Translate this page into:
Antibody profile in post-vaccinated & SARS-CoV-2 infected individuals
For correspondence: Dr Manisha Madkaikar, ICMR-National Institute of Immunohaematology, KEM Hospital Campus, Mumbai 400 012, Maharashtra, India e-mail: madkaikarmanisha@gmail.com
-
Received: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
During the COVID-19 pandemic it was important to assess the antibody profile in individuals vaccinated with Covaxin (BBV152) and Covishield (ChAdOx1 nCoV-19) with both 28 and 84 days gaps between two doses, those infected with SARS-CoV-2 and post-COVID-19-infected individuals vaccinated with only one dose of either of the vaccines. The present study was aimed to assess these objectives.
Methods:
Fifty real time reverse transcription–polymerase chain reaction (qRT-PCR)-confirmed COVID-19-infected individuals, along with 90 COVID-19-naïve (BBV152 and ChAdOx1 nCov-19)–vaccinated individuals, were included in the study. Individuals who received a single dose of either vaccine with a confirmed past diagnosis of SARS-CoV-2 infection (n=15) were also included. Blood samples were collected strictly between the 4th and 5th wk after development of symptoms for SARS-CoV-2 infected individuals and after the first/second vaccination dose. Antibody profile assessment was done using whole-virus, spike-receptor binding domain (RBD) and nucleocapsid-specific ELISA kits along with neutralizing antibody kit.
Results:
There was an overall 97.7 per cent seropositivity rate in vaccinated individuals, and a strong correlation (R2=0.8, P<0.001) between neutralizing and spike-RBD antibodies. Among individuals who received two standard doses of ChAdOx1 nCoV-19 vaccine, the spike antibody levels developed were of higher titre with a longer prime boost interval than in those with shorter intervals (P<0.01). Individuals vaccinated with two doses as well as only one dose post-SARS-CoV-2 infection had high neutralizing and spike-specific antibodies.
Interpretation & conclusions:
High neutralizing and spike-specific antibodies were developed in individuals vaccinated only with one dose of either vaccine post-SARS-CoV-2 infection. With the main priority being vaccinating majority of the population in our country, single-dose administration to such individuals would be a sensible way to make the most of the limited supplies. Furthermore, neutralizing antibody levels observed in COVID-19-naïve vaccinees imply the need for booster vaccination.
Keywords
BBV152
ChAdOx1 nCov-19
neutralizing antibody
SARS-CoV-2 infection
spike-specific antibody
vaccination
For defeating the current global COVID-19 pandemic crisis, vaccines development, their rapid distribution and vaccination of the majority of the citizens was the leading strategy worldwide. The advent of novel vaccines stimulated both infection and vaccination response evaluation. In India, two vaccines, i.e. BBV152 and ChAdOx1 nCov-19, were initiated in 2021 after Emergency Use Authorization (EUA). ChAdOx1 nCoV-19 or AZD1222 contains recombinant SARS-CoV-2 spike antigen acquired from Oxford University and AstraZeneca, produced by Serum Institute of India, Pune, whereas BBV152 is the inactivated whole virion having all the structural SARS-CoV-2 antigens. Studies show that both these vaccines are safe and effective13. However, only a few studies compared the antibody profile in vaccinated individuals, with individuals infected with SARS-CoV-2 infection47. Other studies highlighted that one dose of mRNA vaccine might be sufficient for SARS-CoV-2–infected individuals813. Thus, this study was aimed to analyze and compare the antibody profile in SARS-CoV-2–infected individuals, individuals vaccinated with ChAdOx1 nCoV-19 with both 28 and 84 days gaps between the two doses, as well as individuals vaccinated with BBV152. The antibody profile was also assessed in post-COVID-19–infected individuals vaccinated with only one dose of either of these vaccines.
Material & Methods
Participants: The study was undertaken at ICMR-National Institute of Immunohaematology (NIIH), Mumbai, after approval by the Institutional Ethics Committee. Written informed consent was obtained from all participants. Patients who were referred for real-time reverse transcription–polymerase chain reaction (RT-PCR) and antibody profile to ICMR-NIIH were randomly included in the study.
SARS-CoV-2 infected individuals: The individuals were RT-PCR –confirmed COVID-19 patients at the time when symptoms developed. The blood samples were collected strictly between the 4th and 5th wk after development of symptoms. Fifty such individuals (group 1) were included in the study. With 28-71 yr age range [mean±standard deviation (SD): 41.8±12.5 yr], two individuals had comorbidities (heart condition and diabetes).
