Translate this page into:
Association of rheumatic fever & rheumatic heart disease with plausible early & late-stage disease markers
Reprint requests: Dr. Anuradha Chakraborti, Department of Experimental Medicine & Biotechnology, Postgraduate Institute of Medical Education & Research, Chandigarh 160 012, India e-mail: superoxide14@gmail.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Rheumatic fever (RF) and rheumatic heart disease (RHD) are the autoimmune sequelae caused by Group A Streptococcus. RHD still remains a major concern in the developing countries due to its poor diagnosis, lack of vaccines and social awareness among population. This study was aimed to identify the plausible early- and late-stage disease markers associated with RF/RHD.
Methods:
A total of 84 patients with confirmed pharyngitis (n=18), RF (n=23) and RHD (n=43) were included in the comparative analysis of different factors involved in host-pathogen interaction during RF/RHD pathogenesis.
Results:
This study revealed high titre of serum antistreptolysin O (ASO) antibody in pharyngitis compared to RF and RHD patients, whereas procollagen type 1 C-peptide (PICP) level was elevated in RHD which showed an inverse correlation with serum ASO titre. The significant elevation of serum anti-peptide associated with RF (PARF) antibody in RF patients was correlated as a probable stage-specific determinant. In addition, pro-inflammatory cytokine profile revealed high levels of interleukin-12 (IL-12)/IL-23p40, IL-17A in RF, whereas IL-6 concentration was higher in RHD compared to healthy controls.
Interpretation & conclusions:
The overall assessment of the factors/disease markers involved in host-pathogen interaction in RF/RHD may be suggestive of plausible disease marker in different groups of patients. Further studies with larger sample need to be done to better understand RF/RHD pathogenesis.
Keywords
Antistreptolysin O
cytokines
mannose-binding lectin
peptide associated with rheumatic fever
pharyngitis
procollagen type 1 C-peptide
rheumatic fever
rheumatic heart disease
Rheumatic fever (RF) is autoimmune sequelae of disease affecting children and young adults worldwide. It develops mostly from untreated or poorly treated tonsillopharyngitis caused by β-haemolytic Group A streptococcus (GAS)1. RF further leads to rheumatic heart disease (RHD)2, and may progress to atrial fibrillation, embolic stroke and heart failure. Annually, over 600 million people suffer due to GAS-induced pharyngitis3, of whom 0.3-3 per cent develop RF and 30-45 per cent of RF further acquire RHD4. The poor diagnosis of this disease is due to lack of diagnostic markers as well as availability of radiological set-up in rural health centres5.
Several virulence factors of GAS participate in infection and play a pivotal role in disease progression towards RHD. Streptolysin O, a potent exotoxin and haemolysin, is often used to characterize the streptococcal and post-streptococcal infectious stage6. Surface M protein of GAS and its N-terminal motif i.e., peptide associated with RF (PARF) also plays an imperative role during autoimmune pathogenesis. PARF interacts with the CB3 region of human type IV collagen and induces the autoimmunity in RF7. Procollagen type 1 C-peptide (PICP) is secreted into the circulation during collagen synthesis, and an elevated level is attributed to collagen-related disease. The carboxy-terminal PICP in serum of RHD patient group has been reported to be associated with the severity of heart valve damage8. Therefore, such interacting factors/biomolecules could be used as potential disease markers.
Besides, the host immune system is regulated by the interplay of Th1/Th2 cells in the pharyngeal as well as in the post-pharyngeal state of the disease. The mannose-binding lectin (MBL) of host complement system also plays a pivotal role in valvular injuries during RHD9. Such a complicated mechanism in the pathophysiology of RHD may be explained by a wide range of biomolecular alterations, which can be observed in the patient's serum during the developmental stages of RHD. Hence, this study was undertaken to investigate the host responses against pathogenic factors as well as involvement of different host factors during the RF/RHD pathogenesis.
Material & Methods
This study was conducted from October 2010 to November 2013 and was approved by the Institute Ethics Committee, Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh, India (IEC No. 10/3962, dated 23.09.2010). Only those participants (patients and kin, caretakers or guardians on behalf of the minors/children) who provided written informed consent were included in the study. A total of 84 patients (male: 39, female: 45) including children (n=29), adolescent (n=12), adult (n=37) and middle-aged/elderly (n=6), who visited during the study period to the OPDs of Advanced Cardiac Centre, Advanced Paediatric Centre and Community Clinics of School of Public Health, PGIMER, Chandigarh, from different parts of north India, were included in the study. The patients were divided into three different disease groups; 5 to 15 yr of age for pharyngitis (n=18), 5 to 65 yr of age for RF (n=23) and RHD (n=43). The overall sample collection procedure was done as discussed previously10. Single blood sample (2 ml) was collected from each patient suffering either from pharyngitis or RF/RHD. Serum was separated and stored at −20°C. Only 50 healthy individuals from different departments who volunteered to participate in the study were included as healthy controls. All RF and RHD patients were on prophylactic penicillin during the time of blood collection (duration of prophylaxis was not available) whereas no prophylactic penicillin was given to the pharyngitis patients before blood collection.
