Translate this page into:
Coverage & missed opportunity for Japanese encephalitis vaccine, Gorakhpur division, Uttar Pradesh, India, 2015: Implications for Japanese encephalitis control
Reprint requests: Dr Manoj V. Murhekar, National Institute of Epidemiology, Indian Council of Medical Research, R-127, Tamil Nadu Housing Board, Ayapakkam, Ambattur, Chennai 600 070, Tamil Nadu, India e-mail: mmurhekar@gmail.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Japanese encephalitis (JE) is an important aetiology of acute encephalitis syndrome in Gorakhpur division, Uttar Pradesh, India. Two doses of JE vaccine (first during 9-12 months and second during 16-24 months of age) are administered under the Universal Immunization Programme. We conducted surveys to estimate the coverage of JE vaccine and magnitude of missed opportunity for vaccination (MoV) for JE in Gorakhpur division.
Methods:
To estimate the JE vaccine coverage, cluster surveys were conducted in four districts of Gorakhpur division by selecting 30 clusters by probability proportional to size method in each district, seven children aged 25-36 months were selected from each cluster and their mothers were interviewed about JE vaccination. To estimate the magnitude of MoV, exit surveys were conducted in vaccination clinics in selected health facilities, mothers were interviewed about the vaccination status of their children and vaccines administered to the child on the day of interview.
Results:
A total of 840 children were surveyed, 210 from each district. The coverages of one and two doses of JE vaccine in Gorakhpur division were 75 per cent [95% confidence interval (CI): 71.0-78.9] and 42.3 per cent (95% CI: 37.8-46.8), respectively. Facility-based exit survey indicated that 32.7 per cent of the eligible children missed JE vaccine.
Interpretation & conclusions:
The survey results showed that three of the four children aged 25-36 months in Gorakhpur division had received at least one dose of JE vaccine. The coverage of second dose of JE vaccine, however, was low. Failure to administer vaccination simultaneously was the most common reason for MoV for JE vaccine. Training vaccinators about correct vaccination schedule and removing their misconception about administering vaccines simultaneously would substantially improve JE vaccine coverage in Gorakhpur.
Keywords
Gorakhpur
India
Japanese encephalitis
vaccine
vaccine coverage
Outbreaks of acute encephalitis syndrome (AES) occur frequently in several Indian States. According to the National Vector-borne Disease Control Programme, more than 52,000 cases of AES were reported from India with 7964 (15%) deaths between 2009 and 20151. Nearly half of the total AES cases and deaths were reported from the State of Uttar Pradesh1. Seasonal outbreaks of AES with high case fatality and disability are frequently reported from Gorakhpur division of Uttar Pradesh234567.
Analysis of AES surveillance data from the BRD Medical College Hospital in Gorakhpur indicated that more than 10,000 AES cases were admitted during 2008-2012, of which 8.4 per cent were positive for Japanese encephalitis (JE)5. Live-attenuated JE vaccine (SA-14-14-2) was introduced in the division in two rounds of mass vaccination campaign in 2006 and 2010, targeting children aged between 1 and 15 years. Following this, JE vaccine was introduced in the Universal Immunization Programme in the division in 2011 as a single dose targeting children aged between 16 and 24 months along with Diphtheria-Pertussis-Tetanus /Oral Polio Vaccine (DPT/OPV) booster. The evaluated coverage of one dose of JE vaccine in the division was only 51 per cent, [95% confidence interval (CI): 47.9- 54.2] in 20138. In 2013, a two-dose JE vaccine strategy was introduced, with the first dose (JE-1) to be given between 9 and 12 months along with measles-containing vaccine (MCV-1) and second dose (JE-2) during 16-24 months along with DPT/OPV booster and second dose of MCV (MCV-2)9.
Information about vaccine coverage and reasons for low coverage is necessary to improve the ongoing vaccination programme10. Studies conducted across the globe have shown that missed opportunities for vaccination (MoV) constitute a major obstacle for improving vaccination coverage1112. MoV is defined as ‘any situation in which an eligible child has contact with a health facility, but he/she is not administered an indicated vaccine, despite not having contraindications’1112. It is estimated that about one-third of children and women in childbearing age visiting health facilities for vaccination miss one or more vaccines11. Interventions focussing on reducing MoV have resulted in substantial improvement in vaccination coverage101113. With this background, a study was conducted in Gorakhpur division of Uttar Pradesh, India, to estimate coverage of two doses of JE vaccine. The secondary objective was to estimate the magnitude of MoV for JE.