Vaccinated individuals: Seventy ChAdOx1 nCoV-19-vaccinated individuals, 36 (group 2a) with 28 days gap and 34 (group 2b) with 84 days gap between the two doses, and 20 BBV152-vaccinated individuals (Group 3) with 28 days gap were included in the study. Furthermore, 15 individuals who had received only one dose (group 4) of either ChAdOx1 nCoV-19 (n=8) or BBV152 (n=7) with confirmed past diagnosis of SARS-CoV-2 infection were also included. Blood samples were collected between 4th and 5th wk after the second dose. The age ranges for the groups 2a, 2b, 3 and 4 were 25-65 (mean±SD: 38.9±12.74), 24-71 (41.5±14.4), 24-70 (42.5±11.5) and 29-65 yr (38.8±12.8). Only one individual in each group 2b and group 3 had comorbidities.
Healthy individuals: Twenty healthy individuals who were never positive for SARS-CoV-2 infection by RT-PCR or antibody and unvaccinated with COVID-19 vaccines were also included in the study for determining the cut-offs of all the ELISAs. Enrolment of individuals in groups 1 and 2a began in April 2021 and that of groups 2b and 3 from June 2021 to mid-August 2021.
SARS-CoV-2 antibody tests: The following kits were used for antibody detection (i) Anti-IgG antibody detection ELISA (Covid Kavach™ – Zydus Cadila, Ahmedabad, Gujarat, India); (ii) Anti-SARS-CoV-2 RBD IgG ELISA kit (Vazyme Biotech Co., Ltd, Nanjing, China); (iii) Anti-SARS-CoV-2 nucleocapsid IgG ELISA kit (ImmunoDiagnostics Ltd., Hong Kong, China); and (iv) Anti-SARS-CoV-2 neutralizing antibody titre serologic assay kit (AcroBiosystems Co. Ltd., Newark, Delaware, USA).
Cut-offs of ELISAs: After analyzing the range obtained in 150 samples along with negative and positive controls at different dilutions and using the cut-off specified by the kit, moderate- and high-titre lower limit cut-off was calculated arbitrarily by approximate two-fold, wherein for spike antibodies, the cut-off value for antibodies was <9.50 RU/ml and moderate- and high-titre lower limit cut-offs were set at 100 and 200 RU/ml, respectively, whereas for neutralizing antibodies, the cut-off value was >25 per cent of signal inhibition and moderate- and high-titre lower limit cut-offs were set at 35 and >60 per cent of signal inhibition.
Statistical analysis: The correlation equation graph was made using Microsoft Excel 2021 (Microsoft, USA), and Pearson’s correlation coefficient, and coefficient of determination R2 were calculated using GraphPad Prism 5.0 software (GraphPad, Inc., San Diego, CA, USA). Using Chi-square test of independence for gender and comorbidities comparison and t test for comparison of age in different groups, age, gender and comorbidities were found comparable in all the groups. One-way ANOVA was performed to compare the antibody titres between the different groups using GraphPad Prism 5.0 software. Individuals with a history of infection (exposed vaccinees) were separated for analysis.
Results
Vaccinated individuals and history of COVID-19 disease: Among the 70 individuals vaccinated with ChAdOx1 nCoV-19, 15 had a history of RT-PCR-confirmed SARS-CoV-2 infection; 20 were never tested for RT-PCR or antibodies however tested positive for nucleocapsid-specific antibodies, thus demonstrating a history of COVID-19 disease; when questioned, 12 of these individuals reported mild symptoms but never got tested. Among the 20 individuals vaccinated with BBV152, five had a history of RT-PCR-confirmed COVID-19 disease.
SARS-CoV-2 antibody detection by different protein-based kits:
Anti-IgG antibody detection ELISA (whole-inactivated virus): The cut-off value for antibodies was OD >0.28 (average OD of negative control+0.2). In group 1, of the 50 individuals with SARS-CoV-2 infection, 84 per cent (n=42) were positive for antibodies and four per cent (n=2) were indeterminant. In group 2a, of the 30 naïve ChAdOx1 nCoV-19-vaccinated individuals with a 28 days gap, 83.3 per cent (n=25) were positive for antibodies and 3.3 per cent (n=1) was indeterminant. In group 2b, of the 25 naïve ChAdOx1 nCoV-19-vaccinated individuals with an 84 days gap, all were positive for antibodies. All 15 exposed ChAdOx1 nCoV-19-vaccinated individuals were positive for antibodies. In group 3, of the 15 naïve BBV152-vaccinated individuals, 80 per cent (n=13) and all five exposed BBV152-vaccinated individuals were positive for antibodies, and in group 4, all the individuals were positive for antibodies.
