Translate this page into:
Comparison of clinical effects of beclomethasone dipropionate & budesonide in treatment of children with mild persistent asthma: A double-blind, randomized, controlled study
Reprint requests: Dr Devki Nandan, Department of Pediatrics, PGIMER and Dr. RML Hospital, New Delhi 110 001, India e-mail: devkinandan2002@yahoo.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Various inhaled corticosteroids (ICSs) are available to control the symptoms of asthma. Although beclomethasone dipropionate (BDP) and budesonide (BUD) are one of the oldest ICSs, their wide availability and low cost make them attractive options in developing countries. Due to lack of consensus on which of the two drugs is better for controlling mild persistent asthma, we undertook this study to compare the efficacy of these two drugs by measuring the change in percentage predicted forced expiratory volume in one second (FEV1) from baseline in children with mild persistent asthma.
Methods:
A double-blind, randomized, parallel group study was conducted in children 7-15 yr of age with newly diagnosed asthma. Of the 85 cases of mild persistent asthma, 42 received BUD while 43 received BDP at a dose of 400 µg/day using pressurized metered-dose inhaler with valved spacer for two months. The outcomes measured were change in FEV1, symptom scores and side effects.
Results:
There was a significant (P < 0.05) improvement in FEV1 in BUD group (98.43 ± 4.63%) than in BDP group (95.65 ± 5.66%) at the end of two months of treatment. The mean symptom scores in BUD group (0.28 ± 1.22) and BDP group (0.43 ± 1.52) were comparable after two months. No side effects were seen in either group.
Interpretation & conclusions:
FEV1 was significantly greater in BUD group than BDP group. Improvement in symptoms and incidence of side effects were similar. Our findings indicate that both BDP and BUD can be used effectively in the management of children with mild persistent asthma. [CTRI No: CTRI/2013/03/003495].
Keywords
Beclomethasone dipropionate
budesonide
forced expiratory volume in one second
metered dose inhaler
mild persistent asthma
symptom score
Asthma is a common childhood illness characterized by chronic airway inflammation. This chronic inflammatory nature of the disease needs to be checked on time to prevent long-term irreversible impairment of pulmonary function1. Uncontrolled asthma reduces the quality of life of children by retarding the growth2, inability to exercise3, loss of sleep and frequent absences from school4.
There are various medications available to control the underlying inflammation such as inhaled corticosteroids (ICSs), oral steroids, leukotriene modifiers, chromones and theophylline. Of these, ICSs are considered as the most effective therapy for all levels of persistent asthma1. Their success is based on their ability to improve asthma symptoms and quality of life5, low systemic bioavailability and lesser side effects6. The various ICSs available are beclomethasone dipropionate (BDP), budesonide (BUD), ciclesonide, flunisolide, fluticasone and mometasone. Among these, BUD and BDP are widely used in developing countries due to their better cost-benefit ratio. However, it is still a matter of debate as to which of these two drugs is a better drug in terms of efficacy. Various in vitro studies have shown differences in their pharmacokinetic properties. Receptor affinity of BDP is higher while in vitro potency of BUD is greater78. It is unclear whether these differences translate into any clinical significance.
The available data on the comparison of clinical effects of these two drugs in children have mostly come from small crossover studies that included less than 30 children9101112. Majority of these studies have poorly defined inclusion and exclusion criteria and have used different delivery devices with or without the spacers. One of these studies has shown significantly higher forced expiratory volume in one second (FEV1) during BUD treatment12, while others have shown only slightly higher FEV1 during BUD treatment91011. A meta-analysis of crossover studies done in children and adults did not demonstrate a significant difference between BDP and BUD for FEV1, morning peak expiratory flow (PEF), evening PEF, asthma symptoms or rescue beta-2 agonist use, over a dose range of 400-1000 µg13.
At present, BUD is being used for the treatment of mild persistent asthma in our hospital. However, the low cost and wide availability of BDP make it an attractive alternative14. Moreover, due to lack of consensus on which of the two drugs is better for controlling mild persistent asthma, We conducted this study to compare the clinical effects of the two drugs so as to help to develop standardized guidelines. The objective of our study was to compare the efficacy of these two drugs in terms of improvement in pulmonary function in children with mild persistent asthma, measured by change in predicted FEV1 from baseline.
Material & Methods
This parallel group double-blind, randomized, controlled trial (RCT) was conducted in the department of Pediatrics, Dr. Ram Manohar Lohia Hospital, New Delhi, India between November 2011 and November 2012 after approval by the Institutional Ethical Committee. The trial was registered in the Clinical Trials Registry of India No: CTRI/2013/03/003495.