Material & Methods
Coverage of Japanese encephalitis (JE) vaccines: The JE vaccine coverage survey was conducted between May and August 2015, covering Deoria, Gorakhpur, Kushinagar and Maharajganj districts of Gorakhpur division. The Institutional Ethics Committee of the ICMR- National Institute of Epidemiology, Chennai, approved the study protocol. Written informed consent was obtained from parents or caretakers of the children before interviewing them.
In each district, 30 clusters (villages in rural areas and wards in urban areas) were selected using probability proportion to their sizes. From each cluster, seven children aged 25-36 months were selected. As the list of households in the selected cluster was not available, the following procedure was used for selecting the random starting point for the survey14:First, the approximate geographic centre of the cluster was located and a random direction was chosen from the centre. All households from the centre of the area to the edge of the area were counted and a number was randomly selected between one and the number of households counted. This was the first household to be visited. We enquired about the presence of an eligible child defined as one aged 25-36 months in the household and interviewed the child's mother if eligible child was present in the household. The younger child was interviewed if there were two eligible children in the household. The next household to be visited was the nearest to the first, and this procedure was continued till the required number of children were enrolled from each cluster.
Next, the mother was interviewed to know about JE vaccination status of her child. The mother was asked to recall if her child had received JE vaccine. She was then asked to show the vaccination card and JE vaccination status of the child was noted as per the card. The child was considered vaccinated against JE, if he/she had received JE vaccine/s as per vaccination card or by mother's history in case the card was not available.
Missed opportunity for JE vaccine: In Gorakhpur division, vaccination services in the public sector are provided twice a week (Wednesdays and Saturdays) through vaccination clinics at fixed facilities such as district hospitals, urban health centres, primary health centres (PHCs) and community health centres (CHCs) as well as through outreach sessions at health subcentres and anganwadi schools. The magnitude of MoV was measured by conducting a population-based survey as well as health facility-based exit survey1112.
Population-based survey to estimate MoV for JE vaccine: The data collected for JE vaccine coverage evaluation survey were analysed to estimate the magnitude of MoV. Only children having vaccination cards were considered for the analysis. Missed opportunity for the JE-1 vaccine was evaluated amongst children who had received MCV-1 and/or JE vaccine. Of these children, those who received (i) only MCV-1, or (ii) who received JE-1 vaccine but the date of vaccination was later than MCV-1 were considered to have missed the JE-1 vaccine. Similarly, missed opportunity for the JE-2 vaccine was evaluated amongst children who had received any of these vaccines: MCV-2, JE-2 and DPT/OPV booster. Children who did not receive the JE-2 vaccine or for whom the date of vaccination for MCV-2 or DPT/OPV booster was earlier than JE-2 were considered to have missed the JE-2 vaccine.
Health facility-based survey to estimate MoV for JE vaccine: For the health facility-based survey, 12 blocks (four from each district) were randomly selected from Deoria, Gorakhpur and Kushinagar districts (Maharajganj district could not be covered due to logistic reasons) of the division during August to September 2015. Then one PHC or CHC was selected from each block randomly. All vaccination sessions scheduled on the day of the survey within the selected PHC/CHC as per the routine immunization microplan were line listed and every third vaccination session was systematically sampled for the survey. After obtaining written informed consent, mothers of eligible children (defined as children aged nine months to two years) exiting the vaccination session were interviewed to collect information about vaccination status of the child, vaccines administered on the day of interview (from vaccination card) and reasons for not having received vaccines for which the child was eligible. We confined our enquiry to this age group as children in this group were eligible to receive JE-1 and JE-2 vaccine as per the Universal Immunization Programme. To determine the extent of MoV for JE, the eligibility of the child to receive JE vaccine was assessed, based on child's age and details of previous vaccination as mentioned in vaccination card. Amongst the children eligible to receive JE vaccine, those who did not receive JE vaccine on the day of survey were considered as missed opportunity. At the end of the vaccination session, the list of children who missed any vaccine was shared with the vaccinator and accredited social health activist (ASHA) and explained her that these children were eligible for vaccination and advised to vaccinate them on the same day.