Anti-SARS-CoV-2 RBD IgG ELISA: In group 1, 94 per cent (n=47) were positive for antibodies, of whom 54 (n=27) and 18 per cent (n=9) had high and moderate titres, respectively. In group 2a, of the 30 naïve ChAdOx1 nCoV-19-vaccinated individuals with 28 days gap, 93.3 per cent (n=28) were positive for antibodies, of whom 56.6 (n=17) and 16.6 per cent (n=5) had high and moderate titres, respectively. In group 2b, of the 25 naïve ChAdOx1 nCoV-19-vaccinated individuals with an 84 days gap, all had high titres. All 15 exposed ChAdOx1 nCoV-19-vaccinated individuals had high titres. In group 3, of the 15 naïve BBV152-vaccinated individuals, all were positive for antibodies, of whom 53 (n=8) and 18.8 per cent (n=3) had high and moderate titres, respectively; all five exposed BBV152-vaccinated individuals were positive for antibodies, of whom 80 per cent (n=4) had high titres. In group 4, all the individuals had high antibody titres.
Anti-SARS-CoV-2 nucleocapsid-specific IgG ELISA: The cut-off value for antibodies was >1.2 units. In group 1, 92 per cent (n=46) were positive for antibodies, of whom 56 (n=28) and 22 per cent (n=11) had high and moderate titres, respectively. In group 2a, of the 30 naïve ChAdOx1 nCoV-19-vaccinated individuals with a 28 days gap, 36.1 per cent (n=7) and, in group 2b, of the 25 naïve ChAdOx1 nCoV-19-vaccinated individuals with 84 days gap, 68 per cent (n=17) were positive for antibodies. All 15 exposed ChAdOx1 nCoV-19-vaccinated individuals were positive for antibodies. In group 3, of the 15 naïve BBV152-vaccinated individuals, 75 per cent (n=11) were positive for antibodies and all five exposed BBV152-vaccinated individuals were positive for antibodies. In group 4, all were positive for antibodies, of whom 33.3 (n=5) and 33 per cent (n=4) had high and moderate titres, respectively (Fig. 1).
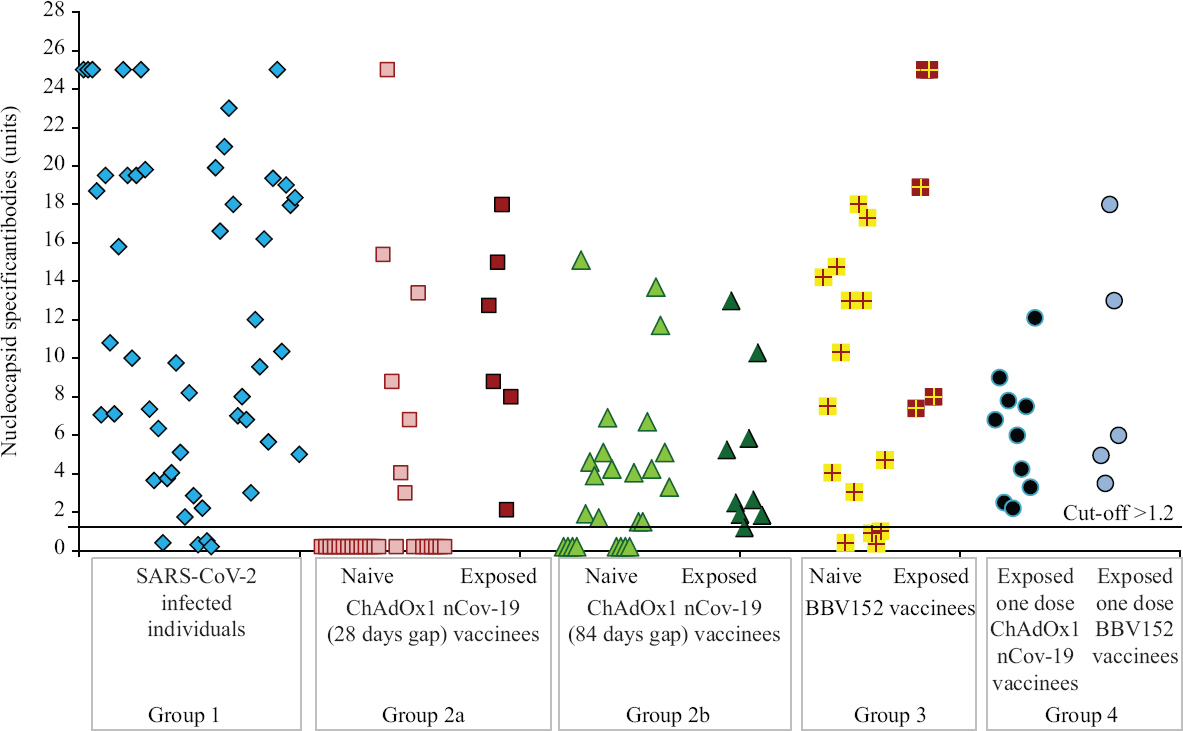
- Nucleocapsid-specific antibody profile among different groups: SARS-CoV-2–infected individuals (group 1), naïve and exposed individuals vaccinated with ChAdOx1 nCoV-19 with 28 (group 2a) and 84 days (group 2b) gap, naïve and exposed individuals vaccinated with BBV-152 (group 3) and individuals who received only one dose (group 4) of either vaccine post-SARS-CoV-2 infection. High titres were observed in individuals with past SARS-CoV-2 infection after either one or both doses of vaccine.