Participants: Children with newly diagnosed mild persistent asthma aged 7-15 yr attending wards, outpatient department and chest clinic were included in this study. Mild persistent asthma was defined as asthma symptoms occurring more than once a week but not daily, exacerbations affecting activity and sleep, nocturnal symptoms greater than or equal to once a month and FEV1 ≥80 per cent of the predicted. Cases fulfilling the above definition and showing an improvement of ≥12 per cent in FEV1 after 15 min of administration of four separate doses of 100 µg of salbutamol metered dose inhaler (MDI) with valved spacer were considered eligible15. Children who received oral, parenteral or inhaled steroids during the last one month; children with any underlying chronic illness including cystic fibrosis, pneumothorax, chronic suppurative lung disease, tuberculosis, congenital heart diseases and any other chronic systemic illness; those unable to use inhaler with spacer or perform spirometry and children with lower respiratory tract infections in the last one month were excluded from the study. Written informed consent was obtained from parents of all the children.
Interventions- how and when they were administered: Children with newly diagnosed asthma were randomly assigned to study (BDP) and control (BUD) groups. Children allocated to BUD group received BUD 200 µg one puff twice a day b.i.d. and children in BDP group received BDP 200 µg one puff b.i.d. from an MDI with a valved spacer. Allocation of children to one of these groups was based on computer-generated simple randomization by an independent person. After selecting the cases and recording the absolute and percentage predicted values of FEV1, patients were given these medicines for two months.
Outcome measures: The primary outcome of our study was to compare the clinical effects of inhaled BDP and BUD by comparing the change in percentage predicted FEV1 after two months of therapy. The secondary outcomes were to compare symptom scoring for day and night time symptoms, limitation in daily activities, absence from school, need for rescue salbutamol inhaler, number of exacerbations since the last visit, need for an emergency visit and incidence of side effects. The definitions used for this study were as follows: FEV1 is the volume of air exhaled in the first second of a forceful expiration. Symptom score was done by the end of each month using a validated Asthma Therapy Assessment Questionnaire (ATAQ)1617. Major side effects observed in the study were cough, hoarseness of voice and candidiasis. These side effects were assessed clinically by history and physical examination on each visit.
The patients’ clinical history and physical examination findings were noted. We used vitalograph portable spirometer which was calibrated for volume once daily with an airtight 3 l calibrated syringe with an accuracy of 15 ml18. Since valid spirometric measures are dependent on patient's ability to perform properly a full, forceful and prolonged expiratory manoeuvres, spirometry was done after properly explaining the correct method to the patients, and the best of three spirometry attempts was recorded. After recording the absolute and percentage predicted values of FEV1, patients were given the medicines for next two months which included random number coded MDI and salbutamol MDI (rescue medication). The same type of valved spacers was used for the delivery of drug from the MDI. Parents were explained to maintain daily symptom diary for day and night symptoms, the number of acute exacerbations, use of rescue medication, visits to the emergency department, limitation in daily activities and absence from school.
Children were followed up at two weeks, first and second month. Compliance to medicines was checked at every visit, as well as telephonically every week. Every month symptom scoring and spirometry were done. The patients were labelled as having controlled asthma if ATAQ score and FEV1 showed improvement during the two months follow up. Those who were not fulfilling these criteria were planned to be stepped up and withdrawn from the study. Any patient developing acute exacerbation was advised to take 2-4 puffs of salbutamol MDI with valved spacer every 20 min for the first hour15 and immediately visit the emergency department for further management. Oral steroids were allowed for acute exacerbation management.
Sample size: There are no previous studies on the comparison of BUD versus BDP in children with mild persistent asthma. Therefore, we hypothesized that if a mean difference in FEV1 in BDP was lower than two per cent in comparison to BUD, it would be considered non-inferior to BUD. Sample size of 39 per group was calculated based on the assumption of at least a mean difference of two per cent in FEV1 (%) at second month between the two groups, a standard deviation (SD) of 32 or effect size of 0.67, a two-sided alpha of 0.05, beta of 0.80, a power of 80 and 10 per cent as lost to follow up.
Randomization:
Sequence generation: (i) Randomized sequence was generated using computerized random number generator. (ii) Randomization list was prepared by biostatisticians not involved in the study and was kept with them.
Randomization-allocation concealment: Serially numbered, sealed, identical opaque envelopes containing the random numbers were kept at the study site as per the allocation sequence.
Randomization-implementation: The randomization was done as soon as the cases were enrolled in the study. The research staff assigned each case the next serial number corresponding to the randomization code of the intervention.