Statistical analysis: The data were analyzed using STATA SE (version 13.0, StataCorp LLC, Texas, USA) software. Using the survey data analysis module, the coverage of one and two doses of JE vaccines was estimated separately for each district along with the corresponding 95 per cent CIs. The weighted coverage for Gorakhpur division was estimated using the proportion of two to three year old children in each of the four districts as the weights14.
Results
Coverage of JE vaccine: Overall 840 children were surveyed; 210 from each district. The median age of the children surveyed was 30 (interquartile range: 27-33) months and 54.8 per cent were boys. Majority belonged to Hindu religion (83%); 32 per cent belonged to scheduled caste/tribes and 59 per cent had below poverty line card (Table I). Nearly two-third (n=555, 66.1%) of the children had a vaccination card.
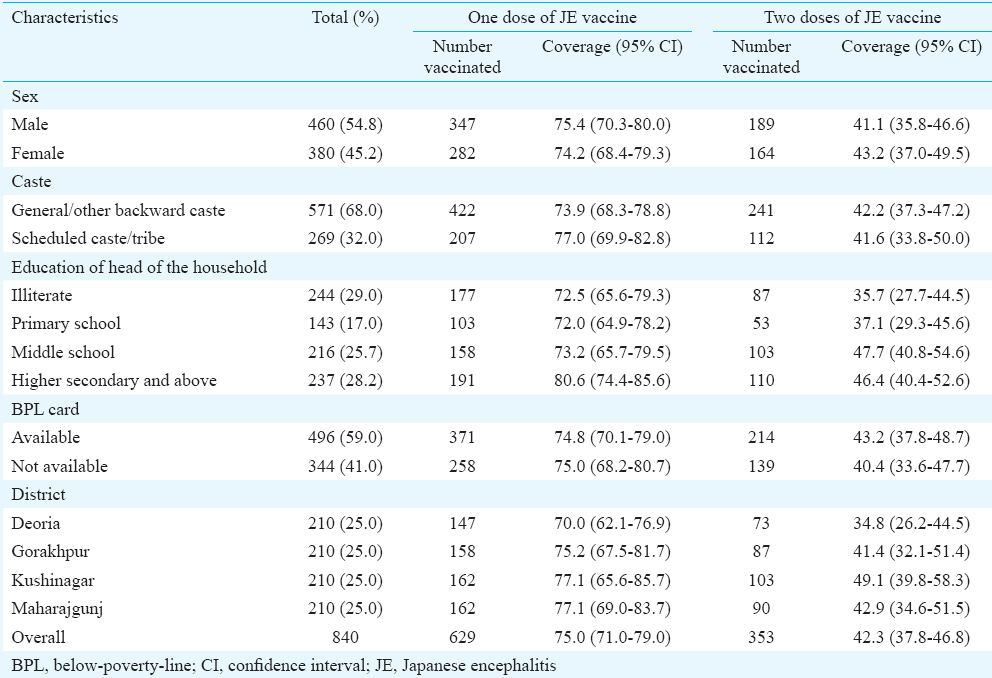
Of the 840 children surveyed, 211 (25%) had not received JE vaccine whereas the remaining 629 children had received at least one dose of JE vaccine and 353 children had received two doses of the vaccine. The weighted coverage of one and two doses of JE vaccine in Gorakhpur division was 75 per cent (95% CI: 71.0-79.0) and 42.3 per cent (95% CI: 37.8-46.8), respectively. The coverage of one and two doses of JE vaccine did not differ by sex, religion, caste, or district (Table I). Amongst the 555 children with a vaccination card, 203 (36.6%) and 224 (40.4%) had received the JE-1 and JE-2 vaccine at the recommended ages of 9-12 months and 16-24 months, respectively.