Anti-SARS-CoV-2 neutralizing antibody titre serological assay: In group 1, 72 per cent (n=36) were positive for neutralizing antibodies, of whom 22 (n=11) and 36 per cent (n=18) had high and moderate titres, respectively. Of the 14 (28%) individuals in group 1 negative for neutralizing antibodies, three were negative for both spike and nucleocapsid-specific antibodies also, three were low titre positive for spike and nucleocapsid-specific antibodies, three had low titre spike antibodies but tested negative for nucleocapsid and five had moderate levels of spike antibodies. In group 2a, of the 30 naïve ChAdOx1 nCoV-19-vaccinated individuals with 28 days gap, 80 per cent (n=24) were positive for neutralizing antibodies, of whom 41.6 (n=9) and 36.6 per cent (n=11) had high and moderate titres, respectively. In group 2b, of the 25 naïve ChAdOx1 nCoV-19-vaccinated individuals with 84 days gap, 92 per cent (n=23) were positive for neutralizing antibodies, of whom 72 (n=18) and 16 per cent (n=4) had high and moderate titres, respectively. All 15 exposed ChAdOx1 nCoV-19-vaccinated individuals had high titres. In group 3, of the 15 naïve BBV152-vaccinated individuals, 80 per cent (n=12) were positive for antibodies, of whom 12.5 per cent (n=2) had high and 37.5 per cent (n=6) had moderate titres, respectively; of the five exposed BBV152-vaccinated individuals, 80 per cent (n=4) had high titres. In group 4, all had high neutralizing titres.
Correlation of neutralizing antibodies with spike-RBD-specific antibodies: The Pearson’s correlation coefficient on comparison of neutralizing antibody and spike-RBD-specific antibody titres showed a strong positive correlation (R2= 0.8) (Fig. 2). However, not all individuals (13.9%) who had spike antibodies had neutralizing antibodies.
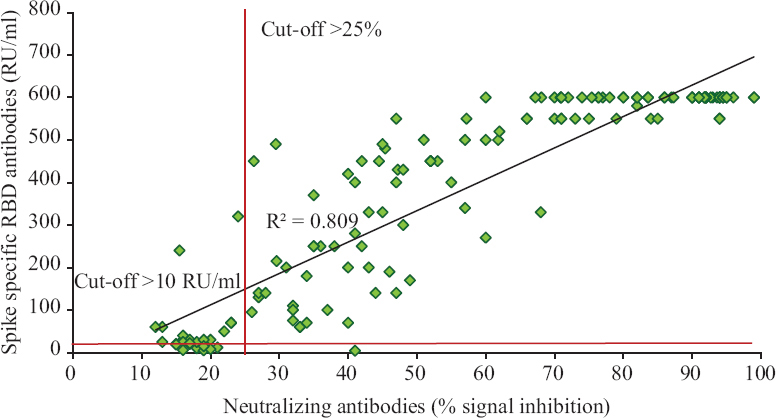
- Correlation of neutralizing antibodies with spike-RBD-specific antibodies. The Pearson correlation coefficient on comparison of neutralizing antibody and spike-RBD-specific antibody titres showed a strong positive correlation (R2= 0.80).
