Translate this page into:
An update on recombinant vaccines against leishmaniasis
For correspondence: Prof. Angamuthu Selvapandiyan, Department of Molecular Medicine, Jamia Hamdard, New Delhi - 110 062, Delhi, India e-mail: selvapandiyan@jamiahamdard.ac.in
-
Received: ,
Accepted: ,
Abstract
Leishmaniasis is a parasitic disease caused by various species of the Leishmania parasite, manifesting in visceral (VL), cutaneous (CL), and mucocutaneous (MCL) forms. To combat this debilitating disease, various vaccines candidates including proteins, DNA, vectors, adjuvants, and recombinant whole parasites have been developed and tested experimentally and preclinically against several Leishmania species. Some vaccines have already entered human clinical trials. These vaccines aim to induce protective immunity using specific antigens. This review examines all efforts to develop recombinant vaccines against the parasite, analyzing successes including commercially available canine vaccines and the overall challenges faced in the quest to eradicate the disease. Additionally, recent advances in vaccine delivery systems, such as viral vectors and non-pathogenic bacteria, offer promising avenues to enhance immunogenicity and improve the targeted delivery of antigens, potentially leading to more effective and long-lasting immune responses. By understanding past and current efforts, future strategies can be refined to create more effective vaccines and ultimately control or eradicate this parasitic disease.
Keywords
DNA vaccine
Leishmania
live attenuated vaccine
protein vaccine
recombinant vaccine
vaccine candidate
Recombinant vaccines against leishmaniasis represent a promising approach to combat this parasitic disease. Leishmaniasis, caused by various species of the Leishmania parasite, it manifests in different forms, including visceral (VL), cutaneous (CL), and mucocutaneous (MCL) leishmaniases1. These vaccines aim to induce protective immunity by using specific antigens derived from the parasite, produced through genetic engineering techniques, and by developing whole live attenuated parasite vaccine candidates2. Various antigens, singly or in combination, are tested with different adjuvants and delivered using diverse vectors to achieve optimal protection and efficacy. This review examines the efforts to develop recombinant vaccines for leishmaniasis, analyzing the successes and challenges in eradicating this debilitating disease. Understanding past and current efforts can refine future strategies to create more effective vaccines and ultimately control or eliminate leishmaniasis. The article reviews current vaccine candidates, including live attenuated, killed, and subunit vaccines, along with their mechanisms of action and efficacy in preclinical and clinical trials2. It also addresses challenges and limitations in vaccine development, such as antigen variability and immune response heterogeneity. Finally, it explores future directions in leishmaniasis vaccine research, emphasizing the role of novel technologies, such as CRISPR-Cas9, in developing more effective vaccine candidates. Figure illustrates the various recombinant antigens developed as vaccines, which aim to induce long-term immunity in the host. Table3-41 lists these recombinant antigens as reported in the literature.
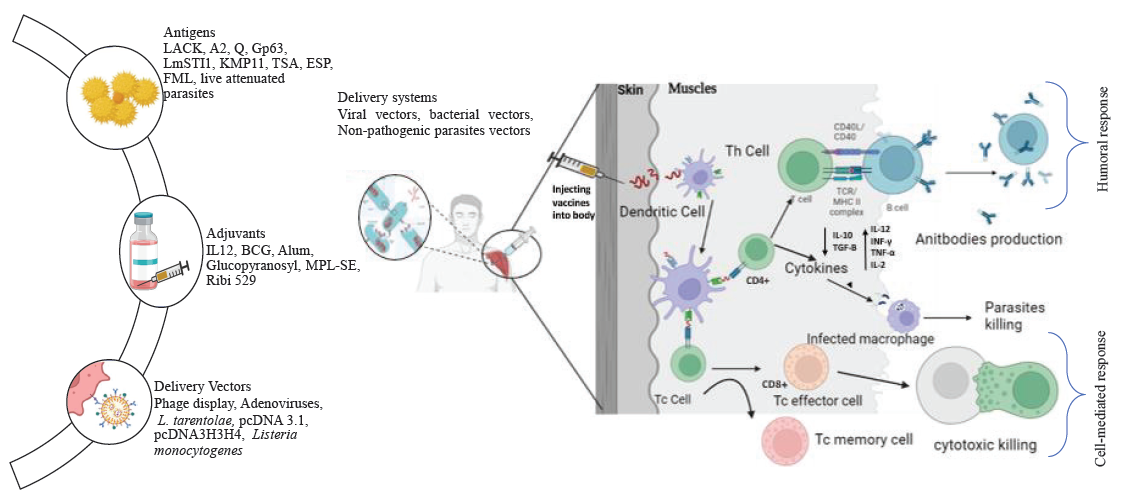
- A schematic diagram depicting the types of recombinant antigens currently being investigated for the development of commercial vaccines against leishmaniasis, along with the potential immunity they may induce in the host. The figure was generated using the programmes ‘Biorender’ and ‘PowerPoint’. LACK, Leishmania activated C kinase; Gp63, Leishmanolysin; LmSTI1, L. major stress-inducible protein; KMP-11, kinetoplastid membrane protein-11; TSA, thiol-Specific Antioxidant; ESP, excreted-secreted proteins; FML, fucose mannose ligand; BCG, Bacillus Calmette-Guerin; IL, interleukin.