Inclusion & exclusion criteria: Patients suffering from RHD were clinically diagnosed by electrocardiography or auscultation and confirmed by echocardiography. Diagnosis of acute RF (ARF) was clinically done by the modified Jones criteria1112 and pharyngitis by severe sore throat with positive GAS culture, high fever, with or without dehydration (10%), nausea and vomiting13. Patients having secondary infections or any other diseases, taking drugs (in case of healthy individuals) and patients who refused to give consent were excluded from the study.
All parameters were measured (in triplicate and in each patient) in different disease groups (pharyngitis, RF and RHD) and healthy controls.
Estimation of antistreptolysin O (ASO) titre: Serum concentration of ASO was estimated by semi-quantitative method based on lysis of RBCs14. In brief, serum samples were inactivated by incubating at 56°C for 30 min in water bath and were 1:10 diluted with phosphate-buffered saline (PBS) containing 0.1 per cent albumin. A volume of 0.5 ml of diluted sample was mixed with 4.5 ml of PBS containing 0.1 per cent albumin and serial dilutions were prepared. A volume of 0.5 ml of reduced streptolysin O containing 0.14 per cent sodium dithionite was added in each tube. After gentle mixing, the tubes were incubated at 37°C for 15 min in water bath. A volume of 0.5 ml of erythrocyte suspension was added to each tube and mixed by gentle stirring. Finally, the tubes were incubated at 37°C for one hour. The ASO titre was calculated by haemolysis and the reciprocal values of their corresponding dilutions.
Estimation of anti-peptide associated with rheumatic fever (PARF) antibody: Antibodies against PARF in the serum samples of different disease groups were assessed by a previously described method6. Consensus sequence (AXYLZZLN) of PARF was commercially synthesized by ISOGEN Life Sciences (Netherlands) and conjugated with Keyhole limpet haemocyanin (KLH). Briefly, the peptide was coated in optimal dilutions on 96-well microtitre plate (Nunc InterMed, Denmark) in carbonate-bicarbonate buffer (pH 9.6) and incubated at 4°C overnight followed by washing. After blocking the non-specific sites with bovine serum albumin (BSA), the serum was added to each well. Following incubation and washing, goat antihuman IgG (Bangalore Genei, India) was added. The coloured product was read at 492 nm in an ELISA reader (Sunrise Tech, Germany).
Procollagen type I C-peptide (PICP): The immunological assay of serum PICP level was carried out by PICP EIA kit following manufacturer's instruction (Takara Bio Inc., Japan).
Cytokine analysis: Serum concentration of interleukin-2 (IL-2), IL-4, IL-6, IL-10, tumour necrosis factor-alpha (TNF-α), interferon gamma (IFN-γ) and IL-12/IL-23p40 was estimated by Human Th1/Th2 Cytokine Kit and Human IL-12/IL-23p40 flex set (BD™ Cytometric Bead Array) following the manufacturer's instructions. The data were analyzed in FACSAria III (Becton, Dickinson and Company, USA).
Mannose-binding lectin (MBL)-2 polymorphism analysis: Genomic DNA was extracted from the patient's blood by HiYield™ Genomic DNA Mini Kit (Blood/Bacteria/Cultured Cells) using manufacturer's instructions (Real Biotech Corporation, Taiwan). Single-nucleotide polymorphism (SNPs) in MBL2 were analyzed by amplifying the partly coding sequence of exon 3 of MBL2 gene using 5’-AGGCAGCCAGGCTACTATCA-3’ and 5’-TTTGGGGTTGGATGGAAATA-3’ primers (NCBI Reference Sequence: NG_008196.1). The temperature cycles used were 94°C for five minutes, 94°C for 45 sec, 58°C for 75 sec, 72°C for 90 sec and 72°C for seven minutes. The amplified products were purified by HiYield™ polymerase chain reaction DNA purification kit (Real Biotech Corporation). The purified products were processed for sequencing reactions in ABI PRISM® dGTP BigDye™ Terminator v3.0 Ready Reaction Cycle Sequencing kit (Applied Biosystems, USA) following manufacturer's instructions. Finally, the sequencing was carried out in 3130×l genetic analyzer of Applied Biosystems. The sequence of the amplified product was analyzed using Multialign software (http://multalin.toulouse.inra.fr/multalin/).
Statistical analysis: Data were expressed as mean±standard deviation along with median and interquartile ranges (IQ Re) wherever appropriate. Student's t test was used to analyze the data. The correlation between different antibody titres was evaluated using Pearson's Chi- square test and correlation coefficient (R). Kolmogorov–Smirnov test was used to analyze the quantitative data of serum cytokines. The data were analyzed by SPSS version 17 (SPSS Inc., Chicago, IL, USA).
Results
Serum ASO titre in different disease groups: The serum ASO titre was found to be maximum in pharyngitis (218.61±148.80 IU; P=0.02). A significant elevation in ASO titre was also observed in RF (202.48±128.17 IU; P=0.09) than RHD (151.30±91.01). However, similar median value (195 IU) was observed due to a wide range of variation in serum ASO titre in pharyngitis as well as in RF which was suggestive of current and/or recurrent stages of infection. The ASO titre in healthy controls (healthy individual) was found to be 121±14.91 IU with a median value of 116.5 IU (Fig. 1).
