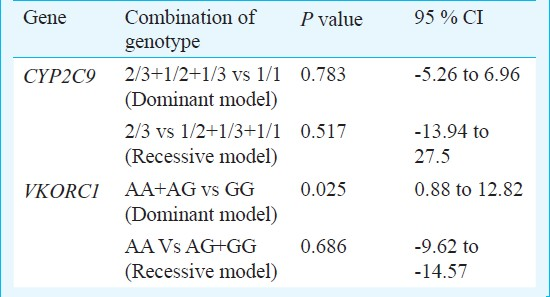
For 111 patients the mean stable therapeutic dose was 23.08 mg/week (range 5.50-71.40 mg/week). The mean weekly stable therapeutic dose for patients carrying various genotypes of CYP2C9 and VKORC1 is as shown in Table II. Pearson correlation coefficient of stable therapeutic dose with the age, body surface area were -0.037 and 0.067, respectively. Considerable variability in dose requirements among all genotypes was noticed (Fig. 2). Statistical analysis of the difference of the mean stable therapeutic dose between combination of genotypes assuming dominant and recessive models of genotypes of CYP2C9 and VKORC1 is shown in Table III.
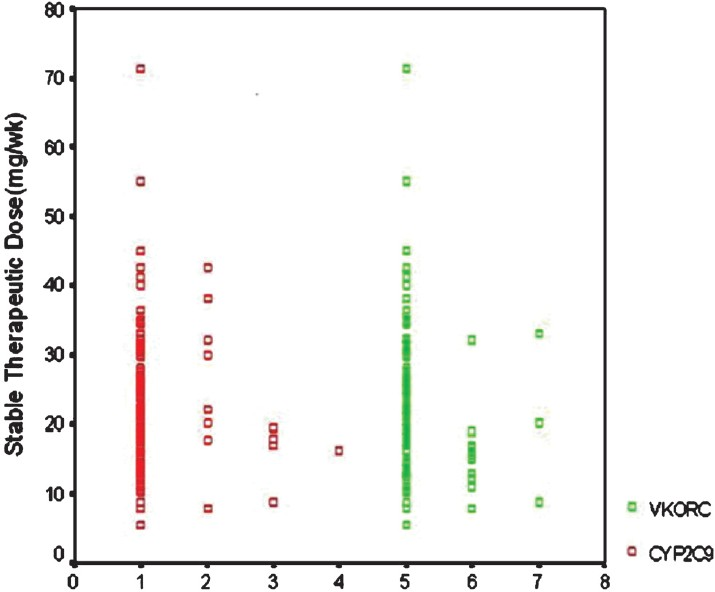
- Depiction of the variability in dose requirements in various genotype 1, 2, 3, 4 = CYP2C9 genotype 1/1; 1/2; 1/3: 2/3, respectively; 5, 6, 7 = VKORC1 genotype GG; GA; AA, respectively.
The patients were divided into two groups based on the stable therapeutic doses - those requiring doses ≤20 mg/week and those requiring >20 mg/week, and calculated the proportion of patients carrying wild allele to the variant alleles in the two groups. The total numbers of patients requiring a dose of ≤20 and >20 mg/week were 53 and 58, respectively. The number of patients with any of the variant allele of CYP2C9 and VKORC1 in heterozygous or homozygous form was 18 out of 53 (34%) in patients receiving a low dose of ≤20 mg/week while it was eight of 58 (13.8%) in those receiving >20 mg/week (P=0.014). The differences were statistically significant when any of the variant allele was compared with the wild type allele, it was observed that of the 26 patients with some variant alleles of either gene, (18/26) (69.2%) needed weekly dose ≤20 mg while of the 85 patients with wild type of allele in both genes (35/85) (41.2%) needed low dose (P=0.01).
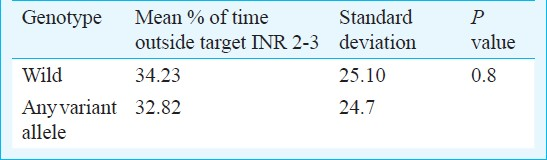
A comparison of the percentage of time of follow up spent outside (above or below) the target therapeutic INR showed that it was similar in patients with wild and minor/variant alleles with as much as 34 per cent time being spent outside the target range (Table IV).