| Vaccine candidate | Adjuvant used | Leishmaniasis against & the host details | Study remarks | Current status in vaccine development | References |
|---|---|---|---|---|---|
| Self-amplifying mRNA (SAM) vaccines | |||||
| Nano vaccine with C1 lipid nanoparticle (LNP) | Self-adjuvant | Localization of the nano vaccine occurred in the lymph nodes & lungs | Highest concentration of CD8+ T cells observed in the lymph nodes, Toll-like receptor 4 (TLR4) activation | Preclinical studies conducted | 3 |
| DNA antigens | |||||
|
Leishmania activated C kinase (LACK) |
None | This antigen is highly conserved among Leishmania strains but lacks in providing cross-protection | LACK is involved in the intracellular signaling pathways of the parasite & generates protective immunity in recombinant vaccines | Preclinical studies conducted | 4,5 |
| Leishmanolysin (GP63) | None |
A major surface protein found in both forms of parasites It rapidly acts on whole range of host cell substrates involved in cell signaling pathways and their functional regulation |
Vaccines targeting GP63 aim to neutralize its function, thereby impairing the parasite’s ability to infect host cells | Preclinical studies conducted | 6,7 |
| Ankara vaccine (LACK) | TRYP | TRYP is tandemly repeated & highly conserved across L. major and highly expressed in promastigotes & amastigotes | It induces protective immunity against virulent challenge with L. major in susceptible BALB/c mice as shown by reduction in footpad lesion size following injection of promastigotes | Preclinical studies conducted | 8 |
| Oligomeric recombinant fusion protein vaccine (ORFF) | None | Presented in both promastigote & amastigote forms | Elicit an Ag-specific cell-mediated immune response | Preclinical studies conducted | 9 |
| Leishdnavax | None | Lipophosphoglycan 3 (LPG3) is essential for the synthesis of glycoconjugates as parasite virulence factors | Vaccination with LPG3 of L. infantum induces parasite-specific protective Th1 responses | Preclinical studies conducted | 10 |
| Leishmune | FML-saponin |
FML inhibits the penetration of promastigotes as well as amastigotes. It provides protection from VL in canines |
It reduces parasite infection incidences in humans | Commercialized as canine vaccine as ‘Leishmune’ | 11 |
| Coctail DNA antigens | |||||
| Pleish-dom | IL-12 | It incorporates antigenic regions from four proteins (LACK, TSA, KMP11, and LmSTI1) | The immunized mice show lower parasite burden &, significant protection against infection | Preclinical studies conducted | 12 |
|
Recombinant Canine Distemper Virus - Leishmania-activated C-kinase (rCDV-LACK) |
A promising vaccine candidate against CL infection | Dogs immunized by the rCDV-LACK protected against L. major infection | Preclinical studies conducted | 13 | |
|
Cocktail antigens (HLA-DR and HLA-A2 from L. major GP63) HLA is human leukocyte antigen |
Montanide | TSA antigen provides protection against L. major infection | Immunized animals demonstrated enhanced IgG levels, lymphoproliferative activity with no cytotoxicity observed in renal & liver tissues | Preclinical studies conducted | 14 |
| Fucose-Mannose Ligand (FML/VR1012-NH36) | SAP |
A vaccine against Nucleoside hydrolase gene (NH36) Immunoprotective against visceral (L. chagasi) and cutaneous (Leishmania mexicana) murine leishmaniasis |
Significant reduction of parasitic load observed | Preclinical studies conducted | 15 |
|
Multicomponent DNA vaccine (with 10 antigens) |
IL-12 or GM-CSF | This induced a delayed type hypersensitivity (DTH) response to viable L. donovani promastigotes and led to a reduction of parasite burden in an in vitro intracellular infection model, & in the draining lymph node of dogs | This multicomponent DNA vaccine primed dogs for a parasite-specific type 1 cellular immune response which restricted parasite growth | Preclinical studies conducted | 16 |
|
ChAd63KH (ChAd63 is adenovirus vector derived from chimpanzees) |
It utilizes a chimpanzee adenovirus vector (ChAd63) to deliver a synthetic gene encoding two key Leishmania antigens: kinetoplastid membrane protein-11 (KMP-11) & hydrophilic acylated surface protein B (HASPB) | This vaccine has shown promise in preclinical and clinical studies in Sudan, Africa, leading to the induction of strong CD4+ and CD8+ T cell responses, especially for therapeutics against persistent Post Kala Dermal Leishmaniasis (PKDL) | Phase III clinical studies in humans conducted | 17 | |
| Protein antigens | |||||
|
L. major Stress-inducible protein (LmSTI1) |
Ag-M720 & Ag-M50 | The STI1 protein is expressed in both L. major promastigotes & amastigotes | Lower parasitic burden & lesion size observed in the Ag-M720 group, indicating better protection against L. major infection | Preclinical studies carried out | 18 |
| Leishmania homolog of eukaryotic ribosomal elongation & initiation Factor 4a (LeIF) | MPL |
LeIF is an important protein for the parasite’s protein synthesis machinery. L. infantum LeIF protein inhibits translation in yeast |
LeIF induces intramacrophage parasite growth inhibition, microbicidal activity; thereby induces a strong immune response. | Preclinical studies conducted | 19 |
| KMP-11 | None | The KMP-11 protein is differentially expressed both in amastigote and promastigote forms | KMP-11 elicits strong immune responses and provides protection in experimental models with Th1 protective response. | Preclinical studies conducted | 20 |
| Leish-Tec | Recombinant A2 protein & saponin |
A2 antigen protects mice, monkeys, dogs. It efficiently protects the canine from VL |
Involvement of A2 antigen enhanced humoral IG especially IG1 and IG2 levels in the vaccinated dogs | Commercialized as canine vaccine as ‘LeishTec’ | 5,21-26 |
| A2 | IL-12, alum & saponin | A2 antigen leads to clearance of the parasites and provides cross-protection against Leishmania species | A2 formulated vaccines protect dogs, mice, and nonhuman- primates against VL | Preclinical & early clinical studies conducted | 27 |
| Q protein/LetiFend | Chimerical multi-component Q protein | Provide immunity against L. infantum in canines |
High levels of anti-Q antibodies, globulin levels observed in vaccinated dogs A DTH response along with the production of NO observed |
Commercialized as canine vaccine as ‘LetiFend’ | 28,29 |
|
Phlebotomus duboscqi salivary protein (PdSP15) |
Glucopyranosyl lipid | A salivary protein of P. duboscqi protects against vector-transmitted cutaneous leishmaniasis | The animal developed humoral mediated DTH response, clear signs of MNC recruitment and exhibited a positive IFN-γ response. Reduction in the parasitic burden and a lower no of lesions also found | Preclinical studies conducted in dogs | 30 |
|
L. infantum excreted-secreted protein (LiESP/QA-21) |
QA-21 | Formulated from the excreted-secreted proteins (LiESP) of L. infantum | Dogs showed IG2 response against PSA Ag and IG1 response against ESP Ag along with cell mediated immunity | Commercialized as ‘CaniLeish’ as cainine vaccine | 31 |
| Polyprotein antigens | |||||
|
Leishmania Glucopyranosyl Lipid A - Stable emulsion (LEISH-F3+/GLA-SE-) |
GLA-SE | It provides protection against VL caused by L. infantum & L. donovani | The vaccine was safe and induced a strong antigen-specific immunity, as evidenced by the cytokine & immunoglobulin subclass | Undergone clinical trials (phase I) | 32,33 |
| LEISH-F3+/GLA-SE- CBP | GLA-SE | It protects against VL in mice & dogs. The addition of the truncated CBP shows a robust immune response | Undergone clinical trials (phase I) | 33-36 | |
|
Leish-111f (TSA, LmSTI1 & LeIF) TSA is Thiol-specific antioxidant |
MPL-SE and Ribi 529 |
Tried against CL & mucocutaneous leishmaniasis Promising to treat both canine & human populations |
Immunized mice showed reduction in parasitic burden, an enhanced humoral response & cell-mediated response when administrated with Ribi whereas MPL generated humoral response Provides cross protection & stability Phase II clinical trial for CL in Brazil, where it was used as an adjunct therapy to enhance the standard treatment with sodium stibogluconate. The trial showed promise in boosting the immune response against L. braziliensis infection |
Undergone clinical trials (phase II) | 37 |
| Live attenuated parasites as antigens | |||||
| L. donovani | |||||
| Laboratory & cGLP grade live attenuated LdCen-/-parasites | None | Challenged against L. donovani, L. major, & L. braziliensis. Tested in BALB/c mice, hamsters, dogs & in human cells | Demonstrated safety and protection against homologous & heterologous challenges with Th1 protective immunity | Preclinical testing conducted | 38 |
| L. major | |||||
| Laboratory & cGLP grade live attenuated LmCen-/-parasites | None | Challenged (sand fly mediated) against L. major & L, donovani in C57BL/6 and BALB/c mice & hamsters | Demonstrated safety & protection against homologous & heterologous challenges with Th1 protective immunity | Preclinical & animal toxicity testing with cGLP grade parasite conducted. Clinical trial in progress | 39 |
| L. mexicana | |||||
| Live attenuated LmxCen-/-parasites | None | Tested against L. mexicana in BALB/c mice | Demonstrated strategy & protection with Th1 protective immunity | Preclinical testing conducted | 40 |
| L. infantum | |||||
| Live attenuated Li Cen-/-parasites | None | Phase 1 study in limited number of dogs | Moderate protective efficacy with immunity was noticed | Preclinical testing conducted | 41 |
Recombinant antigens
Recombinant vaccines exploit various antigens critical to the survival or pathogenicity of the Leishmania parasite. These antigens can include surface-expressed molecules and secretory proteins, which are recognized by the body’s immune cells, thereby stimulating a protective immune response42. By targeting these specific antigens, recombinant vaccines aim to induce immunity that can effectively prevent infection or reduce its severity.