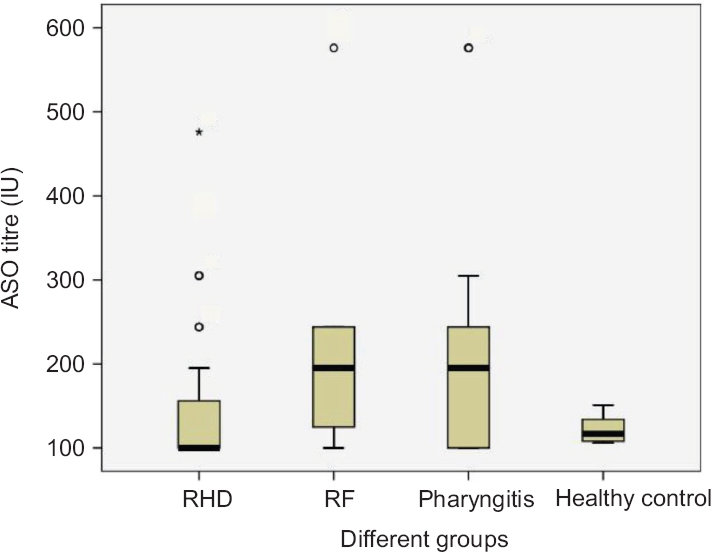
- Box plot showing antistreptolysin O (ASO) titre in the serum samples of different groups. The pharyngitis group shows significantly higher serum antistreptolysin O titre among the different disease groups. Extreme values are represented as ‘o’ and ‘*’.
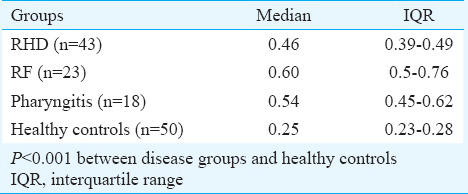
Anti-peptide associated with rheumatic fever (PARF) antibody: The level of circulatory anti-PARF antibody was estimated in different groups, and a wide range of serum antibody titre was observed (Table I). Further analysis of the data by median as well as in I.Q. range showed the maximum level of anti-PARF antibody in RF, followed by pharyngitis and RHD compared to healthy controls (P<0.001).
Concentration of serum procollagen type 1 C-peptide (PICP): A significantly high (P<0.05) concentration of PICP was observed in RHD patient (1011.43±501.221 ng/ml; median=1080 ng/ml) group compared to RF (950.00±500.520 ng/ml; median=1000 ng/ml) and pharyngitis (486.67±75.719 ng/ml; median=520 ng/ml) against healthy individual (median=530 ng/ml) (Fig. 2). A negative correlation coefficient (R=−0.144) was observed between ASO and PICP (Table II and Fig. 3).
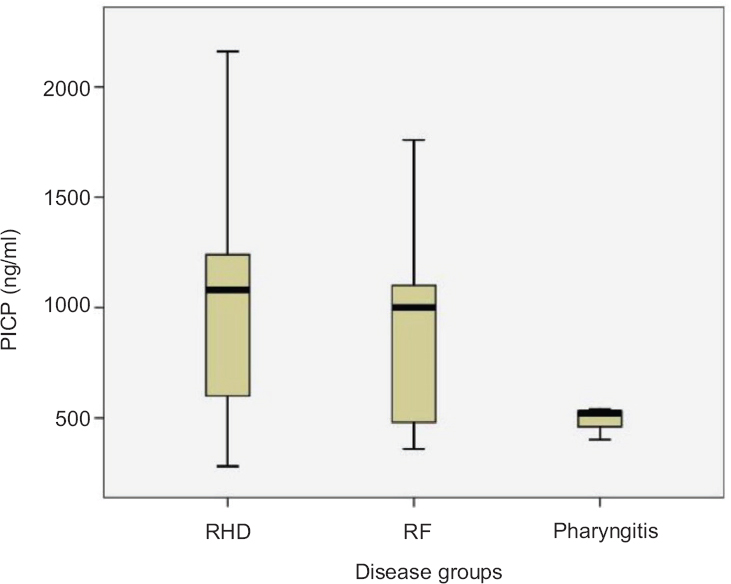
- Serum procollagen type 1 C-peptide (PICP) concentration. Rheumatic heart disease (RHD) and rheumatic fever (RF) groups show elevated serum PICP concentration compared to pharyngitis.
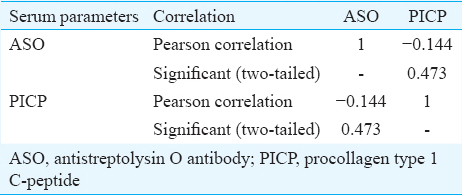
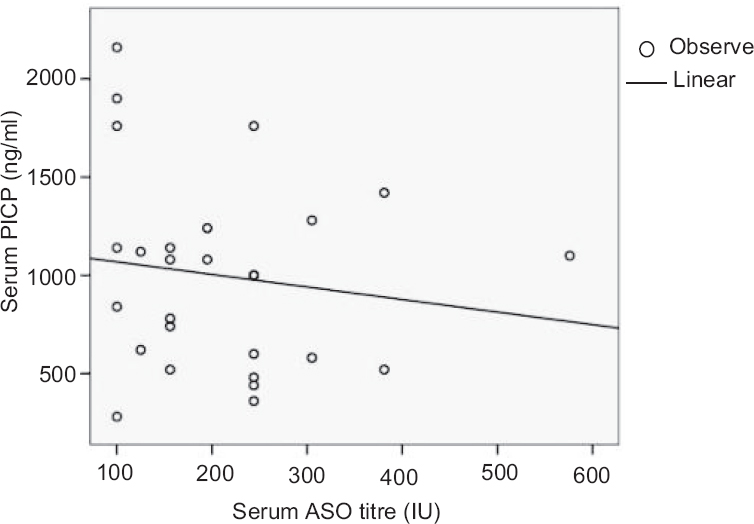
- Correlation between antistreptolysin O (ASO) and procollagen type 1 C-peptide (PICP) in the serum samples of different disease groups. The scatter diagram showing a negative correlation between ASO and PICP.