Discussion
The prescription of an optimal dose of acenocoumarol is difficult due to considerable individual variability in drug response. Our data revealed as much as 15 fold variation in the stable therapeutic dose requirements of our patients. Pharmacogenetic studies on oral coumarin anticoagulants have pointed towards CYP2C9*2,*3 and VKORC1 gene polymorphisms as the most important genetic variants determining the dose requirements of these anticoagulants. The prevalence of these polymorphisms varies across different ethnic groups. For example, the allele frequencies of CYP2C9*2 and CYP2C9*3 have been shown as 0.105 and 0.067 in Russian population10, as 0.125 and 0.085 in Caucasians11, and as 0.165 and 0.095 in Croatians12. However, the prevalence of these polymorphisms in India appears to be less. In our study comprising of patients from Northern India, the allele frequencies of CYP2C9*1,*2,*3 were 0.937, 0.041, 0.022, respectively and the genotype frequencies CYP2C9 *1/*1,*1/*2,*1/*3 were 0.883,0.072,0.036, respectively. Jose et al13 have reported the CYP2C9*1,*2,*3 allele frequencies as 0.88, 0.04, 0.08, respectively in south Indian population. It appears that allele frequencies of CYP2C9*2 in the north Indian population (present study) were more, whereas CYP2C9*3 was more common than CYP2C9*2 in southern India. Recently a study from northern India by Rathore et al4 has reported the CYP2C9*2,*3 frequencies as 0.049 and 0.039. The lower prevalence for the CYP2C9*3 and *2 alleles has also been reported from China14.
In our study, the genotype frequencies for the VKORC1 GG, AG, and AA genotypes were 0.883, 0.090, and 0.027 respectively. For this gene polymorphism also ethnic variations have been demonstrated. Two studies on Indian population415 have reported the GG, AG, AA genotype frequencies of VKORC1 -1639G>A polymorphism as 80, 13, 7 per cent and 73.53, 24.51, 1.96 per cent, respectively. The AA and AG genotype frequencies in our study were slightly lower than these studies.
Regarding the effects of the studied polymorphisms on acenocoumarol dose requirements we found that the carriers of the variant alleles had a tendency for lower dose requirements. The carriers of CYP2C9*3 had the lowest dose requirement. This observation is in concordance with other similar studies on the effects of CYP2C9*2,*3 polymorphisms on acenocoumarol1617. The bearers of CYP2C9*3 allele have been found to have lowest dose requirements. On analyzing the data by the low vs high dose requirement of ≤20 and >20 mg/week, the proportion of patients with wild allele to the patients carrying at least any one of the variant alleles was significantly different in the two groups. The low dose group had a greater proportion of variant alleles. As many as 69.2 per cent of the patients carrying any one of the 3 variant alleles required a stable therapeutic dose of ≤20 mg/week whereas only 41.2 per cent of those who were carrying the wild alleles needed a low dose. Thus, it appears that the presence of variant allele in a patient places him at a higher chance of requiring a low dose. If such a patient is started on the standard dose, there may be a risk of overanticoagulation.
A study on Greek patients by Markatos et al18 showed significant difference in the dose requirement of patients with variant alleles (CYP2C9*3, VKORC1A P<0.001). Similarly in Serbian population studied by Kovac et al19 as much as 2.5-fold higher dose requirements in patients with GG genotype of VKORC1 polymorphism have been reported. In our study, the presence of the A allele of VKORC1 G>A as per the dominant model reached a statistical significance in terms of affecting the dose requirement. However the combination of the other genotypes as per the Dominant and Recessive models for CYPC9 and VKORC1 could not significantly affect the dose of acenocoumarol. A major reason for this could be the small sample size of the study and a lesser number of variant alleles detected in the studied patients. Other factors that might be contributing to the observed differences from other studies can be differences in the methodologies of calculation of stable doses and the analysis done by categorization of patients under differently described low, medium and high dose groups.
To evaluate the association of stability of anticoagulation with the genotypes, we calculated the percentage of time of follow up being spent outside the target INR range. About 30-34 per cent time was being spent outside the target range in our patients with no significant difference between the patients with or without variant alleles. Tassies et al20 noted a statistically significant difference in the percentage of time spent within the target INR by the carriers of CYP2C9*3 vs wild genotype. Although the time being spent outside the target range was comparable, the small number of CYP2C9*3 variants in our study probably led to insignificant results. Also there could be differences in the INR monitoring protocol being followed for the two study groups.
Our data also showed a wide variability in the dose requirements of patients with same genotypes. This suggests that there may be other genetic and non-genetic factors responsible for dose variability in our patients. For example, a search for new genetic variants contributing to inter-individual variation on stabilized acenocoumarol doses was made through Genome-wide association study21. This study identified CYP4F2 and CYP2C18 as additional genetic variants contributing significantly to acenocoumarol doses. Thus, further studies on the effects of these genetic variants on drug doses are indicated. Several environmental factors have also been known to affect the response of the coumarin anticoagulants. The important among them are dietary factors, smoking and drug-drug interactions. Increase in the dietary intake of vitamin K, a high protein diet and smoking have been shown to increase the dose requirements22–24. None of our patients gave history of smoking and patients on concomitant medications well known to interact with coumarin anticoagulants were excluded. However, the influence of dietary factors cannot be ruled out due to the retrospective design of our study.
A major limitation of our study was the small sample size. Hence future studies should be done on a larger sample size. Another limitation was a failure to determine the role of some environmental factors like vitamin K in the diet of our patients.