Comparison of neutralizing and spike-RBD antibody profile between ChAdOx1 nCoV-19 and BBV152 vaccinated individuals and individuals with COVID-19: As shown in Figures 3 and 4, exponential increase in neutralizing and spike antibodies was seen in all individuals vaccinated post-SARS-CoV-2 infection (P<0.01), i.e. exposed vaccinees, when compared to individuals post-COVID-19 infection (unvaccinated) and only vaccinated individuals without a history of COVID-19 infection. In group 4 individuals who had taken only one dose of vaccine, with a history of SARS-CoV-2 infection, had significatly increased antibodies when compared to all other groups (P<0.01) except individuals vaccinated with ChAdOx1 nCoV-19 with 84 days gap wherein the antibody levels were equivalent.
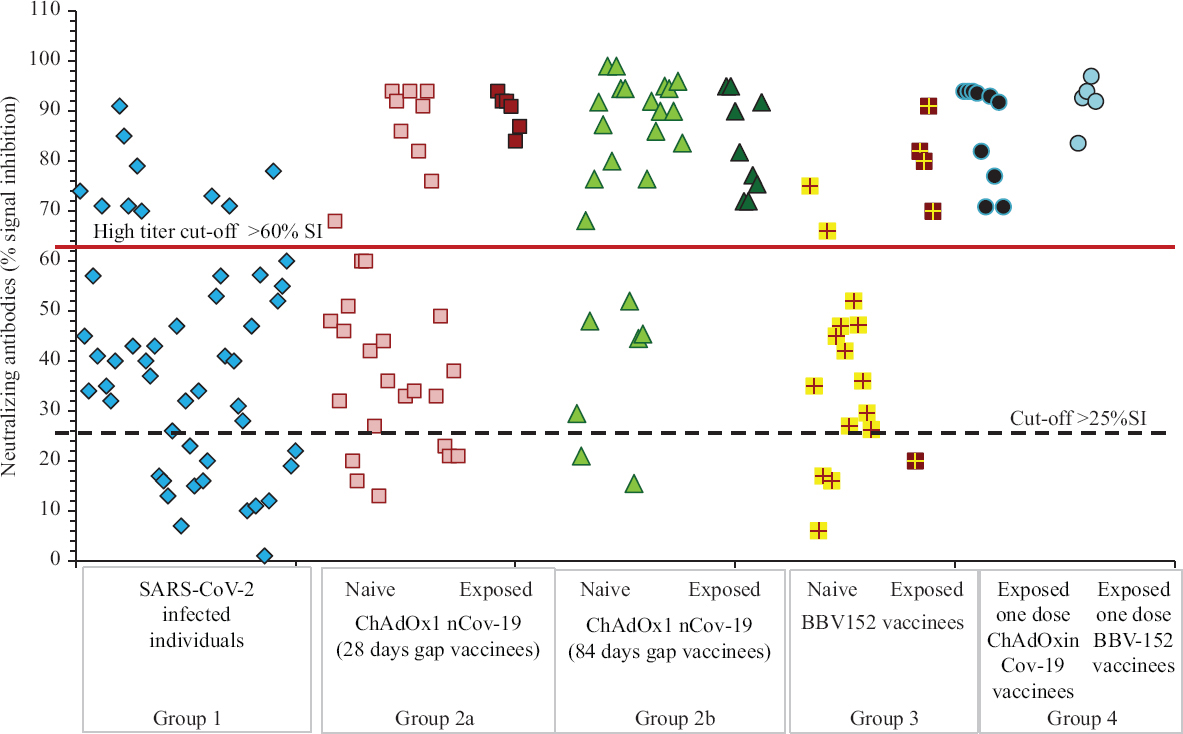
- Neutralizing antibody profile among different groups: SARS-CoV-2–infected individuals (group 1), naïve and exposed individuals vaccinated with ChAdOx1 nCoV-19 with 28 (group 2a) and 84 days (group 2b) gap, naïve and exposed individuals vaccinated with BBV152 (group 3) and individuals who have received only one dose (group 4) of either vaccine post-SARS-CoV-2 infection.