Host immune response: Flow cytometric experiments revealed a significant alteration in various serum cytokines in patients in different disease groups. A total of eight cytokines (IL-12/IL-23p40, IL-17A, IFN-γ, TNF-α, IL-10, IL-6, IL-4, IL-2) were analyzed in all the patient groups and healthy controls. It was observed that IL-12/IL-23p40, IL-17A level was maximum in RF patients followed by RHD, pharyngitis and healthy controls. RHD patients showed significantly (P<0.001) high IL-6 concentrations compared to healthy controls (Table III). Whereas, TNF-α level was found to be significantly higher in RF (P<0.05) than pharyngitis and in RHD, although the level was high, it was not significant. IFN-γ, IL-10, IL-4 and IL-2 in different disease groups did not show any variations (Table III and Figs 4, 5).
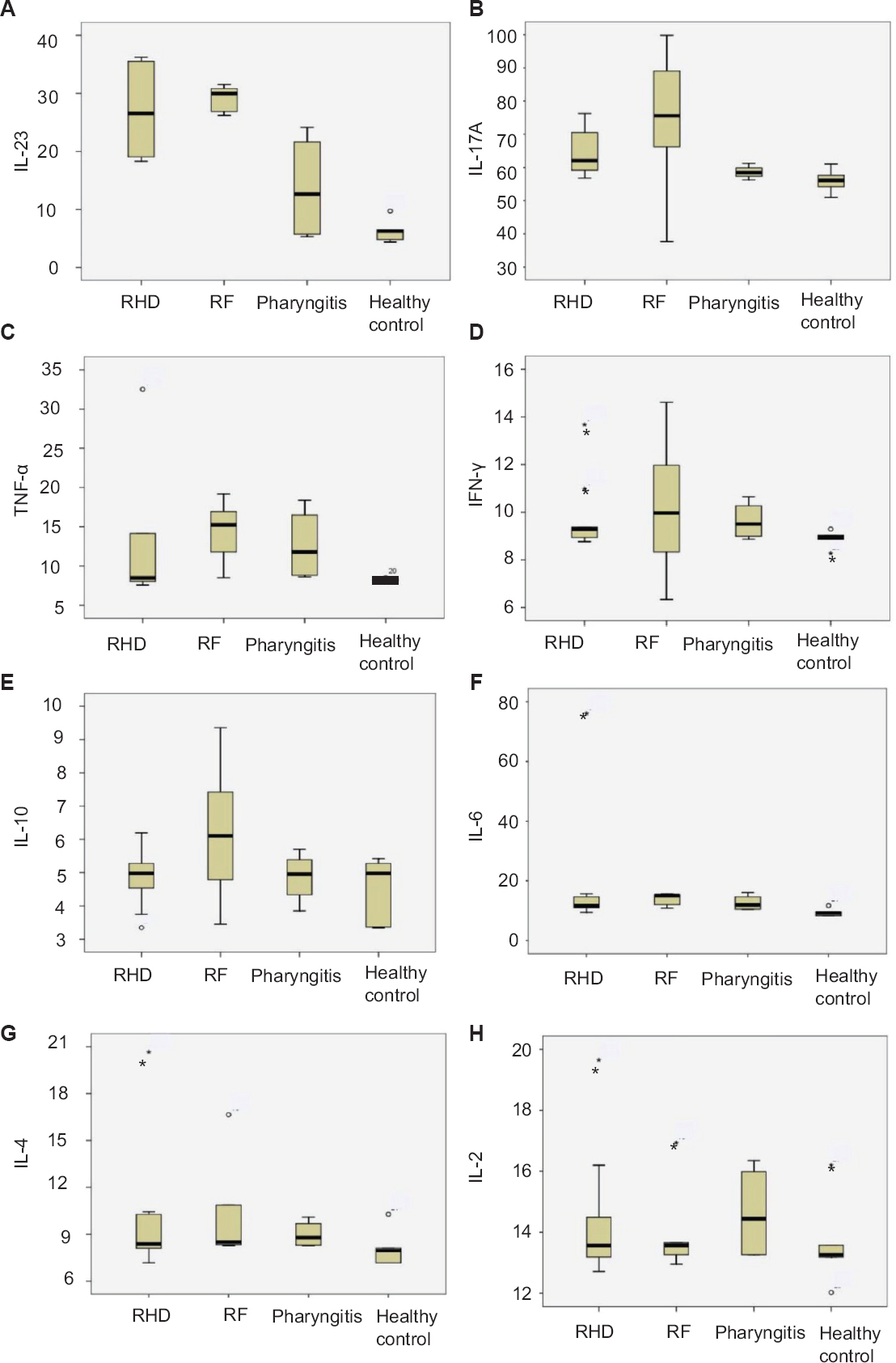
- Box plots elucidating combined serum cytokines data in different groups of patients. (A) interleukin (IL)-12/interleukin-23p40 and (C) tumour necrosis factor-alpha (TNF-α) level showing highest concentration in rheumatic fever (RF) compared to the other groups, (F) rheumatic heart disease (RHD) group showing an elevated level of IL-6 concentration (B) IL-17A showing highest concentration in RF but significantly high in RHD, whereas (D) interferon gamma (INF-γ), (E) IL-10, (G) interleukin-4, and (H) IL-2 showing no comparable difference among the groups.