Stress-inducible protein (STI)
The eukaryotic homolog of STI, is one of the most commonly used antigens in vaccine studies. This conserved protein is expressed in both forms of the parasite and has been shown to protect against Leishmania major infection effectively43. The recombinant LmSTI1 vaccine, formulated with Ag-M720 and Ag-M50 adjuvants, was administered to BALB/c mice at 3-week intervals, and the immune response was monitored. A lower parasitic burden and a smaller lesion size were observed in the Ag-M720 group, indicating better protection18. Results showed a higher level of IFN-γ and a lower induction of IL-4, IL-10, and IL-17 cytokines (and/or a higher IL-10/IL-17 ratio) in the immunized/protected animals compared to the control group. Another approach involved using STI1 from L. major in fusion with SP15 from Phlebotomus papatasi expressed as self-amplifying mRNA (SAM) through alphavirus. These SAM constructs show transient expression and do not integrate into the host genome. Such constructs mimic viral infection and enhance the immune response against the fused antigens44.
Kinetoplastid membrane protein-11 (KMP-11)
A conserved protein found on the surface of Leishmania parasites, KMP-11 is differentially expressed in both amastigote, and promastigote forms and has been shown to elicit strong immune responses, protecting experimental models20 Vaccinated hamsters exhibited a reversal of T cell inactivity, leading to IL-2 production and a strong specific response from cytotoxic T lymphocytes (CTLs). In a separate study, elevated levels of IFN-γ, IL-12, and TNF-α, along with increased splenic CD3+, CD4+ and CD8+ T cells, hepatic granulomas, and an 86 per cent reduction in splenic load were observed in immunized mice post-infection, suggesting the immune-protective nature of the vaccine45.
Leishmania homologue of receptors for activated C kinase (LACK)
This highly conserved antigen among Leishmania strains is involved in the parasite’s intracellular signalling pathways and has been used in recombinant vaccines to generate protective immunity4. Crosslinked chitosan microparticles have also been exploited as a mucoadhesive delivery system for LACK DNA. When delivered intranasally to a mouse model, this vaccine reduced parasitic load upon challenge with L. amazonensis46. Mice that received this vaccine showed an enhanced Th1 immune response compared to those challenged with naked LACK DNA46. The use of LACK antigens as a vaccine was further explored in a cocktail vaccine, which is discussed below.
Leishmania homolog of eukaryotic ribosomal elongation and initiation Factor 4A (LeIF)
This important protein is integral to the parasite’s protein synthesis machinery and is a target for recombinant vaccine development, known for inducing a strong immune response19. The Leishmania infantum LeIF protein is an ATP-dependent RNA helicase and an eIF4A-like factor that inhibits translation in yeast47. Additionally, LieIF inhibits the growth of intramacrophage parasites by promoting the production of TNF-α, which stimulates microbicidal activity through the generation of nitric oxide (NO) and reactive oxygen species (ROS)19. Its use in cocktail vaccines is detailed below.
GP63 (Leishmanolysin)
GP63 is a major surface protease of Leishmania and is critical for the parasite’s virulence and survival. Knockout studies of L. major for GP63 have shown reduced infection in mice6. It is a highly active protease that can rapidly act on a wide range of host cell substrates involved in cell signalling pathways and their functional regulation7. Vaccines targeting GP63 aim to neutralize its function, thereby impairing the parasite’s ability to infect host cells. The catalytic epitope of GP63 combined with the B subunit of heat-labile enterotoxin (LTB) of E. coli as an adjuvant increased complement-mediated lysis of promastigotes in vitro, leading to elevated synthesis of antibodies against GP6348.
Amastigote-specific stress response protein (A2)
The A2 protein has been exploited as a target antigen in recombinant vaccines, and its association with various adjuvants, including IL-12, alum, and saponin, provides both humoral and Th1/Th2 mediated immunity49,50. The recombinant A2(rA2) antigen of L. major was able to cross-protect an animal model against infections from both L. donovani and L. amazonensis, as indicated by high levels of interferon-gamma (IFN-γ), in contrast to rLACK18. The primary role of the A2 antigen is known to be the clearance of the parasites rather than their dissemination. Such formulated vaccines have shown protective effects in dogs, mice, and nonhuman- primates against VL27.
In the past decade, the selection of targets for vaccination has significantly expanded. Sand fly salivary proteins are immunogenic, making them promising candidates for vaccination. These proteins are advantageous because they exhibit minimal homology with human proteins. Efforts have been made to test these salivary proteins as target antigens, with notable examples including PdSP15 from Phlebotomus duboscqi51, and LJL143 and LJM19 from Lutzomyia longipalpis52. The salivary protein PdSP15 of P. duboscqi provides protection against vector-transmitted CL. Animals exposed to bites from uninfected sand flies developed a humoral-mediated delayed-type hypersensitivity (DTH) response (63%), accompanied by clear signs of mononuclear cell (MNC) recruitment. The study also noted a reduction in parasitic burden and fewer lesions. Reverse antigen screening identified PdSP15 as the key protein responsible for significant protection. Non-primate animals immunized with DNA encoding PdSP15 and boosted with rPdPS15 plus glucopyranosyl lipid (as an adjuvant) tested positive for anti-rPdPS15 antibodies. Furthermore, these animals exhibited a positive IFN-γ response and demonstrated enhanced protection against vector-transmitted infections30. Similarly, the rLSA protein of L. infantum, one of the many antigens, efficiently generates a cellular immune response in dogs. The effectiveness of rLSA, in terms of antibody levels, is significantly higher compared to rKMP-1153.