Randomization-blinding: A person independent of the study prepared the medicines. After removing their labels, both the medicines were painted with red colour and covered with similar coloured caps. Study labels containing random numbers were put on the medicines. The treating paediatricians selected the cases, assessed them and advised these medicines. The random number coded medicines were distributed by the staff nurse. Once the statistical analysis was done; the identity of the two drugs was revealed to the treating paediatrician by the person who prepared the medicines. The participants and the treating paediatrician both were blinded to the intervention.
Statistical analysis: Data were collected using a predesigned proforma. Statistical testing was conducted with the Statistical Package for the Social Science system version SPSS 17.0 (Chicago, IL, USA). Chi-square test and Fisher's exact test (used where expected frequency was <5) were used for categorical variables and Wilcoxon rank-sum test or Student's t test was used for continuous variables depending on whether they were normally distributed or not. Analysis was performed according to the intention to treat principle.
Results
Of the 90 patients screened, 85 children with mild persistent asthma were enrolled and randomized. Forty three children received BDP, while 42 received BUD. Three children were lost to follow up from BDP group and two from BUD group (Figure). The baseline demographic and clinical characteristics of the study groups are presented in Table I. The two groups had comparable baseline characteristics including the baseline spirometric parameters, i.e. FEV1.
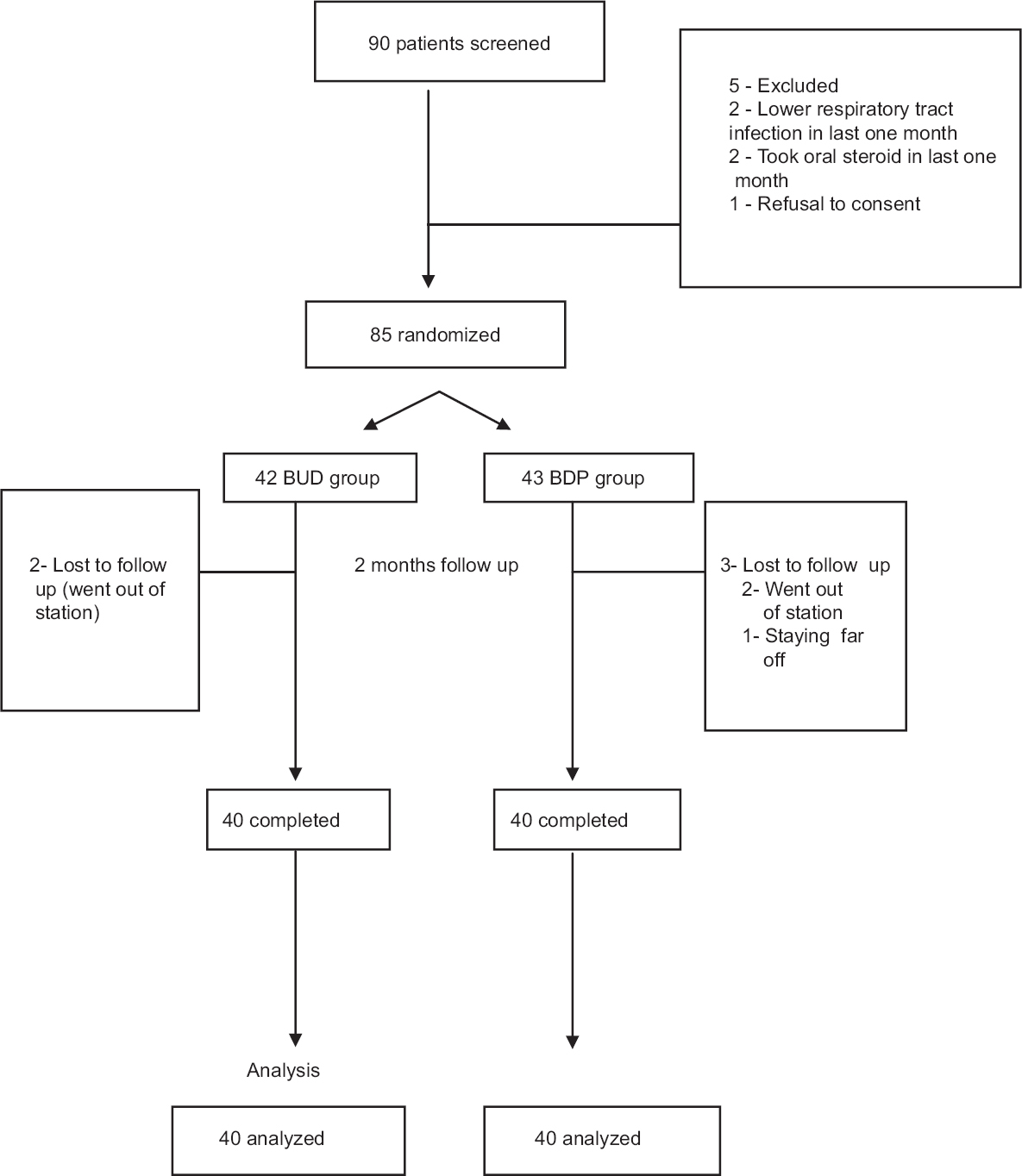
- Flow chart, showing study design. BUD, budesonide; BPD, beclomethasone dipropionate.