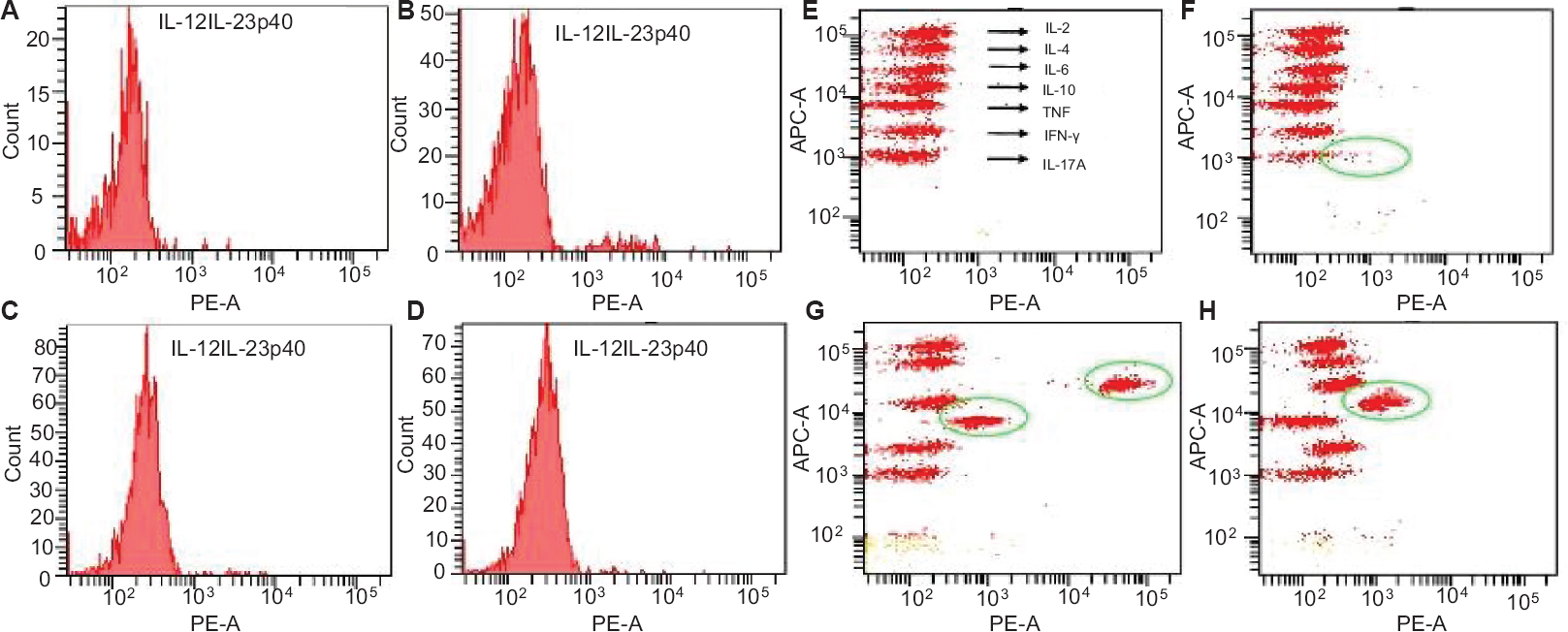
- Fluorescence activated cell sorting analysis of serum cytokines in different disease groups. Two-dimensional plots of fluorescent activated cell sorting analysis represent the concentration of interleukin (IL)-12/interleukin-23p40 in serum samples of (A) healthy controls, (B) pharyngitis (C) rheumatic fever (RF), and (D) rheumatic heart disease (RHD). Three dimensional shifts demonstrate the concentration of (F) IL-17A, and (H) IL-10 in RF; (G) IL-6 and tumour necrosis factor-alpha (TNF-α) in RHD, whereas no shifts have been observed in healthy controls (E).