Missed opportunity for JE vaccine:
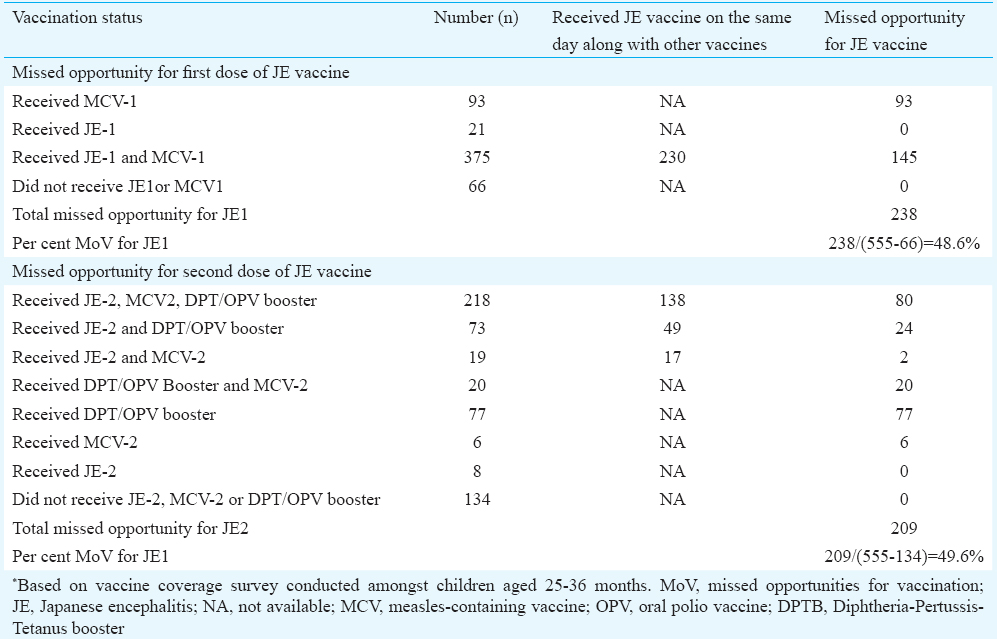
Population-based survey: Of the 840 children surveyed, 555 had vaccination cards and were evaluated for MoV for JE. Of these children, 375 had received both MCV-1 and JE-1, 93 had received MCV-1, 21 had received JE-1 vaccine, whereas 66 did not receive any of these vaccines. Of the 375 children who had received both the vaccines, 230 received these vaccines on the same day whereas 145 received MCV-1 on an earlier date than JE-1. Thus, missed opportunity for JE-1 vaccine was 48.6 per cent [(145+93)/(555-66)]. The missed opportunity for the JE-2 vaccine was 49.6 per cent (Table II).
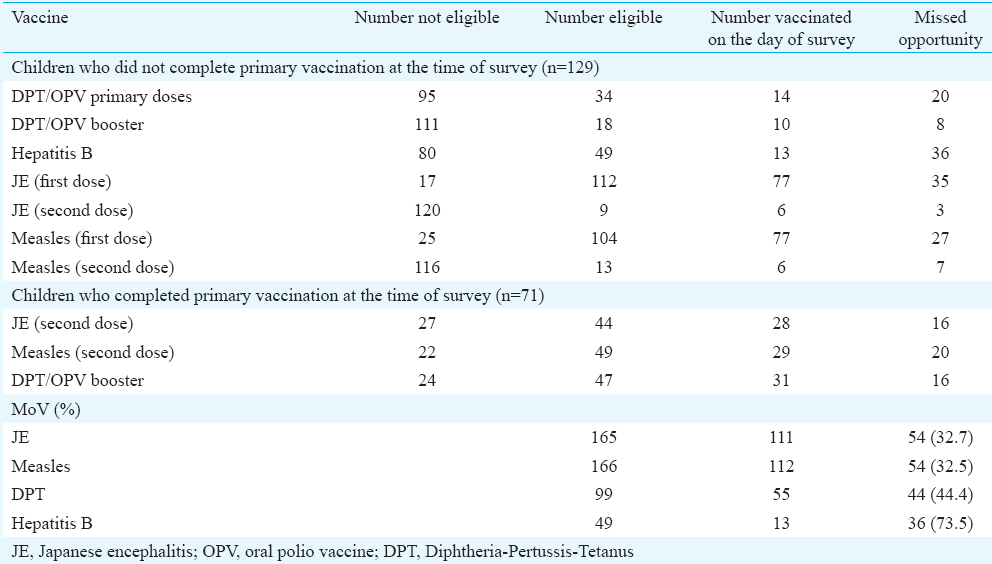
Facility-based exit survey: Mothers of 223 children aged between 9 and 24 months from 51 vaccination sessions in Gorakhpur, Kushinagar and Deoria districts were interviewed. Of these, 200 had vaccination cards and were considered for estimation of MoV for JE vaccine. Based on the vaccination details on the card, 71 children had completed primary vaccination (Bacillus Calmette–Guérin, three doses of DPT/OPV, three doses of hepatitis B vaccine, MCV-1 and JE-1 vaccine), whereas the remaining 129 had not completed their primary vaccination before their visit to the vaccination clinic. Of the 71 children who had completed primary vaccination, 44 were eligible to receive JE-2 vaccine, 28 of whom received JE vaccine on the day of survey. Amongst the remaining 129 children who had not completed primary vaccination, 112 and nine were eligible to receive JE-1 and JE-2 vaccine, respectively, and 77 and six received JE vaccine on the day of survey. The overall MoV for JE was 32.7 per cent (Table III).