Primary outcomes: At the end of two months, mean FEV1 in BDP group was 1.55 ± 0.39 l, while in BUD group was 1.74 ± 0.42 l with corresponding percentages of 95.65 ± 5.66 and 98.43 ± 4.63, respectively (Table II). When the two groups were compared, there was a significant (P<0.05) improvement in FEV1 in BUD group [mean difference: −2.78; 95% confidence interval (CI): -5.08 - -0.48] as compared to BDP group (Table II).

Secondary outcomes: The mean symptom score in BDP group at baseline was 4.30 ± 0.75 and in BUD group was 4.28 ± 0.85. On intergroup comparison of symptom score after two months of treatment, improvement in BDP (0.43 ± 1.52) and BUD groups (0.28 ± 1.22) was similar (mean difference: 0.15; 95% CI: -0.46 - -0.76, P = 0.638) as shown in Table III. No side effects were observed in either group.

Some additional parameters were also observed in our study including PEF rate at the end of the first and second month, number of acute exacerbations, limitation of physical activities, sleep disturbance, school absenteeism, use of rescue medication and visit to emergency department (Table III). There was significant improvement in PEF in both group BDP (3.32 ± 0.89 l, 3.68 ± 1.19 l) and group BUD (3.89 ± 1.14 l, 4.12 ± 1.10 l) after the first and second month of treatment. When both groups were compared, BUD group had significantly better improvement in PEF than BDP group (P<0.05) after first and second month (Table IV). However, other additional parameters did not show significant difference between the two groups (Table III).

Discussion
In this double-blind, randomized, single centre study, greater improvement was reported in patients’ FEV1 treated with 400 µg/day of BUD MDI as compared to 400 µg/day of beclomethasone MDI administered through valved spacer with comparable improvement in symptoms of asthma without any side effect after two months of treatment.
Mild persistent asthma constitutes the largest group of children with persistent asthma1. The previous clinical studies comparing inhaled BDP and BUD in asthmatic children have shown no clear advantage in terms of efficacy of either drug9101112. However, many clinico-pharmacological studies have suggested that BUD has better topical to systemic glucocorticoid activity ratio than BDP, and BUD may be preferred where high doses ICSs are required to control asthma19.
In 1982, Field and colleagues9 conducted a study on 31 severely asthmatic children aged between 4 and 14 yr requiring regular inhaled steroid prophylaxis. Fifteen children used BUD through pressurized aerosol with a spacer inhaler, while 12 used BDP through Rotahaler. FEV1 and symptom score in the BUD group were 83.4 ± 22.9 l and 13.6 ± 16.5, while the values of the same parameters in BDP group were 78.5 ± 25.1 l and 17.2 ± 19.3, respectively. This comparison did not show any significant difference although there was a consistent tendency towards greater benefit from BUD9. In another study conducted by Springer et al.10 on 10 asthmatic children in each group who were already on treatment with steroids were given a total daily dose of 400 µg each of BDP and BUD through a conventional pressurized aerosol without a spacer. The comparison of FEV1 and symptom score between the two groups showed no significant difference10. In a study by Baran et al11 on 21 chronic asthmatic children, aged 4-14 yr who were on regular ICSs for their control, BUD group received 100 µg b.i.d. through spacer inhaler, while BDP group received 100 µg b.i.d. through dry powder inhaler. On analysis, it was found that compared with placebo, FEV1 was significantly better with BUD (P<0.05), but no significant effect could be detected with BDP11.
Our study also reflected improvement in the quality of life of the asthmatic children, especially those receiving BUD. In the BUD group, though there was a reduction in sleep disturbance, school absenteeism, limitation of physical activities, less number of children requiring emergency visits and rescue medication, but this difference was not significant. There was a significant increase in PEF at the end of the first and second month in the group receiving BUD. However, daily home monitoring of PEF rate was not possible as patients could not purchase the peak flow meter.