Cocktail antigens
A cocktail vaccine combining multiple antigens in a single formulation could potentially improve efficacy by targeting various aspects of the immune response and covering multiple pathways essential for the parasite’s survival. For example, formulations containing HLA-DR and HLA-A2 peptides from L. major GP63, using Montanide as an adjuvant, were evaluated both separately and in combination for cytotoxicity and protection against infection. Animals immunized with this formulation demonstrated enhanced IgG levels and lymphoproliferative activity with no cytotoxicity observed in renal and liver tissues14. In another case, mice immunized with the Thiol-Specific Antioxidant (TSA) antigen DNA either alone or in combination with LmSTI1 DNA exhibited CD4 and CD8 T cell-mediated immunity against the infection54. Additionally, the DNA vaccine known as ‘pleish-dom’, which incorporates antigenic regions from four proteins (LACK, TSA, KMP11, and LmSTI1) was tested. These regions were cloned and administered to BALB/c mice alongside IL-12 as an adjuvant (pIL-12). The immunized mice showed a lower parasite burden and, consequently, significant protection against infection12. Similarly, recombinant canine distemper virus (CDV) was cloned with various Leishmania antigens like LACK (rCDV-LACK), Thiol-Specific Antioxidant (rCDV-TSA) and LmSTI1 (rCDV - LmSTI1). These recombinant CDVs were evaluated for their protection against virulent L. major in dogs. Only the dogs immunized by the rCDV-LACK were able to protect dogs against L. major infection, whereas the other two rCDVs did not protect. The rCDV-LACK came out as a promising vaccine candidate for CDV and CL13.
The cocktail of LACKp24, TSA, LmSTI1, and CoPa Leishmania antigens was evaluated for protection against CL. Individual antigens provided only a limited extent of Th1 cell-mediated protection. Different combinations of these antigens were tested for their protective efficacy, and the cocktail containing all four antigens proved to be a more effective approach for vaccination. Mice immunized with this combination demonstrated an enhanced Th1 cell-mediated immune response55. Whether the enhanced protection results from additive or synergistic effects, remains a topic of debate. Overall, the concept behind using multivalent antigens is to present multiple epitopes to immune cells, thereby generating a more robust immune response compared to using single antigens. It is anticipated that these multivalent formulations will exhibit additive, if not synergistic, effects.
Vaccine delivery systems
Various delivery systems have been used for the recombinant vaccines, both preclinically and clinically, as detailed below.
Viral vectors
Viral vectors are engineered viruses that deliver Leishmania antigens into host cells, eliciting a strong immune response. Adenoviruses (AdV) are widely used viral vectors for this purpose. Engineering these viruses to deliver antigens is complex; the gene of interest is integrated into the AdV DNA either by homologous recombination or ligation, followed by delivery into the host. Adenoviruses are also popular for delivering CRISPR-Cas9 machinery for genomic editing56.
Bacteriophages are also commonly used as delivery vectors. The main principle behind this approach is the expression of Leishmania antigens on the phage surface. The mimotopes B10 and C01 from L. infantum were selected and cloned into the phage, either alone or in combination. This formulation has been shown to provide cross-protection in mice against L. amazonensis57.
Bacterial vectors
Bacterial vectors involve recombinant bacteria expressing Leishmania antigens to stimulate an immune response. One example is Listeria monocytogenes, a Gram-positive organism used as a live vector. This model relies on both CD8 and CD4-mediated immune responses58,59. For instance, the LACK antigen of L. major was cloned into this bacterium, and when co-administered with IL-12 in BALB/c mice, it induced a robust immune response and showed promising resistance to infection60.
The most common method of using bacteria for delivery is through the exploitation of their plasmids. Plasmids are small DNA carriers that can replicate independently within the host cell without integrating into the host genome for extended periods. Their small size and ease of replication make them an excellent choice for delivering constructs of interest61. For example, the pcDNA3H3H4 plasmid was used to deliver the H3 and H4 histone proteins of L. major61, and the pcDNA 3.1 vector was utilized for the pLeish-dom vaccine, among other vectors. While viral vectors are effective for delivering larger DNA constructs, they tend to be more complex to use compared to bacterial vectors. In contrast, bacterial vectors are easier to handle and are well-suited for delivery.
Non-pathogenic parasitic vectors
Recently, non-pathogenic parasites like Leishmania tarentolae have been exploited to deliver vaccines or DNA directly to dendritic cells and lymph nodes. Recombinant L. tarentolae expressing pathogenic parasite or viral proteins can induce protection in infected subjects. For instance, L. tarentolae expressing the A2 protein protects against L. donovani infection21,62. Studies have also demonstrated successful immunomodulation by dendritic cells using recombinant L. tarentolae expressing SARS-CoV-2 Spike protein63. Despite being non-pathogenic to mammals, L. tarentolae shares a high genetic similarity (90%) with pathogenic species64, raising questions about its commercial viability.
In the race of the vaccine discovery, the L. amazonensis antigens linked with two nanoformulations was checked for the potential immunological response by taking hamsters as a model. The results have shown that the hamsters injected with LAPSmG and LAPSmP had identical immune responses for the anti-leishmania IgG test, demonstrating exceptional protection against the infection65.
Recombinant vaccine candidates edging for human use and commercially available for dogs
Protein vaccines
ChAd63KH
The ChAd63-KH vaccine is a recombinant vaccine designed to protect against leishmaniasis. It utilizes a chimpanzee adenovirus vector (ChAd63) to deliver a synthetic gene encoding two key Leishmania antigens: kinetoplastid membrane protein-11 (KMP-11) and hydrophilic acylated surface protein B (HASPB)17. The ChAd63-KH vaccine has shown promise in preclinical and clinical studies in Sudan, Africa, leading to the induction of strong CD4+ and CD8+ T cell responses, especially for therapeutics against persistent Post Kala Dermal Leishmaniasis (PKDL)66-68. These responses are crucial for controlling and eliminating Leishmania infections.
LEISH-F3+/GLA-SE
LEISH-F3+/GLA-SE is a di-fusion protein (nucleoside hydrolase and sterol 24-C-methyltransferase) vaccine candidate against Leishmania formulated with the adjuvant GLA-SE (glucopyranosyl lipid adjuvant-stable emulsion). The next-generation vaccine was developed by adding a third antigen, truncated CBP (Leishmania cysteine protease B). This protein is known to protect against VL in mice and dogs34,35. Adding truncated CBP elicited a robust immune response like earlier vaccines, with the truncation not affecting vaccine efficacy. The immune response in Leish-F3 with full-length and truncated CBP showed similar protection rates of 94.9 per cent and 95.2 per cent, respectively. LEISH-F3+/GLA-SE protects mice against VL caused by L. infantum and L. donovani32. Based on such experimental data, the researchers developed cGMP grade of it and conducted a phase 1 study in healthy, uninfected adults in the USA33,36. The vaccine was shown to be safe and induced a strong antigen-specific immunity, as evidenced by the cytokine and immunoglobulin subclass32, indicating its commercial potential.