The most common reasons for MoV for JE as cited by mothers of 54 children were health workers’ advice to bring child for the next session after administering one vaccine (n=46, 79.6%) and illness of child (n=5, 9.3%).
Discussion
Our results indicated that three of the four children in Gorakhpur division had received at least one dose of JE vaccine. Compared to the earlier survey conducted in 2013 which indicated that 51 per cent children received one dose of JE vaccine8, the coverage of one-dose JE vaccine in 2015 had improved significantly. However, the proportion of children receiving the second dose was low, with about 60 per cent children not receiving the second dose. MoV for JE occurred frequently during the vaccination sessions in Gorakhpur division.
In Gorakhpur division, nearly half of eligible children missed either the JE-1 or JE-2 vaccine during 2012-2013. During 2015, about one-third of the eligible children attending vaccination clinics missed JE vaccine. The magnitude of MoV observed in Gorakhpur division was comparable with other studies conducted in India and elsewhere12. In the Universal Immunization Programme, the JE vaccine is co-administered along with measles and DPT vaccine. Data from the published studies have shown that co-administration of JE with other vaccines including live-attenuated vaccines has an acceptable safety profile and does not affect the immunogenicity1516. JE vaccine when co-administered with live-attenuated measles vaccine showed no significant differences between groups in immunogenicity or safety concerns when the vaccines were given on the same day or separated by one month, with high seroprotection rates of 91.8 per cent (95% CI 87.3-95.1) for measles and 90.5 per cent (95% CI 85.9-94.1) for JE1617. In Gorakhpur, failure to co-administer vaccines was the main reason for MoV for JE. More than three-fourth of mothers reported that their children were vaccinated with only one vaccine and they were asked to bring their child for vaccination in the next session. The literature about MoV suggests that there is a misconception amongst health workers that administering multiple vaccinations at the same time can overload child's immune system and increase the risk of side effects1819. Simultaneous administration of most widely used live and inactivated vaccines does not result in decreased antibody responses or increased rates of adverse reaction17. On the other hand, it increases chances that a child will be fully vaccinated as per the age. Hence, this system-related factor for MoV must be addressed by appropriately sensitizing auxiliary nurse midwife (ANMs) about vaccinating child with all eligible vaccines.
Our study had certain limitations. First, vaccination cards were not available for about one-third of children surveyed to accurately estimate vaccination coverage. The vaccination status of these children was assessed based on mother's history. However, this approach has certain limitations with respect to dependable recall. To examine the role of recall bias, we compared the JE vaccination status in 555 children having vaccination card with that of mother's recall. Coverages of one and two dose of JE vaccine amongst these children as per card were 81.4 (95% CI: 77.4-84.9) and 47.2 per cent (95% CI: 41.7-52.8), respectively. The corresponding coverages as per mothers recall were 76.0 (95% CI: 71.8-79.8) and 46.3 per cent (95% CI: 41.7-51.0). As the coverages by the two methods were not significantly different, mother's recall might not have biased our overall coverage estimates in the division. Second, facility-based exit surveys could potentially underestimate actual prevalence of MoV, as the presence of survey team interviewing mothers exiting from vaccination clinics could influence vaccinator's behaviour in favour of minimizing MoV. It is, therefore, possible that the actual magnitude of MoV could be higher than that observed during the survey.