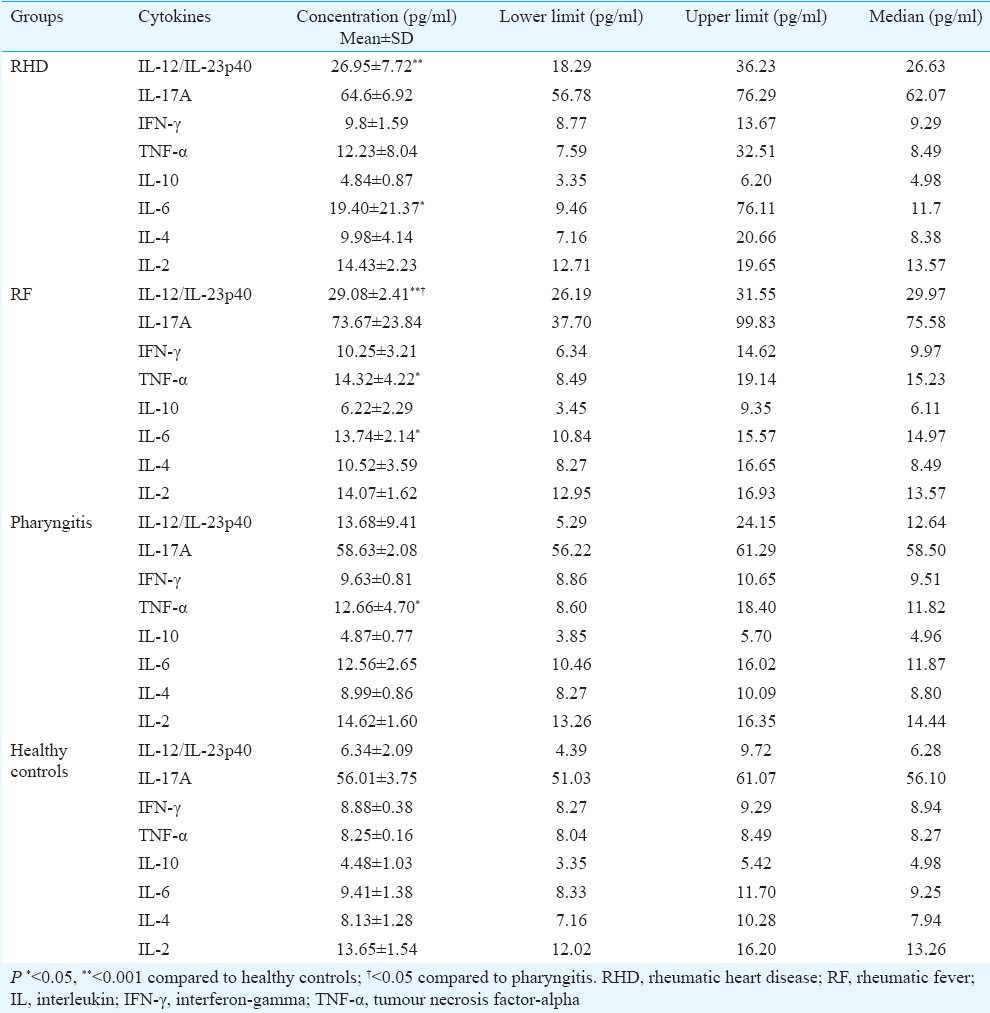
Gene polymorphism in mannose-binding lectin (MBL2): We amplified 219 bp product of partly coding sequence of exon 3 of the MBL2 gene from disease as well as healthy control groups. The amplified product was sequenced and analyzed but no SNPs were observed in exon 3 (Fig. 6).
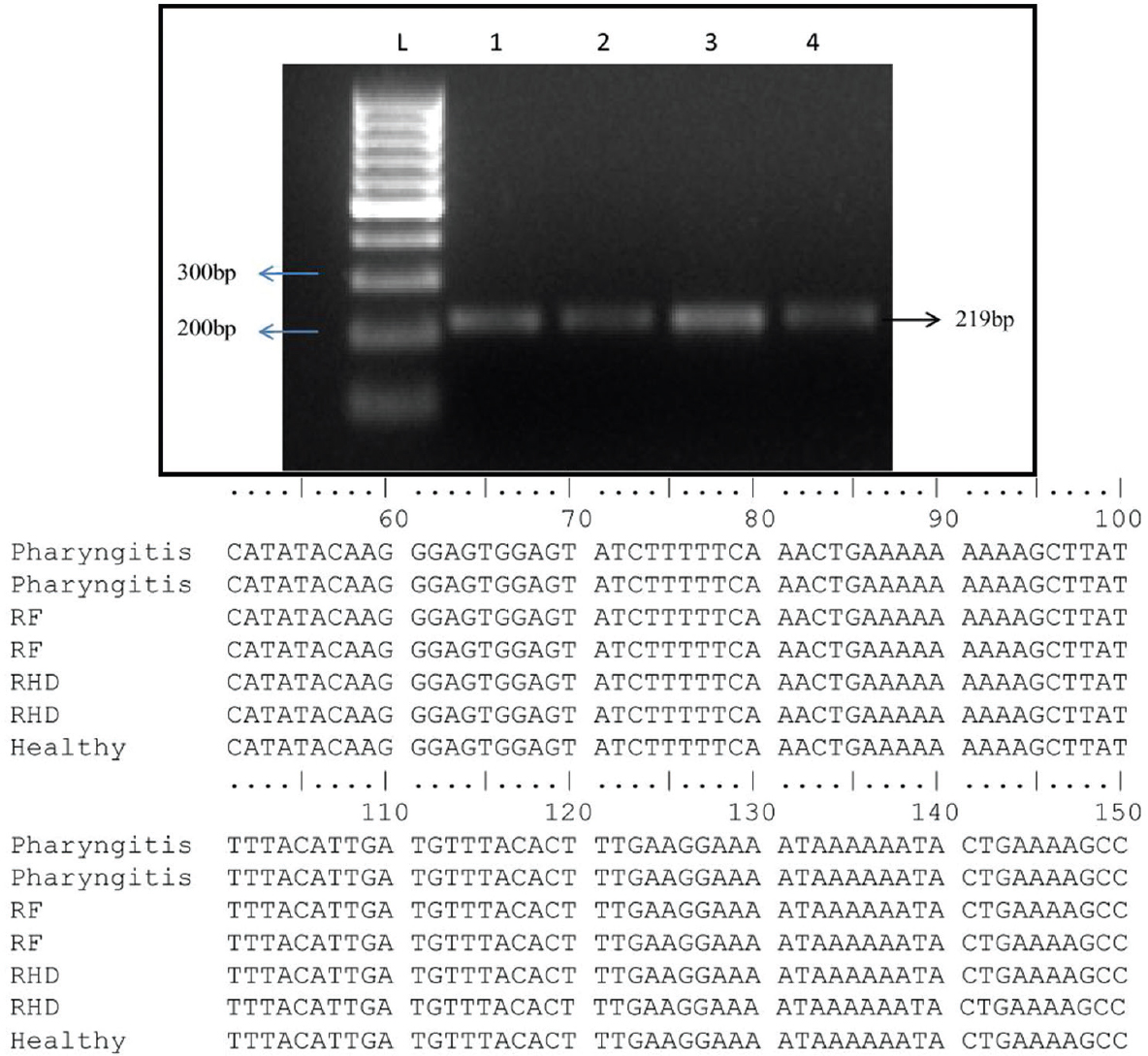
- Amplified polymerase chain reaction (PCR) product along with multiple alignments of exon 3 of mannose-binding lectin 2 (MBL2) gene sequences. Representative gel picture showing 219bp amplified PCR product of exon 3 of MBL2. Lane-1, healthy control; Lane-2, pharyngitis; Lane-3, rheumatic fever; Lane-4, rheumatic heart disease patient DNA; Lane L: 100 bp ladder. The aligned sequences represent no SNPs in exon 3 of MBL2.
Discussion
Group A streptococcal pharyngitis and its sequelae, RF and RHD, are major diseases in developing countries5. Lack of prophylaxis, vaccines and diagnostic marker is the cause of concern for the disease15. Some antibiotics such as penicillin, cephalosporins, amoxicillin are available; however, non-adherence leads to the treatment failure at an early stage16. A previous study suggested the involvement of various serological and genetic factors during the disease progression17, but the pathogenesis of RF and RHD is still not clear.
In this study, an effort was made to correlate host-pathogen interacting factors or different biomolecules associated with different disease groups and involved in RF/RHD pathogenesis. Similar to an earlier report18, this study also demonstrated a high level of ASO titre in pharyngitis patients compared to the other groups by applying semi-quantitative methods of estimation. The RF patients also showed a significantly high ASO titre, which could be due to past or recurrent infection. This confirms the probable role of ASO as a potential marker in pharyngitis and ARF when corroborated with clinical symptoms.
Carboxy-terminal propeptide of type I procollagen is an indicator for collagen synthesis, which is secreted in the blood during any collagen-related remodelling. PICP level has been reported to be elevated in various disease conditions including RHD819. In this study, quantification of serum PICP in three disease groups showed gradual increase from pharyngitis to RF, with highest concentration in RHD patients. This indicates the degree of valvular damage due to ongoing valvular assault, which can be represented by their serum PICP level, in the absence of any other collagen-related diseases. Thus, the correlation between PICP and ASO could be an indicator for establishing the ongoing heart valve damage.
In our study, the maximal concentration of anti-PARF antibody in RF suggested that antibody against PARF was initiated in pharyngitis itself but reached to the highest level in RF. Thus, patients diagnosed with streptococcal pharyngitis could be followed up for anti-PARF antibody estimation to establish progression towards RF stage. Besides ASO, PICP and PARF, the inflammatory cytokines may also provide an insight in the immunomodulatory autoimmune mechanism during the disease progression at post-pharyngeal