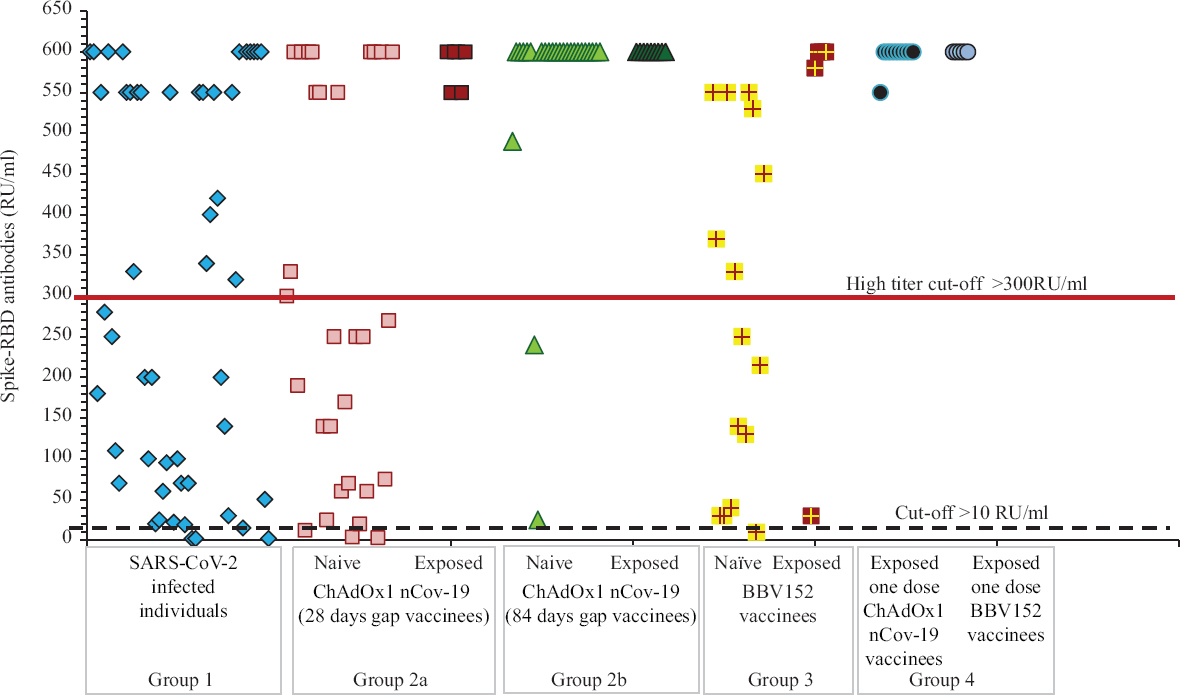
- Spike-RBD antibody profile among different groups: SARS-CoV-2–infected individuals (group 1), naïve and exposed individuals vaccinated with ChAdOx1 nCoV-19 with 28 (group 2a) and 84 days (group 2b) gap, naïve and exposed individuals vaccinated with BBV152 (group 3) and individuals who have received only one dose (group 4) of either vaccine post-SARS-CoV-2 infection.
If individuals with a history of infection (exposed vaccinees) were to be separated, the antibody levels developed in ChAdOx1 nCoV-19 with 84 days gap vaccinees when compared to ChAdOx1 nCoV-19 with 28 days gap were significantly higher (P<0.01); ChAdOx1 nCoV-19 with 28 days gap vaccinees and BBV152 vaccinees were equivalent; ChAdOx1 nCoV-19 with 84 days gap vaccinees were significantly higher than BBV152 vaccines (P<0.01) and post-SARS-CoV-2–infected individuals were equivalent to ChAdOx1 nCoV-19 with 28 days gap and BBV152 vaccinees.
The mean and standard deviation of neutralizing and spike antibody levels in different groups are shown in Table IA, and the comparison of these antibody levels in different groups using ANOVA is shown in Table IB.