The strengths of our study were the study design, proper blinding and objective primary outcome. It was an adequately powered RCT in children with mild persistent asthma and the results favoured BUD which is commonly prescribed in most regions of the world. Thus, our results can be generalized to most settings. However, one important limitation of our study was the short follow up period of two months during which we did not expect the compliance to be as poor as would be the case in case of a lengthier time period.
In conclusion, our study showed improvement in patients’ FEV1 treated with 400 µg/day of BUD MDI as compared to 400 µg/day of beclomethasone MDI administered through spacer with valve over a period of two months. However, comparable improvement in symptoms of asthma was observed in both the groups. No significant safety concerns were identified. BUD was found to be slightly more effective than BDP. It can be suggested that both BDP and BUD can be used effectively in the management of children with mild persistent asthma.
Acknowledgment
Authors acknowledge Dr T.P. Yadav and the hospital administration for providing inhalers, Sister Chanci as the nursing staff for the distribution of medicines and the pharmacist Shrimati Sagar for the preparation of the drug. Authors thank Shrimati Parul Takkar for helping in statistical analysis and Dr. Neha Patharia for collection of review material.
Conflicts of Interest: None.
References
- 2007. National Heart, Lung and Blood Institute. Bethesda, MD: National Asthma Education and Prevention Program; c2001. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma – Summary Report Available from: http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm
- Maturational delay and temporal growth retardation in asthmatic boys. J Allergy Clin Immunol. 1977;59:200-6.
- [Google Scholar]
- Sport and the asthmatic child: a study of exercise-induced asthma and the resultant handicap. J R Coll Gen Pract. 1988;38:253-5.
- [Google Scholar]
- Morbidity and school absence caused by asthma and wheezing illness. Arch Dis Child. 1983;58:777-84.
- [Google Scholar]
- Effect of long-term treatment with an inhaled corticosteroid (budesonide) on airway hyperresponsiveness and clinical asthma in nonsteroid-dependent asthmatics. Am Rev Respir Dis. 1990;142:832-6.
- [Google Scholar]
- Inhaled glucocorticoids in childhood asthma. In: Godfrey S, ed. Glucocorticoids in childhood asthma. Amsterdam: Exerpta Medica; 1987. p. :78-84.
- [Google Scholar]
- Beclomethasone dipropionate: absolute bioavailability, pharmacokinetics and metabolism following intravenous, oral, intranasal and inhaled administration in man. Br J Clin Pharmacol. 2001;51:400-9.
- [Google Scholar]
- Clinical pharmacokinetics of inhaled budesonide. Clin Pharmacokinet. 2001;40:427-40.
- [Google Scholar]
- Asthma treatment with a new corticosteroid aerosol, budesonide, administered twice daily by spacer inhaler. Arch Dis Child. 1982;57:864-6.
- [Google Scholar]
- Comparison of budesonide and beclomethasone dipropionate for treatment of asthma. Arch Dis Child. 1987;62:815-9.
- [Google Scholar]
- A comparison of inhaled budesonide and beclomethasone dipropionate in childhood asthma. Br J Dis Chest. 1987;81:170-5.
- [Google Scholar]
- Endocrine and lung function in asthmatic children on inhaled corticosteroids. Am J Respir Crit Care Med. 1994;150:624-8.
- [Google Scholar]
- Inhaled beclomethasone versus budesonide for chronic asthma. Cochrane Database Syst Rev. 2002;1:CD003530.
- [Google Scholar]
- Wheezing in older children: Asthma. In: Chernick V, Boat TF, Wilmott RW, Bush A, eds. Kendig and Chernick's disorders of the respiratory tract in children (8th ed). Philadelphia: Saunders; 2012. p. :701-30.
- [Google Scholar]
- 2002. Global Strategy for Asthma Management and Prevention, Global Initiative for Asthma (GINA) 2006. Available from: http://www.ginasthma.org
- c2008-2010. Asthma Therapy Assessment Questionnaire. New Jersey: Merck Sharp and Dohme Corp; Available from: http://www.asthmacontrolcheck.com/asthma_control/asthmacontrolcheck/consumer/index.jsp
- Association of asthma control with health care utilization and quality of life. Am J Respir Crit Care Med. 1999;160:1647-52.
- [Google Scholar]
- Budesonide. An updated review of its pharmacological properties, and therapeutic efficacy in asthma and rhinitis. Drugs. 1992;44:375-407.
- [Google Scholar]