Leish-111f
Leish-111f is a recombinant polyprotein vaccine that combines three antigens: TSA, LmSTI1, and LeIF, with adjuvants such as MPL-SE and Ribi 529. Initially developed to target CL and MCL, this formulation was tested across various forms of leishmaniasis. Mice immunized with Leish-111f were protected against CL, showing enhanced humoral and cell-mediated responses when administered with Ribi 529, whereas MPL69 primarily induced a humoral response. When tested against L. infantum infection, the vaccine also elicited a strong Th1-type immune response in immunized mice, resulting in up to a 99.6 per cent reduction in parasitic burden MPL37,69. In canine trials, Leish-111f + MPL-SE proved effective in mild cases of VL but showed limited efficacy in dogs with severe disease70. Despite this, Leish-111f remains a promising candidate for treating both canine and human populations.
LiESP/QA-21
The LiESP/QA-21 vaccine commercially available in Europe is formulated for canines from the excreted-secreted proteins (LiESP) of L. infantum. During trials, naive dogs vaccinated with LiESP/QA-21 were monitored for serological humoral responses. Some vaccinated dogs experienced local swelling with minor pain, and a few unrelated deaths occurred during the transfer and vaccination period; these dogs were excluded from the final analysis31. Overall, the vaccine demonstrated 68.4 per cent efficacy and 92.7 per cent protection. Serological studies indicated that vaccinated dogs developed antibodies against ESP and PSA, two main vaccine antigens, with 96 per cent showing an IgG2 response against PSA, and 89 per cent showing an IgG1 response against ESP23. Additional studies also showed cell-mediated immune responses induced by this vaccine in dogs71,72, supporting its promising commercial use.
Q protein/LetiFend
An effort to provide immunity against L. infantum in canines involved administering a chimeric multi-component Q protein in either single or double doses. Results indicated that vaccinated dogs exhibited no or only minor clinical symptoms. Serum analysis revealed high levels of anti-Q antibodies, along with increased globulin levels in both single- and double-dose Q-vaccinated dogs. Furthermore, spleen and lymph node examinations confirmed an absence of parasitic burden. A delayed-type hypersensitivity (DTH) response and nitric oxide (NO) production were also observed28. This work led to the development of the commercial canine vaccine LetiFend® by LETI Pharma, Barcelona29, for use in Europe. The overall efficacy of LetiFend® in preventing confirmed cases of L. infantum visceral leishmaniasis in dogs within high endemic areas was reported to be 72 per cent.
Leish-Tec
The A2 protein, mentioned previously5,21-25,50, has been shown to protect mice, monkeys24, and dogs25 against Leishmania infection. Studies indicate that vaccinated dogs exhibit enhanced humoral immunity, with increased IgG, particularly IgG1 and IgG2, compared to placebo and untreated groups, in the vaccinated dogs compared to the placebo and untreated ones. The efficacy of the vaccine was reported at 71.4 per cent, with a protection rate of 96.4 per cent in vaccinated animals73. These findings led to the development of a commercial canine vaccine in Brazil. Formulated with recombinant A2 protein and saponin as an adjuvant, this vaccine, marketed as Leish-Tec® by CevaSanté Animale73, effectively protects canines from VL.
DNA-based Leishmania vaccine candidates
DNA antigens from Leishmania, designed to stimulate cellular immunity, are also relevant to this discussion. Leishmune®, an FML-saponin-based vaccine, was the first licensed veterinary vaccine in Brazil to protect against canine VL and also helped reduce parasite transmission to humans in endemic regions. With preclinical and clinical trials demonstrating 92-95 per cent protection and 76-80 per cent efficacy, Leishmune® proved highly effective11. The FML component, a glycoprotein fraction present on the parasite’s surface throughout its life cycle, is known to inhibit the penetration of promastigotes and amastigote11 forms into host cells. The success of this vaccine underscored its importance in leishmaniasis control, offering substantial canine protection and contributing to lower infection rates in human populations. However, due to the lack of blinded evaluation during clinical trials, Leishmune® was not approved for commercial use, leading to the termination of its marketing license in 201426.
Pleish-dom
Pleish-dom is a DNA vaccine combining four protein-encoding genes. Antigenic regions of the LACK, TSA, KMP11, and LmSTI1 proteins were cloned into the vector pcDNA 3.1, creating a chimeric construct administered to mice. Results demonstrated extensive protection against infection in vaccinated mice, with a strong Th1-mediated immune response12. It remains unclear whether this protection is due to synergy or the additive effect of the genes.
Genetically attenuated live Leishmania recombinant parasite vaccines
Genetically attenuated live Leishmania vaccines present a promising approach for combating leishmaniasis by utilizing genetically modified parasites rendered non-pathogenic74-76. These vaccines are designed to stimulate robust and long-lasting immunity by exposing presenting the immune system to live parasites that mimic natural infection without causing disease. Genetic modifications focus on deleting or inactivating specific genes, particularly those expressed in the amastigote stage77,78 that are essential for parasite virulence, thus ensuring host safety and enhancing vaccine efficacy. Both conventional homologous recombination79 and the more advanced CRISPR Cas9 approach77 have been used to delete the virulence genes in Leishmania strains associated with VL or CL. These genetically attenuated parasites have been tested in various animal models, including the industry-grade GLP parasites, confirming their safety and protective efficacy38-40,80-83. Notably, the centrin gene-deleted L. major knockout parasite, developed through CRISPR as a marker-free gene deletion and originating from a CL-causing strain, protects against both homologous and heterologous strains of Leishmania.
Discussion
Recombinant vaccines represent a promising strategy for inducing immunity against leishmaniasis. These vaccines aim to generate protective immunity by using specific antigens derived from the parasite, produced through genetic engineering techniques77. These antigens are recognized by the immune system, triggering a targeted response. The key players in this response are CD4 and CD8 T cells: CD4 T cells activate immune cells like macrophages, dendritic cells, and B cells, while CD8 T cells directly kill infected cells, helping to control the parasite42. This immune response can be either humoral or cell-mediated, involving T-helper (Th) and Cytotoxic T (Tc) cells. Tc cells can kill infected cells directly and produce cytokines that support pathogen elimination by other immune mechanisms (Figure).
The response is mediated mainly by two types of T-helper cells, Th1 and Th2, depending on antigen interaction. Th2 responses primarily generate a humoral response marked by antibody production, while Th1 responses activate both cellular and humoral immunity. Th1 cells are essential for activating macrophages and producing cytotoxic T lymphocytes, which work to eliminate intracellular pathogens. They also stimulate the production of antigen-specific antibodies, enhancing the overall immune response84. Additionally, memory T-cells are crucial for long-term immunity against leishmaniasis, enabling rapid and effective responses upon re-infection. Various vaccine platforms, including live attenuated, recombinant protein, DNA, mRNA, and viral vector vaccines, are designed to harness the potential of these cells by promoting robust T-cell activation and memory formation.