| Groups | G1: SARS-CoV-2 infection | G2a: Naive ChAdOx1 nCoV-19 (28 days gap) | G2b: Naive ChAdOx1 nCoV-19 (84 days gap) | G3: Naïve BBV152 | G4: Exposed one-dose vaccinees | Total |
| Neutralizing antibodies (per cent signal inhibition) | ||||||
| n | 50 | 30 | 25 | 15 | 15 | 135 |
| Mean | 41.44 | 50.80 | 74 | 37.80 | 88.03 | 54.32 |
| SD | 22.92 | 26.74 | 25.93 | 18.65 | 8.84 | 28.40 |
| Spike-RBD antibodies (RU/ml) | ||||||
| Mean | 312.44 | 302.30 | 558.20 | 278.33 | 557.33 | 379.12 |
| SD | 238.41 | 236.96 | 133.64 | 211.02 | 146.50 | 238.09 |
G, group; SD, standard deviation; RBD: receptor binding domain
| Comparison of neutralizing antibodies | |
| Pairwise comparisons | (Q0.05=3.9123, Q0.01=4.7002) |
|---|---|
| G1:G2a | P=0.65 (Q=1.91) |
| G1:G2b | P<0.001 (Q=6.66) |
| G1:G3 | P=0.98 (Q=0.74) |
| G1:G4 | P<0.001 (Q=9.53) |
| G2a: G2b | P<0.01 (Q=4.74) |
| G2a: G3 | P=0.33 (Q=2.66) |
| G2a: G4 | P<0.001 (Q=7.61) |
| G2a: G3 | P<0.001 (Q=7.40) |
| G2b: G4 | P=0.25 (Q=2.87) |
| G3:G4 | P<0.001 (Q=10.27) |
| Comparison of spike antibodies | |
| Pairwise comparisons | (Q0.05=3.9123, Q0.01=4.7002) |
| G1:G2a | P=0.999 (Q=0.23) |
| G1:G2b | P=0.001 (Q=5.63) |
| G1:G3 | P=0.98 (Q=0.78) |
| G1:G4 | P<0.001 (Q=6.51) |
| G2a: G2b | P<0.001 (Q=5.86) |
| G2a: G3 | P=0.995 (Q=0.55) |
| G2a: G4 | P<0.001 (Q=6.74) |
| G2a: G3 | P<0.001 (Q=6.41) |
| G2b: G4 | P=0.97 (Q=0.88) |
| G3:G4 | P<0.001 (Q=7.29) |
G, group; M, mean; Q, P value that has been adjusted for the false discovery rate
Discussion
This study reported an overall 97.7 per cent (88/90) seropositivity rate after the completion of two doses of both the vaccines (ChAdOx1 nCoV-19 - 97.1% and BBV152 - 100%) when compared to 94 per cent observed in unvaccinated individuals post-SARS-CoV-2 infection. If vaccinated individuals who had a history of COVID-19 disease were to be removed from the analysis, neutralizing antibodies were seen in 80 and 75 per cent of individuals vaccinated with BBV152 and ChAdOx1 nCoV-19, respectively. These findings were similar to other studies and published evidence in randomized controlled trials17.
Another interesting observation in our study was that since we strictly used kits which contained calibrators for determining exact titres and different antigen-specific kits, 36.1 and 68 per cent of individuals vaccinated with ChAdOx1 nCoV-19 having 28 and 84 days gap, respectively, were positive for nucleocapsid antibodies, which demonstrated SARS-CoV-2 infection in these individuals which might have occurred either in the past or during the 28 and 84 days gap highlighting the extent of herd immunity or cross-reactivity with other coronaviruses. ChAdOx1 nCoV-19, which is a spike-based vaccination, will only induce formation of spike-specific antibodies and not nucleocapsid antibodies. It could be possible that a fraction of these individuals were exposed to other coronaviruses since anti-N antibodies are cross-reactive among human coronaviruses.
Voysey et al1 observed that in individuals who received two standard doses of ChAdOx1 nCoV-19, vaccine efficacy was higher (81.3%) with a longer prime boost level than in those with shorter intervals (55.1%). In our study, it was found that the spike-RBD antibody levels developed in individuals vaccinated with ChAdOx1 nCoV-19 with 84 days gap were higher than ChAdOx1 nCoV-19 with 28 days gap. However, this observation may be due to many of them (approximately 68%) being infected either in the past or during the 28 and 84 days gap.
Another observation in the study was that high titres of neutralizing and spike antibodies were seen in completely vaccinated individuals with a history of SARS-CoV-2 infection which were also seen after one dose of either vaccination in individuals having a past SARS-CoV-2 infection. The limitation of the study was the small sample size of naïve vaccinees and that the gold standard plaque reduction neutralization test (PRNT) was not performed.