state. Various cytokine levels are altered from the initiation of infection i.e., pharyngitis to RHD20. Significantly higher concentration of IL-12/IL-23p40, IL-17A in RF patients indicated the triggering of pro-inflammatory responses in the early stage of RHD. IL-12/IL-23p40 is a pro-inflammatory cytokines, which contributes in the development of Th1 and Th17 cells and is secreted by dendritic cells, macrophages and B-cells in response to microbial pathogens, highlighting its maximum level in RF stage21. IL-17A plays a key role in protection against various Gram-positive bacteria such as Streptococcus pneumoniae22, and dysregulated IL-17A may cause chronic inflammation. A previous study has indicated the role of IL-23 and IL-17 during inflammation; IL-17 is activated by IL-23 and thus it recruits the neutrophils for contributing in the ongoing inflammation23 as also found in the present study. On the other hand, IL-6, an important pro-inflammatory cytokine and a driving factor in the differentiation of TH cells, was found to be high in RF and RHD compared to healthy control, but RHD patients showed significantly higher secretion of IL-6 than RF group. This indicates that perhaps the pro-inflammatory response of IL-6 is generated initially, its level increases transiently with valvular damage. Although the level of TNF-α was found to be significantly higher in RF and RHD, the level was also elevated in pharyngitis state. This indicates that TNF-α secretion is initiated during the GAS infection by the resident macrophages, which further targets IL-1 and IL-6 gene activation as a part of normal inflammatory reaction and persists throughout the disease24. It has been demonstrated that both the CD4+ and CD8+ T-cells secrete high concentration of IL-2 and IFN-γ in ARF while low concentration of IL-4 is secreted in RHD patients through Th2-type immune response25. However, we did not find any significant variation in IL-2, IFN-γ and IL-4 level. IL-10 is as an anti-inflammatory cytokine, which can efficiently suppress and negatively regulate inflammation by inhibiting IL-12, IFN-γ, IL-12p40 and decreasing NFkB (nuclear factor kappa B subunit 1), AP-1 (activator protein 1) activation26. However, no differences were observed in terms of serum concentrations of IL-10 in the different patient groups. To further understand the immunological data, we analyzed the polymorphism in MBL2 gene. MBL2 is an important part of complement cascade of immune system and plays an important role in pathogen recognition and complement system activation. Polymorphism in this gene may lead to increased susceptibility to various diseases including RF and RHD827, where alteration in the immune response is linked to disease pathogenesis. We have earlier reported association of HLA class II genes with the genetic susceptibility in RHD28. However, the present data did not correlate with genetic susceptibility as SNPs were not detected in exon 3 of MBL2 whereas, four novel mutations in exon-1 of RHD patient were reported in south Indian population27. However, experiments on large cohort may be useful to understand genetic susceptibility in more detail.