Incorporating an appropriate adjuvant is crucial, as it enhances the body’s immune response to the vaccine69. Adjuvants can increase the strength and duration of the immune response, ensure proper activation of immune cells, and help direct the immune response toward a more effective pathway, such as promoting Th1 cell-mediated immunity, which is essential for combating intracellular pathogens like Leishmania.
Utilizing a multi-valent antigen cocktail to induce a robust immune response involving CD4 and CD8 T cells54 is a highly effective strategy for vaccine development. This approach enhances immunization by presenting multiple epitopes from various antigens, thereby stimulating a broader and more potent immune response55. Multi-valent vaccines can target different aspects of the pathogen’s lifecycle, increasing the chances of effective immunity.
Delivery vectors play a vital role in ensuring that antigens reach the appropriate cells and tissues in the body. Various delivery methods, such as viral, bacterial, and nanoparticle-based systems, can enhance the uptake and presentation of antigens to the immune system. These vectors also facilitate the sustained release of antigens, providing prolonged exposure to the immune system and enhancing the overall efficacy of vaccines.
Live attenuated Leishmania vaccine candidates would allow the parasites to replicate within the host, triggering both humoral and cellular immune responses. This approach typically leads to longer-lasting immunity and may reduce the need for multiple immunizations42,83. In contrast, dead parasite vaccines or other single antigen immunizations usually induce primarily a humoral immune response, often requiring multiple doses and resulting in shorter-lasting immunity. By eliciting strong and sustained CD4 and CD8 T cell responses, live attenuated vaccines have the potential to achieve higher levels of efficacy and long-lasting immunity, ultimately contributing to better control and eradication of the disease.
Conclusion
The lack of a single effective vaccine against leishmaniasis after decades of research stems from a combination of scientific, immunological, economic, and logistical challenges. The complexity of the Leishmania parasite, its immune evasion mechanisms, the diverse forms of the disease, and the socio-economic context in which leishmaniasis occurs have all contributed to the slow progress. However, recombinant vaccines represent a significant potential in combating this disease. Continued research and development are crucial to addressing current challenges and delivering effective vaccines to those in need.
By targeting specific antigens and employing innovative delivery systems, recombinant vaccines could become critical tools for preventing and managing leishmaniasis. Nonetheless, a major challenge remains in the partial protection offered by a few formulations. Other obstacles include regional variability in protection, lower efficacy rates, and potential side effects. CRISPR-based approaches for creating live attenuated vaccines represent a promising advancement in the field of vaccine development, particularly for diseases like leishmaniasis caused by Leishmania parasites. While the precision, efficiency, and potential for rapid development are significant advantages, researchers must navigate challenges related to delivery, safety, stability, and ethical considerations. As this field continues to evolve, ongoing research will be crucial to addressing these challenges and harnessing the full potential of CRISPR technology in the fight against leishmaniasis. Also, utilizing nanoparticles as delivery vehicles for antigens or adjuvants can improve the stability and targeting of vaccine components. This approach enhances antigen presentation to the immune system and can be tailored to promote specific immune responses. The use of mRNA and DNA vaccine platforms allows for a rapid response to emerging strains of Leishmania.
Financial support & sponsorship
None.
Conflicts of Interest
None.
Use of Artificial Intelligence (AI)-Assisted Technology for manuscript preparation
The authors confirm that there was no use of AI-assisted technology for assisting in the writing of the manuscript and no images were manipulated using AI.
References
- Immunity to visceral leishmaniasis using genetically defined live-attenuated parasites. J Trop Med. 2012;2012:631460.
- [Google Scholar]
- Beta1 and beta2 receptor mechanisms in rat jugular veins: differences between norepinephrine and isoproterenol-induced relaxation. Life Sci. 1978;23:1997-2006.
- [Google Scholar]
- Delivery of mRNA vaccine with a lipid-like material potentiates antitumor efficacy through Toll-like receptor 4 signaling. Proc Nat Acad Sci. 2021;118:e2005191118.
- [Google Scholar]
- A comprehensive analysis of LACK (Leishmania homologue of receptors for activated C kinase) in the context of Visceral Leishmaniasis. Bioinformation. 2013;9:832-7.
- [Google Scholar]
- Immune responses induced by the Leishmania (Leishmania) donovani A2 antigen, but not by the LACK antigen, are protective against experimental Leishmania (Leishmania) amazonensis Infection. Infect Immun. 2003;71:3988-94.
- [Google Scholar]
- The role of Leishmania GP63 in the modulation of innate inflammatory response to Leishmania major infection. PLoS One. 2021;16:e0262158.
- [Google Scholar]
- Leishmania virulence factors: focus on the metalloprotease GP63. Microbes Infect. 2012;14:1377-89.
- [Google Scholar]
- A prime/boost DNA/Modified vaccinia virus Ankara vaccine expressing recombinant Leishmania DNA encoding TRYP is safe and immunogenic in outbred dogs, the reservoir of zoonotic visceral leishmaniasis. Vaccine. 2009;27:1080-6.
- [Google Scholar]
- Ubiquitin conjugation of open reading frame F DNA vaccine leads to enhanced cell-mediated immune response and induces protection against both antimony-susceptible and -resistant strains of Leishmania donovani. J Immunol. 2009;183:7719-31.
- [Google Scholar]
- Immune response in susceptible <scp>BALB</scp> /c mice immunized with <scp>DNA</scp> encoding Lipophosphoglycan 3 of Leishmania infantum. Parasite Immunol. 2014;36:700-7.
- [Google Scholar]
- FML vaccine against canine visceral leishmaniasis: from second-generation to synthetic vaccine. Expert Rev Vaccines. 2008;7:833-51.
- [Google Scholar]
- Immunization against Leishmania major infection in BALB/c mice using a subunit-based DNA vaccine derived from TSA, LmSTI1, KMP11, and LACK predominant antigens. Iran J Basic Med Sci. 2019;22:1493-501.
- [Google Scholar]
- Efficacy of recombinant canine distemper virus expressing leishmania antigen against leishmania challenge in dogs. PLoS Negl Trop Dis. 2015;9:e0003914.
- [Google Scholar]
- Immunogenicity of HLA‐DR1 and HLA‐A2 peptides derived from Leishmania major Gp63 in golden hamsters. Parasite Immunol. 2020;42:e127801.
- [Google Scholar]
- Immunotherapy against visceral leishmaniasis with the nucleoside hydrolase-DNA vaccine of Leishmania donovani. Vaccine. 2006;24:4863-73.