There are similar studies with other vaccines; in one study post-- mRNA vaccine, a dramatic increase in spike-specific and neutralizing antibodies was seen following a single vaccination after SARS-CoV-2 infection, which significantly exceeded values seen with SARS-CoV-2 infection alone8. Another immunogenicity study following vaccination with BNT162b2 mRNA COVID-19 vaccine among Israeli healthcare workers observed very high titres in those with past SARS-CoV-2 infection9. Other similar studies suggested that only one dose of RNA-based vaccine had a classic booster response1013. A recent study showed that a single dose of BBV152-induced antibody levels in previously infected individuals was equivalent to infection-naïve individuals with two vaccine doses14. Thus, our data support the studies which state that one dose may be sufficient for individuals who have had COVID-19 disease1516. With the main priority being vaccinating majority of the country’s population, single-dose administration to such individuals would be a way to make the most of the limited supplies. It has been observed that the neutralizing antibody level decreases with time, thus decreasing the vaccine’s protective efficacy17. Thus, the levels of neutralizing antibodies observed in COVID-19 naïve vaccinees imply the need for booster vaccination with priority given to high-risk groups such as geriatric and immunodeficient individuals.
Among individuals who received two standard doses of ChAdOx1 nCoV-19 vaccine, the antibody levels developed were of higher titre with a longer prime-boost level (three months) than in those with shorter intervals (one month). Individuals vaccinated post-SARS-CoV-2 infection had high neutralizing and spike-specific antibodies accentuating the point that one dose may be sufficient for individuals who have had SARS-CoV-2 infection. It is also important to highlight that neutralizing antibody levels observed in COVID-19-naïve vaccinees imply the need for booster vaccination.
Financial support & sponsorship: Funding was provided by the Indian Council of Medical Research, New Delhi, India.
Conflicts of Interest: None.
References
- Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine:A pooled analysis of four randomised trials. Lancet. 2021;397:881-91.
- [Google Scholar]
- Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2:An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99-111.
- [Google Scholar]
- Bharat Biotech announces phase 3 results of COVAXIN®:India's first COVID-19 vaccine demonstrates interim clinical efficacy of 81%. Available from: https://www.bharatbiotech.com/images/press/covaxin-phase3-efficacy-results.pdf
- Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152:A double-blind, randomised, phase 1 trial. Lancet Infect Dis. 2021;21:637-46.
- [Google Scholar]
- Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152:Interim results from a double-blind, randomised, multicentre, phase 2 trial, and 3-month follow-up of a double-blind, randomised phase 1 trial. Lancet Infect Dis. 2021;21:950-61.
- [Google Scholar]
- Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2:A preliminary report of a phase ½, single-blind, randomised controlled trial. Lancet. 2020;396:467-78.
- [Google Scholar]
- Antibody response after first and second-dose of ChAdOx1-nCOV (Covishield™®) and BBV-152 (Covaxin™®) amongst health care workers in India:The final results of cross-sectional coronavirus vaccine-induced antibody titre (COVAT) study. Vaccine. 2021;39:6492-509.
- [Google Scholar]
- Exponential increase in neutralizing and spike specific antibodies following vaccination of COVID-19 convalescent plasma donors. Transfusion. 2021;61:2099-106.
- [Google Scholar]
- Convalescent plasma from people vaccinated after COVID-19 infection. Lancet Microbe. 2021;2:e171-2.
- [Google Scholar]
- Impact of age, ethnicity, sex and prior infection status on immunogenicity following a single dose of the BNT162b2 mRNA COVID-19 vaccine:Real-world evidence from healthcare workers, Israel, December 2020 to January 2021. Euro Surveill. 2021;26:2100096.
- [Google Scholar]
- Antibody responses boosted in seropositive healthcare workers after single dose of SARS-CoV-2 mRNA vaccine. medRxiv 2021 Doi:10.1101/2021.02.03.21251078
- [Google Scholar]
- Robust spike antibody responses and increased reactogenicity in seropositive individuals after a single dose of SARS-CoV-2 mRNA vaccine. medRxiv 2021 Doi:10.1101/2021.01.29.21250653
- [Google Scholar]
- Single dose vaccination in healthcare workers previously infected with SARS-CoV-2. medRxiv 2021 Doi:10.1101/2021.01.29.21250653
- [Google Scholar]
- Antibody responses to the BBV152 vaccine in individuals previously infected with SARS-CoV-2:A pilot study. Indian J Med Res. 2021;153:671-6.
- [Google Scholar]
- Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection. Nature. 2021;595:426-31.
- [Google Scholar]
- Immunological memory and neutralizing activity to a single dose of COVID-19 vaccine in previously infected individuals. Int J Infect Dis. 2021;108:183-6.
- [Google Scholar]
- Booster vaccination strategy:Necessity, immunization objectives, immunization strategy, and safety. J Med Virol. 2022;94:2369-75.
- [Google Scholar]