In conclusion, our findings provided information on various factors/biomolecules as probable marker involved in the progression of RF/RHD. Further, follow up study is required with large population setting, which may help the clinicians in predicting any predilection towards RHD.
Acknowledgment
The study was financially supported by the Indian Council of Medical Research, New Delhi, India.
Conflicts of Interest: None.
References
- Pathogenesis of group A streptococcal infections. Clin Microbiol Rev. 2000;13:470-511.
- [Google Scholar]
- Rheumatic heart disease: Key points on valve lesions development. J Clin Exp Cardiolog. 2013;S3:006.
- [Google Scholar]
- The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005;5:685-94.
- [Google Scholar]
- World Health Organization. Rheumatic fever and rheumatic heart disease. WHO Tech Rep Ser. 2004;923:1-122.
- [Google Scholar]
- Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis. 2012;55:1279-82.
- [Google Scholar]
- Crucial role of the CB3-region of collagen IV in PARF-induced acute rheumatic fever. PLoS One. 2009;4:e4666.
- [Google Scholar]
- Circulating carboxy-terminal propeptide of type I procollagen is increased in rheumatic heart disease. Int J Cardiol. 2012;156:117-9.
- [Google Scholar]
- The association between mannose-binding lectin gene polymorphism and rheumatic heart disease. Hum Immunol. 2006;67:991-8.
- [Google Scholar]
- Group A streptococcal sore throat in a periurban population of Northern India: A one-year prospective study. Bull World Health Organ. 2001;79:528-33.
- [Google Scholar]
- Group A streptococcal pharyngitis. Curr Opin Otolaryn Head Neck Surg. 2002;10:449-54.
- [Google Scholar]
- WHO manual on laboratory diagnosis of group A streptococcal infections. Geneva: World Health Organization; 1996.
- World Heart Federation criteria for echocardiographic diagnosis of rheumatic heart disease – An evidence-based guideline. Nat Rev Cardiol. 2012;9:297-309.
- [Google Scholar]
- Diagnosis and treatment of streptococcal pharyngitis. Am Fam Physician. 2009;79:383-90.
- [Google Scholar]
- Prevention of rheumatic fever and diagnosis and treatment of acute Streptococcal pharyngitis: A scientific statement from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young, the Interdisciplinary Council on Functional Genomics and Translational Biology, and the Interdisciplinary Council on Quality of Care and Outcomes Research: Endorsed by the American Academy of Pediatrics. Circulation. 2009;119:1541-51.
- [Google Scholar]
- Comprehensive analysis of antibody responses to streptococcal and tissue antigens in patients with acute rheumatic fever. Int Immunol. 2008;20:445-52.
- [Google Scholar]
- Clinical significance of markers of collagen metabolism in rheumatic mitral valve disease. PLoS One. 2014;9:e90527.
- [Google Scholar]
- Responses of innate immune cells to group A Streptococcus. Front Cell Infect Microbiol. 2014;4:140.
- [Google Scholar]
- Regulation of interleukin-12 production in antigen-presenting cells. Adv Immunol. 2001;79:55-92.
- [Google Scholar]
- Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 2009;30:108-19.
- [Google Scholar]
- Prolonged activation of jun and collagenase genes by tumour necrosis factor-alpha. Nature. 1989;337:661-3.
- [Google Scholar]
- Immune responsiveness during disease progression from acute rheumatic fever to chronic rheumatic heart disease. Microbes Infect. 2012;14:1111-7.
- [Google Scholar]
- Genes, autoimmunity and pathogenesis of rheumatic heart disease. Ann Pediatr Cardiol. 2011;4:13-21.
- [Google Scholar]
- Four novel mutations detected in the exon 1 of MBL2 gene associated with rheumatic heart disease in South Indian patients. Int J Genet Mol Biol. 2010;2:165-70.
- [Google Scholar]
- Association of HLA-DRB1*14 with rheumatic heart disease patients from Chandigarh, North India. Biomarkers. 2012;17:160-5.
- [Google Scholar]