- [Google Scholar]
- Immunogenicity of a multicomponent DNA vaccine against visceral leishmaniasis in dogs. Vaccine. 2006;24:1928-40.
- [Google Scholar]
- Therapeutic vaccination with recombinant adenovirus reduces splenic parasite burden in experimental visceral leishmaniasis. J Infect Dis. 2012;205:853-63.
- [Google Scholar]
- Comparing Montanide ISA 720 and 50-V2 adjuvants formulated with LmSTI1 protein of Leishmania major indicated the potential cytokine patterns for induction of protective immune responses in BALB/c mice. Mol Immunol. 2016;76:108-15.
- [Google Scholar]
- Leishmania Eukaryotic Initiation Factor (LeIF) Inhibits parasite growth in murine macrophages. PLoS One. 2014;9:e97319.
- [Google Scholar]
- Kinetoplastid Membrane Protein-11 DNA vaccination induces complete protection against both pentavalent antimonial-sensitive and -resistant strains of Leishmania donovani that correlates with inducible nitric oxide synthase activity and IL-4 generation: evidence for mixed Th1- and Th2-like responses in visceral Leishmaniasis. J Immunol. 2005;174:7160-71.
- [Google Scholar]
- Recombinant Leishmania tarentolae expressing the A2 virulence gene as a novel candidate vaccine against visceral leishmaniasis. Vaccine. 2009;28:53-62.
- [Google Scholar]
- Epitope mapping and protective immunity elicited by adenovirus expressing the Leishmania amastigote specific A2 antigen: Correlation with IFN-γ and cytolytic activity by CD8+ T cells. Vaccine. 2008;26:4585-93.
- [Google Scholar]
- Evaluation of immune responses and protection induced by A2 and nucleoside hydrolase (NH) DNA vaccines against Leishmania chagasi and Leishmania amazonensis experimental infections. Microbes Infect. 2007;9:1070-7.
- [Google Scholar]
- Clinical and parasitological protection in a leishmania infantum-macaque model vaccinated with adenovirus and the recombinant A2 antigen. PLoS Negl Trop Dis. 2014;8:e2853.
- [Google Scholar]
- Protective immunity against challenge with Leishmania (Leishmania) chagasi in beagle dogs vaccinated with recombinant A2 protein. Vaccine. 2008;26:5888-95.
- [Google Scholar]
- Commercially approved vaccines for canine leishmaniosis: a review of available data on their safety and efficacy. Trop Med Int Health. 2020;25:540-57.
- [Google Scholar]
- Making an anti-amastigote vaccine for visceral leishmaniasis: rational, update and perspectives. Curr Opin Microbiol. 2012;15:476-85.
- [Google Scholar]
- The chimerical multi-component Q protein from Leishmania in the absence of adjuvant protects dogs against an experimental Leishmania infantum infection. Vaccine. 2009;27:5964-73.
- [Google Scholar]
- A large-scale field randomized trial demonstrates safety and efficacy of the vaccine LetiFend® against canine leishmaniosis. Vaccine. 2018;36:1972-82.
- [Google Scholar]
- A sand fly salivary protein vaccine shows efficacy against vector-transmitted cutaneous leishmaniasis in nonhuman primates. Sci Transl Med. 2015;7:290ra90.
- [Google Scholar]
- A randomized, double-blind, controlled efficacy trial of the LiESP/QA-21 vaccine in naïve dogs exposed to two leishmania infantum transmission seasons. PLoS Negl Trop Dis. 2014;8:e3213.
- [Google Scholar]
- From mouse to man: safety, immunogenicity and efficacy of a candidate leishmaniasis vaccine LEISH-F3+GLA-SE. Clin Transl Immunology. 2015;4:e35.
- [Google Scholar]
- Leishmania vaccine development: A comprehensive review. Cell Immunol. 2024;399-400:104826.
- [Google Scholar]
- Protective vaccination against experimental canine visceral leishmaniasis using a combination of DNA and protein immunization with cysteine proteinases type I and II. Vaccine. 2005;23:3716-25.
- [Google Scholar]
- Prime-boost vaccination using cysteine proteinases type I and II of Leishmania infantum confers protective immunity in murine visceral leishmaniasis. Vaccine. 2006;24:2169-75.
- [Google Scholar]
- Advances in Leishmania vaccines: current development and future prospects. Pathogens. 2024;13:812.
- [Google Scholar]
- Leish-111f, a recombinant polyprotein vaccine that protects against visceral leishmaniasis by elicitation of CD4 + T Cells. Infect Immun. 2007;75:4648-54.
- [Google Scholar]
- Gene deleted live attenuated Leishmania vaccine candidates against visceral leishmaniasis elicit pro-inflammatory cytokines response in human PBMCs. Sci Rep. 2016;6:33059.
- [Google Scholar]
- Preclinical validation of a live attenuated dermotropic Leishmania vaccine against vector transmitted fatal visceral leishmaniasis. Commun Biol. 2021;4:929.
- [Google Scholar]
- Centrin-deficient Leishmania mexicana confers protection against New World cutaneous leishmaniasis. NPJ Vaccines. 2022;7:32.
- [Google Scholar]
- Live attenuated Leishmania infantum centrin deleted mutant (LiCen) as a novel vaccine candidate: A field study on safety, immunogenicity, and efficacy against canine leishmaniasis. Comp Immunol Microbiol Infect Dis. 2023;97:101984.
- [Google Scholar]
- Zooming in on common immune evasion mechanisms of pathogens in phagolysosomes: potential broad-spectrum therapeutic targets against infectious diseases. FEMS Microbiol Rev. 2023;47:Fuac041.
- [Google Scholar]
- Molecular characterization of the heat-inducible LmSTI1 protein of Leishmania major. Mol Biochem Parasitol. 1997;89:179-93.
- [Google Scholar]
- An alphavirus-derived self-amplifying mRNA encoding PpSP15-LmSTI1 fusion protein for the design of a vaccine against leishmaniasis. Parasitol Int. 2022;89:102577.
- [Google Scholar]
- Development of dominant epitope-based vaccines encoding Gp63, Kmp-11 and Amastin against visceral leishmaniasis. Immunobiology. 2021;226:152085.
- [Google Scholar]
- Crosslinked chitosan microparticles as a safe and efficient DNA carrier for intranasal vaccination against cutaneous leishmaniasis. Vaccine X. 2023;15:100403.
- [Google Scholar]
- Leishmania infantum LeIF protein is an ATP‐dependent RNA helicase and an eIF4A‐like factor that inhibits translation in yeast. FEBS J. 2006;273:5086-100.
- [Google Scholar]
- Immunogenicity and neutralization potential of recombinant chimeric protein comprising the catalytic region of Gp63 of Leishmania and LTB against Leishmania donavani. Protein Pept Lett 2024 Epub2024 Sep 19
- [Google Scholar]
- Localization and induction of the A2 virulence factor in Leishmania: evidence that A2 is a stress response protein. Mol Microbiol. 2010;77:518-30.