In conclusion, our study points towards the role of CYP2C9 and VKORC1 gene polymorphisms in determining the inter-individual dose variability of acenocoumarol in the north Indian patients with mechanical heart valve replacement. This knowledge shall be significant considering the wide use of this oral anticoagulant with a narrow therapeutic index. Further studies with larger sample size are needed to determine the strength and the significance of emerging data.
Acknowledgment
Authors acknowledge the SGPGIMS, Lucknow for granting funds to carry out this work, and thank Dr S.K. Mandal for statistical analysis of the data. Authors also thank all the patients for their cooperation.
References
- The future prospects of pharmacogenetics in oral anticoagulation therapy. Br J Clin Pharmacol. 2006;61:746-51.
- [Google Scholar]
- Comparative pharmacokinetics of vitamin K antagonists: warfarin, phenprocoumon and acenocoumarol. Clin Pharmacokinet. 2005;44:1227-46.
- [Google Scholar]
- Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose. N Engl J Med. 2005;352:2285-93.
- [Google Scholar]
- Frequencies of VKORC1 -1639 G>A, CYP2C9*2 and CYP2C9*3 genetic variants in the Northern Indian population. BioScience Trends. 2010;4:333-7.
- [Google Scholar]
- The impact of VKORC1-1639G>A polymorphism on the maintainence dose of oral anticoagulants for thromboembolic prophylaxis in North India: A pilot study. Indian J Hum Genet. 2011;17:54-7.
- [Google Scholar]
- Systematic overview of warfarin and its drug and food interactions. Arch Intern Med. 2005;165:1095-106.
- [Google Scholar]
- The International Warfarin Pharmacogenetics Consortium.Estimation of the warfarin dose with clinical and pharmacognetic data. N Engl J Med. 2009;360:753-64. International Warfarin Pharmacogenetics Consortium
- [Google Scholar]
- Frequency of cytochrome P450 CYP2C9 variants in a Turkish population and functional relevance for phenytoin. Br J Clin Pharmacol. 1999;48:409-15.
- [Google Scholar]
- Rapid Single-Nucleotide Polymorphism Detection of Cytochrome P450 (CYP2C9) and Vitamin K Epoxide Reductase (VKORC1) Genes for the Warfarin Dose Adjustment by the SMart-Amplification Process Version 2. Clin Chem. 2009;55:4804-12.
- [Google Scholar]
- Polymorphisms of drug-metabolizing enzymes CYP2C9, CYP2C19, CYP2D6, CYP1A1, NAT2 and of P-glycoprotein in a Russian population. Eur J Clin Pharmacol. 2003;59:303-12.
- [Google Scholar]
- Genetic analysis of the human cytochrome P450 CYP2C9 locus. Pharmacogenetics. 1996;6:429-39.
- [Google Scholar]
- Genetic polymorphisms of cytochromes P450: CYP2C9, CYP2C19, and CYP2D6 in Croatian population. Croat Med J. 2003;44:425-8.
- [Google Scholar]
- CYP2C9 and CYP2C19 genetic polymorphisms: frequencies in the South Indian population. Fundam Clin Pharmacol. 2005;19:101-5.
- [Google Scholar]
- Frequency of cytochrome P450 2C9 allelic variants in the Chinese and French populations. Fundam Clin Pharmacol. 2003;17:373-6.
- [Google Scholar]
- Contribution of VKORC1 and CYP2C9 polymorphisms in the interethnic variability of warfarin dose in Malaysian populations. Ann Hematol. 2011;90:635-41.
- [Google Scholar]
- Pharmacogenetics of Oral Anticoagulants: A basis for Dose Individualization. Clin Pharmacokinet. 2008;47:565-94.
- [Google Scholar]
- Cytochrome P450 2C9 polymorphism and acenocoumarol therapy. Kardiol Pol. 2006;64:397-402.
- [Google Scholar]
- VKORC1 and CYP2C9 allelic variants influence acenocoumarol dose requirements in Greek patients. Pharmacogenomics. 2008;9:1631-8.
- [Google Scholar]
- The c.-1639G>A polymorphism of the VKORC1 gene in Serbian population: retrospective study of the variability in response to oral anticoagulant therapy. Blood Coagul Fibrinolysis. 2010;1:558-63.
- [Google Scholar]
- Pharmacogenetics of acenocoumarol: cytochrome P450 CYP2C9 polymorphisms influence dose requirements and stability of anticoagulation. Haematologica. 2002;87:1185-91.
- [Google Scholar]
- A Genome-wide association study of acenocoumarol maintenance dosage. Hum Mol Genet. 2009;18:3758-68.
- [Google Scholar]
- Vitamin K intake and sensitivity to warfarin in patients consuming regular diets. Thromb Haemost. 1999;81:396-9.
- [Google Scholar]
- Warfarin and Vitamin K intake in the era of pharmacogenetics. Br J Clin Pharmacol. 2010;70:164-70.
- [Google Scholar]
- Assessing evidence of interaction between smoking and warfarin: a systematic review and metaanalysis. Chest. 2011;139:1130-9.
- [Google Scholar]