In conclusion, MoV for JE was an important reason for low coverage for JE vaccine in Gorakhpur. MoV occurred frequently in the vaccination clinics in Gorakhpur division, with about one-third of children attending vaccination clinics missing JE vaccine doses. Training vaccinators about correct vaccination schedule and removing their misconception about simultaneous vaccination would substantially improve coverage of JE vaccine in Gorakhpur division.
Acknowledgment
Authors acknowledge the support and cooperation extended by the Additional Director, Gorakhpur division and Dr A. K. Pandey, District Malaria Officer, Gorakhpur, Uttar Pradesh, India, in the conduct of the survey. Authors thank the Expert Group on Research cum Intervention Project of the Indian Council of Medical Research, New Delhi, for valuable comments.
Conflicts of Interest: None.
References
- 2015. Government of India. National Vector Borne Disease Control Programme, Annual Report 2014-15. Details of AES/JE Cases and Deaths from 2009-2015. Available from: http://www.nvbdcp.gov.in/Doc/je-aes-cd-March17.pdf
- Acute encephalitis syndrome surveillance, Kushinagar district, Uttar Pradesh, India, 2011-2012. Emerg Infect Dis. 2013;19:1361-7.
- [Google Scholar]
- Introduction of Japanese encephalitis virus genotype I, India. Emerg Infect Dis. 2011;17:319-21.
- [Google Scholar]
- Changing clinico-laboratory profile of encephalitis patients in the Eastern Uttar Pradesh region of India. Trop Doct. 2012;42:106-8.
- [Google Scholar]
- Changes in acute encephalitis syndrome incidence after introduction of Japanese encephalitis vaccine in a region of India. J Infect. 2014;69:200-2.
- [Google Scholar]
- AES: Clinical presentation and dilemmas in critical care management. J Commun Dis. 2014;46:50-65.
- [Google Scholar]
- A review of Japanese encephalitis in Uttar Pradesh, India. WHO South East Asia J Public Health. 2012;1:374-95.
- [Google Scholar]
- Low coverage and acceptable effectiveness of single dose of Japanese encephalitis vaccine, Gorakhpur division, Uttar Pradesh, India, 2013. J Infect. 2014;69:517-20.
- [Google Scholar]
- Government of India. National Vector Borne Disease Control Programme, National Programme for Prevention and Control of Japanese Encephalitis/Acute Encephalitis Syndrome – Operational guidelines. Available from: http://nvbdcp.gov.in/Doc/JE-AES-Prevention-Control(NPPCJA).pdf
- The use of evaluation to improve the Expanded Programme on Immunization in Mozambique. Bull World Health Organ. 1990;68:199-208.
- [Google Scholar]
- Studies of missed opportunities for immunization in developing and industrialized countries. Bull World Health Organ. 1993;71:549-60.
- [Google Scholar]
- 2013. Pan American Health Organization. Methodology for the evaluation of missed opportunities for vaccination. Washington, DC: Pan American Health Organization; Available from: http://www.paho.org/immunization/toolkit/resources/paho-publication/MissedOpportunity-Vaccination-Protocol-2013.pdf?ua=1
- Avoiding missed opportunities for immunization in the Central African Republic: Potential impact on vaccination coverage. Bull World Health Organ. 1995;73:47-55.
- [Google Scholar]
- World Health Organization. Immunization Coverage Cluster Survey – Reference Manual. Geneva: WHO; 2005.
- Potential impact on vaccination coverage levels by administering vaccines simultaneously and reducing dropout rates. Arch Pediatr Adolesc Med. 1994;148:943-9.
- [Google Scholar]
- Corrigendum to “Comparison of the immunogenicity and safety of measles vaccine administered alone or with live, attenuated Japanese encephalitis SA 14-14-2 vaccine in Philippine infants. Vaccine. 2014;2:306-8.
- [Google Scholar]
- Japanese encephalitis vaccines: WHO position paper – February 2015. Wkly Epidemiol Rec. 2015;90:69-87.
- [Google Scholar]
- Why children are not vaccinated: a review of the grey literature. Int Health. 2012;4:229-38.
- [Google Scholar]
- WHO. Six common misconception about immunization. Geneva: WHO; Available from: http://www.who.int/vaccine_safety/initiative/detection/immunization_misconceptions/en/index6.html