- [Google Scholar]
- Immunization with A2 protein results in a mixed Th1/Th2 and a humoral response which protects mice against Leishmania donovani infections. Vaccine. 2001;20:59-66.
- [Google Scholar]
- What’s behind a sand fly bite? The profound effect of sand fly saliva on host hemostasis, inflammation and immunity. Infect Genet Evol. 2014;28:691-703.
- [Google Scholar]
- Immunity to a salivary protein of a sand fly vector protects against the fatal outcome of visceral leishmaniasis in a hamster model. Proc Natl Acad of Sci USA. 2008;105:7845-50.
- [Google Scholar]
- Humoral responses and ex vivo IFN-γ production after canine whole blood stimulation with Leishmania infantum antigen or KMP11 recombinant protein. Vet Sci. 2022;9:116.
- [Google Scholar]
- Vaccination with plasmid DNA encoding TSA/LmSTI1 Leishmanial fusion proteins confers protection against Leishmania major infection in susceptible BALB/c Mice. Infect Immun. 2002;70:2828-36.
- [Google Scholar]
- DNA based vaccination with a cocktail of plasmids encoding immunodominant Leishmania (Leishmania) major antigens confers full protection in BALB/c mice. Vaccine. 2009;27:99-106.
- [Google Scholar]
- Adenovirus vector system: construction, history and therapeutic applications. Biotechniques. 2022;73:297-305.
- [Google Scholar]
- Phage-fused epitopes from Leishmania infantum used as immunogenic vaccines confer partial protection against Leishmania amazonensis infection. Parasitology. 2015;142:1335-47.
- [Google Scholar]
- Murine listeriosis as a model of antimicrobial defense. Immunol Rev. 1997;158:27-36.
- [Google Scholar]
- Cytokines in the induction and expression of T‐cell‐mediated granuloma formation and protection in the murine model of listeriosis. Immunol Rev. 1997;158:79-93.
- [Google Scholar]
- Listeria monocytogenes as a short-lived delivery system for the induction of type 1 cell-mediated immunity against the p36/LACK antigen of Leishmania major. Infect Immun. 2000;68:1498-506.
- [Google Scholar]
- Vaccines for leishmaniasis and the implications of their development for American tegumentary leishmaniasis. Hum Vaccin Immunother. 2020;16:919-30.
- [Google Scholar]
- Leishmania tarentolae: a vaccine platform to target dendritic cells and a surrogate pathogen for next generation vaccine research in leishmaniases and viral infections. Parasit Vectors. 2023;16:35.
- [Google Scholar]
- Leishmania tarentolae as an antigen delivery platform: Dendritic cell maturation after infection with a clone engineered to express the SARS-CoV-2 spike protein. Vaccines (Basel). 2022;10:803.
- [Google Scholar]
- Genome sequencing of the lizard parasite Leishmania tarentolae reveals loss of genes associated to the intracellular stage of human pathogenic species. Nucleic Acids Res. 2012;40:1131-47.
- [Google Scholar]
- Vaccination with formulation of nanoparticles loaded with Leishmania amazonensis antigens confers protection against experimental visceral leishmaniasis in hamster. Vaccines (Basel). 2023;11:111.
- [Google Scholar]
- Safety and immunogenicity of ChAd63-KH vaccine in post-kala-azar dermal leishmaniasis patients in Sudan. Mol Ther. 2021;29:2366-77.
- [Google Scholar]
- A third generation vaccine for human visceral leishmaniasis and post kala azar dermal leishmaniasis: First-in-human trial of ChAd63-KH. PLoS Negl Trop Dis. 2017;11:e0005527.
- [Google Scholar]
- LEISH2b - A phase 2b study to assess the safety, efficacy, and immunogenicity of the Leishmania vaccine ChAd63-KH in post-kala azar dermal leishmaniasis. Wellcome Open Res. 2022;7:200.
- [Google Scholar]
- Immunization with a polyprotein vaccine consisting of the T-cell antigens thiol-specific antioxidant, Leishmania major stress-inducible protein 1, and Leishmania elongation initiation factor protects against Leishmaniasis. Infect Immun. 2002;70:4215-25.
- [Google Scholar]
- Treatment of canine visceral leishmaniasis by the vaccine Leish-111f+MPL-SE. Vaccine. 2010;28:3333-40.
- [Google Scholar]
- Primary vaccination with the LiESP/QA-21 vaccine (CaniLeish) produces a cell-mediated immune response which is still present 1 year later. Vet Immunol Immunopathol. 2014;158:199-207.
- [Google Scholar]
- The protective immune response produced in dogs after primary vaccination with the LiESP/QA-21 vaccine (CaniLeish®) remains effective against an experimental challenge one year later. Vet Res. 2014;45:69.
- [Google Scholar]
- Field randomized trial to evaluate the efficacy of the Leish-Tec ® vaccine against canine visceral leishmaniasis in an endemic area of Brazil. Vaccine. 2016;34:2233-9.
- [Google Scholar]
- Revival of leishmanization and leishmanin. Front Cell Infect Microbiol. 2021;11:639801.
- [Google Scholar]
- Generation of growth arrested Leishmania amastigotes: a tool to develop live attenuated vaccine candidates against visceral leishmaniasis. Vaccine. 2014;32:3895-901.
- [Google Scholar]
- Live attenuated-nonpathogenic Leishmania and DNA structures as promising vaccine platforms against leishmaniasis: innovations can make waves. Front Microbiol. 2024;15:1326369.
- [Google Scholar]
- A second generation leishmanization vaccine with a markerless attenuated Leishmania major strain using CRISPR gene editing. Nat Commun. 2020;11:3461.
- [Google Scholar]
- Identification of protein biomarkers of attenuation and immunogenicity of centrin or p27 gene deleted live vaccine candidates of Leishmania against visceral leishmaniasis. Parasitol Int. 2023;92:102661.
- [Google Scholar]
- Centrin gene disruption impairs stage-specific basal body duplication and cell cycle progression in Leishmania. J Biol Chem. 2004;279:25703-10.
- [Google Scholar]
- Centrin-deficient Leishmania mexicana confers protection against Old World visceral leishmaniasis. NPJ Vaccines. 2022;7:157.
- [Google Scholar]
- Manufacturing and preclinical toxicity of GLP grade gene deleted attenuated Leishmania donovani parasite vaccine. Sci Rep. 2024;14:14636.
- [Google Scholar]
- Live attenuated Leishmania donovani centrin knock out parasites generate non-inferior protective immune response in aged mice against visceral leishmaniasis. PLoS Negl Trop Dis. 2016;10:e0004963.
- [Google Scholar]
- Intracellular replication-deficient Leishmania donovani induces long lasting protective immunity against visceral leishmaniasis. J Immunol. 2009;183:1813-20.
- [Google Scholar]






